Abstract
It is increasingly recognized that the mechanisms underlying ischemic cell death are sexually dimorphic. Stroke-induced cell death in males is initiated by the mitochondrial release of apoptosis-inducing factor, resulting in caspase-independent cell death. In contrast, ischemic cell death in females is primarily triggered by mitochondrial cytochrome c release with subsequent caspase activation. Because X-linked inhibitor of apoptosis (XIAP) is the primary endogenous inhibitor of caspases, its regulation may play a unique role in the response to injury in females. XIAP mRNA levels were higher in females at baseline. Stroke induced a significant decrease in XIAP mRNA in females, whereas no changes were seen in the male brain. However, XIAP protein levels were decreased in both sexes after stroke. MicroRNAs (miRNAs) predominantly induce translational repression and are emerging as a major regulators of mRNA and subsequent protein expression after ischemia. The miRNA miR-23a was predicted to bind XIAP mRNA. miR-23a directly bound the 3′ UTR of XIAP, and miR-23a inhibition led to an increase in XIAP mRNA in vitro, demonstrating that XIAP is a previously uncharacterized target for miR-23a. miR-23a levels differed in male and female ischemic brains, providing evidence for sex-specific miRNA expression in stroke. Embelin, a small-molecule inhibitor of XIAP, decreased the interaction between XIAP and caspase-3 and led to enhanced caspase activity. Embelin treatment significantly exacerbated stroke-induced injury in females but had no effect in males, demonstrating that XIAP is an important mediator of sex-specific responses after stroke.
Keywords: middle cerebral artery occlusion, estrogen, ovariectomy, spectrin cleavage, second mitochondria-derived activator of caspases/direct inhibitor of apoptosis binding protein with low Pi
Cerebral ischemia activates both caspase-independent and caspase-dependent cell death pathways (1). Sex differences exist in these pathways as evidenced by the fact that genetic deletion or pharmacological inhibition of neuronal nitric oxide synthase or poly(ADP-ribose) polymerase 1 (PARP-1) is robustly neuroprotective in males but paradoxically increases damage in females in experimental stroke models (2–6). In contrast, caspase inhibition reduced injury after middle cerebral artery occlusion (MCAO) in females but had minimal effects in males (7). Sex differences have also been well documented in neonatal and in vitro models (2, 8, 9), eliminating the possibility that these effects are secondary solely to activational effects of gonadal hormones (10, 11).
The molecular mechanisms underlying sex differences in the response to stroke have not yet been elucidated. One candidate molecule is X-linked inhibitor of apoptosis (XIAP), the most potent and versatile member of the inhibitors of apoptosis (IAP) family of proteins, which inhibits caspases-3, -7, and -9 (12, 13). Cellular regulation of XIAP occurs via its endogenous inhibitor, second mitochondria-derived activator of caspase (Smac/DIABLO). This mitochondrial protein inhibits XIAP's ability to bind caspases (13–17), leading to enhanced activation of caspase pathways. Because caspase activation and cytochrome c release occur preferentially in females after experimental ischemia, and females are differentially responsive to caspase inhibition (7, 18), XIAP regulation could be a major contributor to sex differences in stroke. Posttranscriptional mechanisms are increasingly recognized as important contributors to protein regulation (19). MicroRNAs (miRNAs) are noncoding 18- to 24-nt transcripts that bind to a target mRNA, causing translational repression (20). Multiple miRNAs have been implicated in regulation of cell death (21, 22), ischemic preconditioning (23, 24), and angiogenesis (25), in addition to experimental stroke (26, 27), but these have yet to be specifically evaluated in females. Many X-linked miRNAs escape X inactivation (28), and sex-specific miRNA expression occurs in the gonads (29) and brain (30), although differential regulation during ischemia has not been investigated. Using in silico methods as well as both the miRanda and TargetScan databases (31, 32), we determined that miR-23a binding sites reside in the 3′ UTR of XIAP mRNA. To determine whether XIAP played a role in ischemic sexual dimorphism, we used embelin, a small-molecule XIAP inhibitor, to directly assess the effects of XIAP inhibition after experimental stroke (33). We hypothesized that XIAP, because of its unique and integral role in caspase-mediated cell death, was regulated by translational repression by miR-23a and was a key mediator of ischemic sexual dimorphism.
Results
XIAP and Smac/DIABLO mRNA Expression.
At 6 h after stroke, XIAP mRNA levels differed dramatically based on the sex of the animal examined. Baseline XIAP mRNA levels were significantly higher in females compared with age-matched males (Fig. 1A, bar 1 vs. bar 3; P < 0.05). Stroke induced a decrease in XIAP mRNA in gonadally intact (GI) females, with no effect in males (Fig. 1A, bar 3 vs. bar 4; P < 0.05). Because it is well documented that estrogen is neuroprotective after experimental stroke, at least in young animals (34), we also assessed XIAP levels in ovariectomized (Ovx) females and Ovx females replaced with 17β-estradiol (E2) to determine whether changes in XIAP were secondary to intrinsic sex differences or related to circulating E2 levels. Similar to what was seen in intact females, both Ovx and Ovx + E2 groups had high baseline levels of XIAP mRNA and exhibited significant stroke-induced decreases in XIAP mRNA expression (Fig. 1A, bar 5 vs. bar 6 and bar 7 vs. bar 8; P < 0.05), suggesting that XIAP mRNA regulation was independent of ovarian hormone levels.
Fig. 1.
XIAP and Smac/DIABLO mRNA expression at 6 h after ischemia. (A) XIAP mRNA expression was decreased after stroke in females with no change in males or Ovx or Ovx + E2 females (n = 3, P < 0.05). (B) Smac/DIABLO mRNA expression was decreased after stroke in females with no change in males or Ovx or Ovx + E2 females (n = 3, P < 0.05). Values were normalized to GAPDH.
Smac/DIABLO mRNA levels were also examined at 6 h after ischemia, and a pattern emerged that was similar to that seen with XIAP mRNA. GI females had significantly higher baseline Smac/DIABLO mRNA expression compared with males (Fig. 1B, bar 1 vs. bar 3; P < 0.05). Stroke induced a significant decrease in Smac/DIABLO in GI females but had no effect in males (Fig. 1B, bar 3 vs. bar 4; P < 0.05). Ovx and Ovx + E2 females exhibited the same pattern as GI females did, with high baseline mRNA levels and a significant decrease in Smac/DIABLO mRNA expression after stroke (Fig. 1B, bar 5 vs. bar 6 and bar 7 vs. bar 8; P < 0.05).
XIAP and Smac/DIABLO Protein Expression.
Because changes in mRNA are not always reflected at the protein level (35), we subsequently examined cytosolic and mitochondrial XIAP and Smac/DIABLO protein at 6 h after ischemia in male and female mice. To manipulate levels of XIAP and assess stroke-induced protein changes, we administered the small-molecule pharmacological XIAP inhibitor embelin (33). Cytosolic purity was determined by presence of macrophage migration inhibitory factor (MIF) and absence of cytochrome c oxidase (COXIV), whereas mitochondrial purity was determined by presence of COXIV and absence of MIF. XIAP protein levels were decreased in both cytosolic and mitochondrial fractions in females compared with males at baseline (Fig. 2 A and B, lane 1 vs. lane 5; P < 0.05), suggesting the possibility of a more activated caspase pathway in the female brain. Stroke induced a decrease in XIAP protein in males and females in both fractions (Fig. 2 A and B, lane 1 vs. lane 2 and lane 5 vs. lane 6; P < 0.05). Embelin treatment also led to increased XIAP protein in the cytosolic fraction (Fig. 2A, lane 5 vs. lane 7; P < 0.05) and no effect in the mitochondrial fraction (Fig. 2B, lane 5 vs. lane 7; P > 0.05) in noninjured sham females and no effect in either fraction in males (Fig. 2 A and B, lane 1 vs. lane 3; P > 0.05). Embelin eliminated the stroke-induced decrease in XIAP protein in the cytosolic fraction in females (Fig. 2A, lane 7 vs. lane 8; P > 0.05). These data suggest that embelin alters XIAP protein expression selectively in females, whereas males show decreased XIAP protein regardless of embelin treatment. We subsequently examined the endogenous inhibitor of XIAP, Smac/DIABLO.
Fig. 2.
XIAP protein expression at 6 h after ischemia. (A) Cytosolic XIAP (57 kDa) was decreased after stroke in all groups except embelin-treated females (n = 3, P < 0.05). Protein expression was normalized to MIF (15 kDa). No COXIV (17 kDa) was seen in the cytosolic fraction to ensure purity. (B) Mitochondrial XIAP (57 kDa) was decreased in all groups (n = 3, P < 0.05). Protein expression was normalized to COXIV (17 kDa). No MIF (15 kDa) was seen in the mitochondrial fraction to ensure purity.
Smac/DIABLO protein levels were decreased in the cytosolic fractions in females compared with males at baseline (Fig. 3 A and B, lane 1 vs. lane 5; P < 0.05), whereas no differences between the sexes were seen in the mitochondria (Fig. 3B, lane 1 vs. lane 5; P > 0.05). Stroke induced a decrease in Smac/DIABLO protein in both males and females in both fractions (Fig. 3 A and B, lane 1 vs. lane 2 and lane 5 vs. lane 6; P < 0.05). No significant differences in Smac/DIABLO protein in the cytosolic and mitochondrial fractions were seen in either sex after embelin treatment in sham mice; however, embelin eliminated the stroke-induced decrease of Smac/DIABLO protein in both fractions in females (Fig. 3 A and B, lane 7 vs. lane 8; P > 0.05), whereas the stroke-induced decrease was still evident in both fractions in males (Fig. 3 A and B, lane 3 vs. lane 4; P < 0.05). Similar to XIAP, Smac/DIABLO protein levels were altered with embelin treatment in the female brain, whereas the male brain showed similar results irrespective of embelin treatment. These data suggest that females are more sensitive to the effects of manipulation of XIAP signaling than males.
Fig. 3.
Smac/DIABLO protein expression at 6 h after ischemia. (A) Cytosolic Smac/DIABLO (25 kDa) was decreased in all groups except embelin-treated females (n = 3, P < 0.05). Protein expression was normalized to MIF (15 kDa). No COXIV (17 kDa) was seen in the cytosolic fraction to ensure purity. (B) Mitochondrial Smac/DIABLO (25 kDa) was decreased in all groups except for embelin-treated females (n = 3, P < 0.05). Protein expression was normalized to COXIV (17 kDa). No MIF (15 kDa) was seen in the mitochondrial fraction to ensure purity.
Coimmunoprecipitation of XIAP and Caspase-3.
Coimmunoprecipitation studies were performed to evaluate sex differences in the protein interaction between XIAP and Caspase-3 and to confirm the specificity and efficacy of embelin to reduce XIAP activity. Because XIAP is the endogenous inhibitor of caspases, less of an interaction between XIAP and caspase-3 would allow for enhanced caspase availability. Stroke induced an increase in cleaved (active) caspase-3 that was bound to XIAP in males (Fig. S1A, lane 2 vs. lane 3 and SI Materials and Methods), whereas females exhibited a stroke-induced decrease in the XIAP/Caspase-3 interaction (Fig. S1A, lane 4 vs. lane 5). The interaction of both full-length and cleaved caspase-3 with XIAP was decreased in stroke females compared with stroke males (Fig. S1A, lane 3 vs. lane 5). The increased interaction of XIAP with caspase-3 in males and the decrease in females was sex-specific and would be expected to lead to enhanced caspase activation in females because of the loss of the restraining effect of XIAP (12, 13). Embelin decreased both full-length and cleaved caspase-3 in sham (Fig. S1B, lane 2 vs. lane 4) and stroke (Fig. S1B, lane 3 vs. lane 5) males. Embelin also decreased both full-length and cleaved caspase-3 in sham (Fig. S1C, lane 2 vs. lane 4) and stroke (Fig. S1C, lane 3 vs. lane 5) females. This finding suggested that embelin is in fact functioning to inhibit XIAP by decreasing the association between XIAP and Caspase-3 in sham and stroke conditions in both sexes.
Caspase-3 Activity Measured by αII-Spectrin Cleavage.
Coimmunoprecipitation studies revealed less caspase-3 is bound to XIAP (Fig. S1A, lane 3 vs. lane 5) and is more readily available to activate apoptosis in females. We confirmed the hypothesized activation of caspases by assessing cleaved αII-spectrin levels as a measure of caspase-3 activity. αII-spectrin has a specific caspase-3 cleavage site, and the 150-kDa cleavage product was examined to assess caspase-3 activity (36). An increase in cleaved αII-spectrin was seen after embelin treatment (Fig. S2, lane 1 vs. lane 3 and lane 5 vs. lane 7) as well as after stroke (Fig. S2, lane 5 vs. lane 6) in females, both demonstrating increased caspase activity and confirming the effect of embelin on caspase activation.
miR-23a Targets XIAP.
The mechanism by which XIAP is regulated in the ischemic brain is largely unknown. Results indicated that XIAP is differentially regulated in females versus males, with a stroke-induced decrease in mRNA (Fig. 1A) and protein (Fig. 2) in females. Interestingly, Smac/DIABLO exhibited a similar trend of decreased mRNA (Fig. 1B) and protein (Fig. 3) after stroke. These data suggest Smac/DIABLO is not responsible for the regulation of XIAP in our model; therefore, posttranscriptional regulation of XIAP was evaluated. miR-23a was predicted to bind and inhibit XIAP expression in silico. To confirm that miR-23a directly and specifically regulated XIAP expression, we examined HeLa cells, which provided us with a controlled, easily transfectable system for analysis. A single-stranded oligonucleotide designed to bind miR-23a (anti–miR-23a) was used to inhibit miR-23a binding to its target mRNA. Inhibition of miR-23a by anti–miR-23a led to a significant increase in XIAP mRNA expression (Fig. 4A, bars 1 and 2 vs. bar 3; P < 0.05). Scrambled anti-miRNA had no effect on XIAP mRNA expression (Fig. 4A, bar 1 vs. bar 2; P > 0.05).
Fig. 4.
miR-23a targets XIAP. (A) HeLa cells were mock-transfected or transfected with scrambled anti-miRNA or anti–miR-23a. XIAP mRNA was increased with anti–miR-23a treatment (n = 3 per group, P < 0.05 normalized to GAPDH). (B) The predicted miR-23a binding site on XIAP mRNA was inserted into pIS-0 to make pIS-XIAP vector. Grayed out area is the TargetScan predicted binding site at positions 2,675–2,698 of the XIAP 3′ UTR. The mutant sequence was inserted as a negative control for the binding experiment. HeLa cells were transfected with Renilla, a control luciferase vector, for normalization and pIS-0, pIS-XIAP, or pIS-XIAP mutant for 24 h. Cells were also treated with pre–miR-23a (200 nM) or anti–miR-23a (40 nM) and then lysed, and pIS luciferase expression was measured followed by Renilla expression using a Dual-Glo assay system. Luciferase expression was decreased with pIS-XIAP, suggesting that miR-23a binds the 3′ UTR of XIAP mRNA (n = 3 per group, P < 0.05). (C) miR-23a expression was decreased in males and increased in females at 6 h after stroke (n = 4 sham, n = 4 stroke, P < 0.05 normalized to 5s).
To provide direct evidence of the miR-23a interaction with XIAP, a luciferase-based binding assay was performed. The predicted sequence on the 3′ UTR of XIAP was inserted into the pIS-0 vector (37) (Fig. 4B), yielding pIS-XIAP. Binding of miR-23a to XIAP mRNA was measured by a decrease in luciferase activity. Luciferase activity was measured at 24 h after transfection and normalized to activity of Renilla, a control luciferase vector (38). miR-23a bound to the predicted sequence in the vector and led to translational repression as seen by a decrease in luciferase activity. pIS-XIAP had significantly decreased luciferase activity compared with pIS-0, and overexpression of miR-23a further decreased the luciferase activity, confirming binding of miR-23a to XIAP. Use of anti–miR-23a to inhibit miR-23a activity partially rescued the luciferase activity by inhibiting the ability of miR-23a to bind to its target sequence (Fig. 4B), which confirmed that miR-23a directly binds to positions 2,675–2,698 of the 3′ UTR of XIAP.
Sex Differences in miR-23a.
Although miR-23a bound to the 3′ UTR of XIAP mRNA, which reduced XIAP mRNA expression in vitro, the importance of this regulation in vivo was unknown. We assessed whether expression of miR-23a was differentially regulated in male and GI female brain after MCAO. No differences in miR-23a expression between males and females were identified at baseline (Fig. 4C, bar 1 vs. bar 3; P > 0.05); however, ischemia induced a significant decrease in miR-23a expression in males (Fig. 4C, bar 1 vs. bar 2; P < 0.05). In contrast, miR-23a expression was significantly increased after stroke in GI females compared with sham GI females (Fig. 4C, bar 3 vs. bar 4; P < 0.05). Enhanced miR-23a expression is likely responsible for the decreased XIAP protein expression, despite elevations in XIAP mRNA, that was seen in sham females (Fig. 1A).
Embelin's Effect on Infarct Size.
To directly assess the physiological consequences of decreasing XIAP in the ischemic brain, we administered embelin (33) to males, GI females, and Ovx females subjected to MCAO. Because embelin has never been shown to cross the blood–brain barrier, liquid chromatography/tandem mass spectrometry (LC-MS/MS; Charles River Laboratories) was performed on male and female sham and stroke brains at 24 h after a 3-d dosing regimen. Embelin concentrations were elevated in the brains of both male and female sham and stroke mice (Table S1). The levels were significantly higher in stroke mice compared with sham (Table S1) and were near the documented IC50 for embelin (4.1 ± 1.1 μM) (33). Importantly, the previous coimmunoprecipitation studies confirmed that embelin decreased the association between XIAP and Caspase-3 in both sexes and was acting as a XIAP inhibitor.
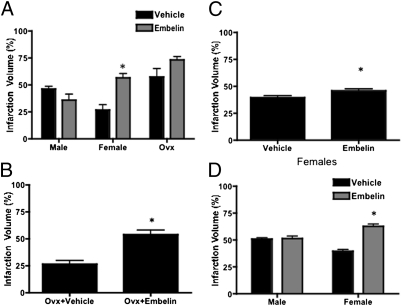
Embelin (20 mg/kg) significantly exacerbated ischemic damage in females (56.6 ± 3.9% and 26.7 ± 4.8%, P < 0.01, n = 10) but had no effect in males (Fig. 5A). Ovx females had significantly larger infarcts than GI females did, secondary to the loss of estrogen (Fig. 5A). Embelin-treated Ovx females showed no significant difference in infarct size from vehicle-treated Ovx females after a 90-min MCAO, although there was an apparent trend toward infarct exacerbation (73.2 ± 3.2% and 57.4 ± 7.9%, n = 10, Fig. 5A). Because of the high stroke volumes in the Ovx groups and the possibility of a ceiling effect, infarct duration was decreased to 60 min. Embelin significantly exacerbated ischemic damage in Ovx mice after a 60-min MCAO (53.8 ± 4.1% and 26.5 ± 3.5%, n = 7, P < 0.05, Fig. 5B), suggesting that the effects of embelin on infarct exacerbation were independent of circulating estrogen levels. A dose–response curve for embelin was then obtained. Either low-dose (10 mg/kg) or high-dose (40 mg/kg) embelin or vehicle was administered for 3 d s.c. before stroke to both GI females and males. Embelin significantly exacerbated stroke-induced injury at both doses in females (10 mg/kg, 48.8 ± 4.2% and 37.4 ± 4.6%, n = 10, P < 0.05, Fig. 5C; 40 mg/kg, 62.8 ± 6.2% and 39.6 ± 4.6%, n = 10, P < 0.05, Fig. 5D) but not in males (40 mg/kg, 51.4 ± 6.5% and 50.8 ± 3.9%, n = 10, P > 0.05, Fig. 5D). The detrimental effects of embelin were reflected in the neurological deficit scores, with embelin-treated Ovx and embelin-treated GI females demonstrating more severe deficits than males did (Table S2). Serum estrogen levels were significantly decreased in Ovx females compared with GI females (Table S3). No differences were seen in physiological parameters (Table S4) or blood flow (Table S5) in female mice treated with vehicle or embelin. We have previously shown that no sex differences exist in physiological parameters or blood flow in our model (39).
Fig. 5.
Embelin's effect on infarct volume. (A) Embelin exacerbated stroke in females, with no effect in males or Ovx females (20 mg/kg s.c. 3 d before 90-min MCAO, n = 10 per group, P < 0.05). (B) A 60-min MCAO illustrated that embelin exacerbated infarct in Ovx females (n = 7, P < 0.05). (C) A dose–response was performed in females because males had no effect at 20 mg/kg (10 mg/kg s.c. 3 d before 90-min MCAO). Females exhibited an increased stroke volume (n = 10, P < 0.05). (D) Stroke was exacerbated in females, with no effect in males (40 mg/kg s.c. 3 d before stroke, n = 10, P < 0.05).
Discussion
Multiple cell death pathways are activated in stroke (1, 11). Data from our laboratory and others suggest that ischemic cell death in male and female animals occurs predominately via caspase-independent and caspase-dependent mechanisms, respectively (4, 6–8, 11, 18). This work demonstrates that a sexual dichotomy exists in XIAP and Smac/DIABLO mRNA expression (Fig. 1). miR-23a was discovered as an inhibitor of XIAP (Fig. 4 A and B) that also exhibited sex differences (Fig. 4C). Sex differences in miRNA regulation have been seen in rodent liver, testes, and ovaries (40, 41), and this report shows sex-specific miRNA regulation in the ischemic brain. The specificity of miR-23a–induced changes in XIAP was confirmed in vitro when XIAP mRNA levels rose after inhibition of miR-23a with anti–miR-23a treatment (Fig. 4A). The interaction between miR-23a and XIAP was confirmed because miR-23a was shown to bind to the 3′ UTR of XIAP (Fig. 4B). XIAP contributes to the pathogenesis of sex differences in cell death because pharmacological inhibition of XIAP with the small-molecule inhibitor embelin exacerbated stroke in both GI and Ovx females but not in males (Fig. 5).
Levels of mRNA for both XIAP and Smac/DIABLO, the endogenous inhibitor of XIAP, were higher in sham females (Fig. 1) compared with males, suggesting that increased activity of caspase-mediated pathways, even at baseline, in the female brain. Stroke induced a significant loss of both XIAP and Smac/DIABLO mRNA in GI, Ovx, and Ovx + E2 females (Fig. 1). No changes were observed in males, suggesting that XIAP is selectively responsive to ischemia in the female brain, an effect that was independent of acute circulating estrogen levels. These findings suggest that an intrinsic sex difference exists in XIAP regulation, and, as a result, subsequent experiments focused on males and GI females.
The mRNA changes (Fig. 1) were reflected in changes at the protein level in females because MCAO induced a loss of both XIAP (Fig. 2) and Smac/DIABLO (Fig. 3) mRNA and protein in GI female mice. Cytosolic Smac/DIABLO protein levels (Fig. 3A) were increased in embelin-treated GI females, which likely contributed to their severe exacerbation of infarct by further increasing caspase activation (Fig. 5). Importantly, female mice exhibited a significant stroke-induced decrease in XIAP mRNA (Fig. 1) and protein (Fig. 2), which is consistent with posttranscriptional regulation of XIAP by miR-23a in females (Fig. 4 A and B), leading to translational repression of XIAP. Embelin treatment, which prevented the interaction between XIAP and Caspase-3 (Fig. S1), led to increased XIAP protein in the female sham brain (Fig. 2A, lane 7) and eliminated the stroke-induced decrease of XIAP protein in females. To confirm that the sex-specific effects of embelin on stroke outcome were specifically related to its interaction with XIAP, we performed coimmunoprecipitation studies. Embelin treatment decreased the XIAP/Caspase-3 interaction in both sexes in sham animals (Fig. S1 A–C), thus confirming that embelin functionally inhibits XIAP (33). An increased XIAP/caspase-3 interaction was seen in males after stroke, but this interaction was decreased in females. The loss of the interaction between XIAP and Caspase-3 led to enhanced caspase signaling in the ischemic female brain as measured by the caspase-specific cleavage of αII-spectrin (Fig. S2). This finding demonstrated that caspase activity was increased in females (Fig. S2, bar 1 vs. bar 5) and showed that activity was further increased after embelin treatment (Fig. S2, bar 4 vs. bar 8), which lead to an exacerbation in infarct in females.
miRNAs and their processing enzymes have been implicated in ischemia, neurodegeneration, angiogenesis, and vascular disease (42–45). miR-92a targets proangiogenic proteins, as shown by the fact that inhibition of miR-92a led to increased angiogenesis in a model of limb ischemia and acute myocardial infarction (25). It has recently been described that stroke can induce changes in miRNA expression (22, 23, 26). The above studies suggest that inhibition of miR-23a reduces caspase activation by enhancing XIAP levels, decreasing cell death. Therefore, any development of therapies targeting miRNAs should consider the potential for differential regulation by sex.
We used the small-molecule, cell-permeable XIAP inhibitor embelin as a tool to examine the downstream effects of XIAP in cerebral ischemia. The use of embelin allowed us to determine the physiological significance of inhibition of XIAP in males and females. We performed LC-MS/MS to confirm embelin's presence in the brain of both sexes. Embelin was shown to cross the blood–brain barrier in both males and females (Table S1) at a concentration similar to the IC50 of embelin (4.1 ± 1.1 μM) (33). Although pharmacological inhibitors may have “off-target” effects (46), XIAP knockout mice compensate for XIAP loss with an associated increase in other IAP protein family members, making them less useful for our studies (47, 48). Embelin was shown to alter transcription of genes such as NF-κB (49) in one in vitro study; therefore, we cannot completely exclude effects on alternative pathways. However, the dose used in that study was significantly higher than both the dose administered here and the IC50 of embelin. Coimmunoprecipitation studies confirmed the specificity of embelin on XIAP, with a reduction in the association of XIAP with Caspase-3 (Fig. S1).
The complete lack of response in males treated with embelin was surprising because previous studies have implicated XIAP in neuroprotection (50). Overexpression of XIAP inhibited apoptosis in hippocampal cultures exposed to oxygen–glucose deprivation (51–53), decreased cytochrome c release, prevented oxidative stress, and reduced injury in males after stroke in vivo (54, 55). However, genetic models produced supraphysiological levels of XIAP, a human XIAP sequence was used, and these animals may have other compensatory changes from life-long XIAP overexpression (47, 54). In addition, XIAP was selectively overexpressed in neurons, and embelin presumably inhibits XIAP in all cell types.
The selective exacerbation of infarct seen in embelin-treated females confirmed the hypothesis that females are uniquely sensitive to manipulation of pathways that normally restrain ischemic caspase activation. This effect was independent of circulating estrogen levels because it was present in both GI and Ovx animals (Fig. 5). Both Ovx and GI females had similar changes in XIAP mRNA expression (Fig. 1), and embelin exacerbated infarction volume in both GI and Ovx females but had no effect in males. These data suggest that the selective vulnerability to caspase-mediated cell death appears to depend on sex rather than on hormones. However, a developmental contribution of ovarian hormones to sex-specific cell death cannot be completely excluded because the organizational effects of steroids during prenatal and early postnatal development are not reversed by acute ovariectomy (10, 11). Because XIAP is located on the X chromosome (56), and up to 20% of genes on the second X chromosome are transcribed and translated, chromosomal dosage effects may also be a factor (57).
Although females are more sensitive to caspase-induced cell death, there is considerable evidence that caspases are also activated in the male brain, but perhaps to a lesser extent (7, 58). Caspase inhibition was neuroprotective in several studies (59), but the sex of the animals was often not specified (60) and the agents were administered i.c.v. with variable dosing, often without positive controls (61–63). Interestingly, caspase-3 induction was not seen in focal or transient forebrain ischemia in male rats (64), and caspase inhibition by z-DEVD-fmk may actually protect by attenuation of calpain rather than caspase inhibition after stroke (65). We propose that, although caspase activation occurs in both males and females (as seen by αII-spectrin cleavage; Fig. S2), this activation leads to cell death preferentially in females. This finding is reminiscent of studies examining PARP-1 activation. Both sexes activate PARP-1, leading to equivalent nuclear apoptosis-inducing factor translocation, which is selectively detrimental to males, as shown by PARP-1 inhibition or deletion. Similarly, recent studies with quinoline-Val-Asp(Ome)-CH2-O-phenoxy (Q-VD-OPh), a pan-caspase inhibitor, led to neuroprotection in females with no effect in males (7, 18). Embelin was used to manipulate the caspase-mediated cell death pathway to expose these sex differences in XIAP in ischemic cell death.
In conclusion, these data support the hypothesis that ischemic cell death in females occurs predominantly by caspase-dependent mechanisms (1). Although miRNAs are promiscuous molecules (40) with poor blood–brain barrier (66) penetration, they may be targets for therapeutic development (25). However, neuroprotective strategies should be developed with these sex differences in mind. Most importantly, a mechanism contributing to sex differences, miRNA regulation of XIAP, has now been identified (Fig. 6) that may also play a role in other sexually dimorphic diseases.
Fig. 6.
Model of XIAP regulation in stroke. miR-23a levels were decreased in males, which increased the XIAP/Caspase-3 association, decreased caspase activity, and subsequently decreased apoptosis in the male ischemic brain. Increased miR-23a in females decreased XIAP mRNA, decreased the XIAP/Caspase-3 association, and allowed increased caspase activity and subsequent apoptosis in the female ischemic brain.
Materials and Methods
C57BL/6 male, GI female, and Ovx female mice were given 60- or 90-min MCAO with 4.5- or 22.5-h reperfusion. Embelin treatment was administered for 3 d before MCAO and was quantified in the brain by LC-MS/MS. miR-23a binding was confirmed by using HeLa cells transfected with anti–miR-23a and a miR-23a luciferase binding assay. Total RNA was extracted at 6 h after ischemia, and quantitative RT-PCR was performed for XIAP, GAPDH, miR-23a, and 5s. Infarction volume was measured brains stained with by triphenyltetrazolium chloride taken 24 h after ischemia. Protein was extracted and fractionated at 6 h after ischemia, and Western blot analyses were performed probing for XIAP, Caspase-3, Smac/DIABLO, αII-spectrin, MIF, and COXIV. Coimmunoprecipitation with Protein A-Sepharose beads, immunopurified XIAP, and Western blot analyses determined XIAP-associated proteins. Data were presented as mean ± SEM, and ANOVA (with Tukey's post hoc correction, when appropriate) was used for comparison between groups. Neurological deficit scores were analyzed by Mann–Whitney U test (P < 0.05 was considered statistically significant). Induction of ischemia, behavioral assessments, and histological assessments were performed by an investigator blinded to drug treatment.
Full descriptions of methods are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Bruce White and Dr. Brian Adams for supplying the HeLa cells, pIS-0, and Renilla vectors as well as for their technical assistance with the miRNA studies. We also thank Dr. Elizabeth Eipper for supplying Protein A beads and Dr. Eipper and Dr. Stephen Crocker for their helpful comments. This research was funded by National Institutes of Health Grants 5F31-NS062608-02 and 5T32-NS041224-08 (to C.S.) and National Institute of Neurological Disorders and Stroke Grants NS055215 and NS050505 (to L.D.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102635108/-/DCSupplemental.
References
- 1.Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: Are sex differences relevant? J Transl Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagberg H, et al. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- 3.Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab. 2009;29:670–674. doi: 10.1038/jcbfm.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: Male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 5.Nijboer CH, Kavelaars A, van Bel F, Heijnen CJ, Groenendaal F. Gender-dependent pathways of hypoxia-ischemia-induced cell death and neuroprotection in the immature P3 rat. Dev Neurosci. 2007;29:385–392. doi: 10.1159/000105479. [DOI] [PubMed] [Google Scholar]
- 6.Yuan M, et al. Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke. Exp Neurol. 2009;217:210–218. doi: 10.1016/j.expneurol.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu F, et al. Sex differences in caspase activation after stroke. Stroke. 2009;40:1842–1848. doi: 10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du L, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- 9.Li H, et al. Sex differences in cell death. Ann Neurol. 2005;58:317–321. doi: 10.1002/ana.20538. [DOI] [PubMed] [Google Scholar]
- 10.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel C, Turtzo C, McCullough LD. Sex differences in cerebral ischemia: Possible molecular mechanisms. J Neurosci Res. 2010;88:2765–2774. doi: 10.1002/jnr.22406. [DOI] [PubMed] [Google Scholar]
- 12.Holcik M, Korneluk RG. XIAP, the guardian angel. Nat Rev Mol Cell Biol. 2001;2:550–556. doi: 10.1038/35080103. [DOI] [PubMed] [Google Scholar]
- 13.Silke J, Verhagen AM, Ekert PG, Vaux DL. Sequence as well as functional similarity for DIABLO/Smac and Grim, Reaper and Hid? Cell Death Differ. 2000;7:1275. doi: 10.1038/sj.cdd.4400790. [DOI] [PubMed] [Google Scholar]
- 14.Deveraux QL, Stennicke HR, Salvesen GS, Reed JC. Endogenous inhibitors of caspases. J Clin Immunol. 1999;19:388–398. doi: 10.1023/a:1020502800208. [DOI] [PubMed] [Google Scholar]
- 15.Saito A, Hayashi T, Okuno S, Ferrand-Drake M, Chan PH. Interaction between XIAP and Smac/DIABLO in the mouse brain after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:1010–1019. doi: 10.1097/01.WCB.0000080702.47016.FF. [DOI] [PubMed] [Google Scholar]
- 16.Saito A, Hayashi T, Okuno S, Nishi T, Chan PH. Oxidative stress is associated with XIAP and Smac/DIABLO signaling pathways in mouse brains after transient focal cerebral ischemia. Stroke. 2004;35:1443–1448. doi: 10.1161/01.STR.0000128416.28778.7a. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasula SM, et al. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–116. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 18.Renolleau S, et al. Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: A role for gender. J Neurochem. 2007;100:1062–1071. doi: 10.1111/j.1471-4159.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- 19.Siomi H, Siomi MC. Posttranscriptional regulation of microRNA biogenesis in animals. Mol Cell. 2010;38:323–332. doi: 10.1016/j.molcel.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 21.Jovanovic M, Hengartner MO. miRNAs and apoptosis: RNAs to die for. Oncogene. 2006;25:6176–6187. doi: 10.1038/sj.onc.1209912. [DOI] [PubMed] [Google Scholar]
- 22.Yin KJ, et al. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis. 2010;38:17–26. doi: 10.1016/j.nbd.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee ST, et al. MicroRNAs induced during ischemic preconditioning. Stroke. 2010;41:1646–1651. doi: 10.1161/STROKEAHA.110.579649. [DOI] [PubMed] [Google Scholar]
- 24.Lusardi TA, et al. Ischemic preconditioning regulates expression of microRNAs and a predicted target, MeCP2, in mouse cortex. J Cereb Blood Flow Metab. 2010;30:744–756. doi: 10.1038/jcbfm.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonauer A, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 26.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 27.Liu DZ, et al. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab. 2010;30:92–101. doi: 10.1038/jcbfm.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song R, et al. Many X-linked microRNAs escape meiotic sex chromosome inactivation. Nat Genet. 2009;41:488–493. doi: 10.1038/ng.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bannister SC, Tizard ML, Doran TJ, Sinclair AH, Smith CA. Sexually dimorphic microRNA expression during chicken embryonic gonadal development. Biol Reprod. 2009;81:165–176. doi: 10.1095/biolreprod.108.074005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koturbash I, Zemp F, Kolb B, Kovalchuk O. Sex-specific radiation-induced microRNAome responses in the hippocampus, cerebellum and frontal cortex in a mouse model. Mutat Res. 2011;722:114–118. doi: 10.1016/j.mrgentox.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008;36:D149–D153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 33.Nikolovska-Coleska Z, et al. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J Med Chem. 2004;47:2430–2440. doi: 10.1021/jm030420+. [DOI] [PubMed] [Google Scholar]
- 34.Alkayed NJ, et al. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165, discussion 166. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 35.Taniguchi Y, et al. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science. 2010;329:533–538. doi: 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams ST, Smith AN, Cianci CD, Morrow JS, Brown TL. Identification of the primary caspase 3 cleavage site in αII-spectrin during apoptosis. Apoptosis. 2003;8:353–361. doi: 10.1023/a:1024168901003. [DOI] [PubMed] [Google Scholar]
- 37.Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- 38.Adams BD, Cowee DM, White BA. The role of miR-206 in the epidermal growth factor (EGF) induced repression of estrogen receptor-α (ERα) signaling and a luminal phenotype in MCF-7 breast cancer cells. Mol Endocrinol. 2009;23:1215–1230. doi: 10.1210/me.2009-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Lang J, Zeng Z, McCullough LD. Akt1 gene deletion and stroke. J Neurol Sci. 2008;269:105–112. doi: 10.1016/j.jns.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung L, Gustavsson C, Norstedt G, Tollet-Egnell P. Sex-different and growth hormone-regulated expression of microRNA in rat liver. BMC Mol Biol. 2009;10:13. doi: 10.1186/1471-2199-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishima T, et al. MicroRNA (miRNA) cloning analysis reveals sex differences in miRNA expression profiles between adult mouse testis and ovary. Reproduction. 2008;136:811–822. doi: 10.1530/REP-08-0349. [DOI] [PubMed] [Google Scholar]
- 42.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 43.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 44.Saugstad JA. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J Cereb Blood Flow Metab. 2010;30:1564–1576. doi: 10.1038/jcbfm.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics. 2011;43:521–528. doi: 10.1152/physiolgenomics.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegelin MD, Gaiser T, Siegelin Y. The XIAP inhibitor Embelin enhances TRAIL-mediated apoptosis in malignant glioma cells by down-regulation of the short isoform of FLIP. Neurochem Int. 2009;55:423–430. doi: 10.1016/j.neuint.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB. Characterization of XIAP-deficient mice. Mol Cell Biol. 2001;21:3604–3608. doi: 10.1128/MCB.21.10.3604-3608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vischioni B, et al. Expression and localization of inhibitor of apoptosis proteins in normal human tissues. Hum Pathol. 2006;37:78–86. doi: 10.1016/j.humpath.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Ahn KS, Sethi G, Aggarwal BB. Embelin, an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein, blocks nuclear factor-κB (NF-κB) signaling pathway leading to suppression of NF-κB-regulated antiapoptotic and metastatic gene products. Mol Pharmacol. 2007;71:209–219. doi: 10.1124/mol.106.028787. [DOI] [PubMed] [Google Scholar]
- 50.Robertson GS, Crocker SJ, Nicholson DW, Schulz JB. Neuroprotection by the inhibition of apoptosis. Brain Pathol. 2000;10:283–292. doi: 10.1111/j.1750-3639.2000.tb00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan YF, Lu CZ, Xie J, Zhao YX, Yang GY. Apoptosis inhibition in ischemic brain by intraperitoneal PTD-BIR3-RING (XIAP) Neurochem Int. 2006;48:50–59. doi: 10.1016/j.neuint.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Li T, Fan Y, Luo Y, Xiao B, Lu C. In vivo delivery of a XIAP (BIR3-RING) fusion protein containing the protein transduction domain protects against neuronal death induced by seizures. Exp Neurol. 2006;197:301–308. doi: 10.1016/j.expneurol.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 53.Guégan C, et al. PTD-XIAP protects against cerebral ischemia by anti-apoptotic and transcriptional regulatory mechanisms. Neurobiol Dis. 2006;22:177–186. doi: 10.1016/j.nbd.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 54.Trapp T, et al. Transgenic mice overexpressing XIAP in neurons show better outcome after transient cerebral ischemia. Mol Cell Neurosci. 2003;23:302–313. doi: 10.1016/s1044-7431(03)00013-7. [DOI] [PubMed] [Google Scholar]
- 55.Zhu C, et al. X chromosome-linked inhibitor of apoptosis protein reduces oxidative stress after cerebral irradiation or hypoxia-ischemia through up-regulation of mitochondrial antioxidants. Eur J Neurosci. 2007;26:3402–3410. doi: 10.1111/j.1460-9568.2007.05948.x. [DOI] [PubMed] [Google Scholar]
- 56.Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis: There is more to life than Bcl2. Oncogene. 2003;22:8568–8580. doi: 10.1038/sj.onc.1207101. [DOI] [PubMed] [Google Scholar]
- 57.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 58.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 59.Rami A, Bechmann I, Stehle JH. Exploiting endogenous anti-apoptotic proteins for novel therapeutic strategies in cerebral ischemia. Prog Neurobiol. 2008;85:273–296. doi: 10.1016/j.pneurobio.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Hara H, et al. Inhibition of interleukin 1β converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci USA. 1997;94:2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Endres M, et al. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J Cereb Blood Flow Metab. 1998;18:238–247. doi: 10.1097/00004647-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Ma J, Endres M, Moskowitz MA. Synergistic effects of caspase inhibitors and MK-801 in brain injury after transient focal cerebral ischaemia in mice. Br J Pharmacol. 1998;124:756–762. doi: 10.1038/sj.bjp.0701871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schulz JB, et al. Extended therapeutic window for caspase inhibition and synergy with MK-801 in the treatment of cerebral histotoxic hypoxia. Cell Death Differ. 1998;5:847–857. doi: 10.1038/sj.cdd.4400420. [DOI] [PubMed] [Google Scholar]
- 64.Gill R, et al. Role of caspase-3 activation in cerebral ischemia-induced neurodegeneration in adult and neonatal brain. J Cereb Blood Flow Metab. 2002;22:420–430. doi: 10.1097/00004647-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Knoblach SM, et al. Caspase inhibitor z-DEVD-fmk attenuates calpain and necrotic cell death in vitro and after traumatic brain injury. J Cereb Blood Flow Metab. 2004;24:1119–1132. doi: 10.1097/01.WCB.0000138664.17682.32. [DOI] [PubMed] [Google Scholar]
- 66.Krützfeldt J, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.