Abstract
Ecologists have long hypothesized that fragmentation of tropical landscapes reduces avian nest success. However, this hypothesis has not been rigorously assessed because of the difficulty of finding large numbers of well-hidden nests in tropical forests. Here we report that in the East Usambara Mountains in Tanzania, which are part of the Eastern Arc Mountains, a global biodiversity hotspot, that daily nest survival rate and nest success for seven of eight common understory bird species that we examined over a single breeding season were significantly lower in fragmented than in continuous forest, with the odds of nest failure for these seven species ranging from 1.9 to 196.8 times higher in fragmented than continuous forest. Cup-shaped nests were particularly vulnerable in fragments. We then examined over six breeding seasons and 14 study sites in a multivariable survival analysis the influence of landscape structure and nest location on daily nest survival for 13 common species representing 1,272 nests and four nest types (plate, cup, dome, and pouch). Across species and nest types, area, distance of nest to edge, and nest height had a dominant influence on daily nest survival, with area being positively related to nest survival and distance of nest to edge and nest height being both positively and negatively associated with daily nest survival. Our results indicate that multiple environmental factors contribute to reduce nest survival within a tropical understory bird community in a fragmented landscape and that maintaining large continuous forest is important for enhancing nest survival for Afrotropical understory birds.
Keywords: avian conservation, demography, nest predators
Reduced nest survivorship, due to elevated rates of nest predation, has long been hypothesized as an important contributory factor to population declines and local extinctions of birds in fragmented tropical landscapes (1, 2). Habitat fragmentation results in a reduction in area, an increase in remnant isolation, the creation of edge, and an alteration in the habitat structure of the remnants, all of which may contribute either directly or indirectly to changes in avian nest survival (3–6). Given that nearly two thirds of all bird species are endemic to the tropics (7, 8) and that moist tropical forests are being lost worldwide at a rate of 0.52% annually (9), understanding the impact of habitat fragmentation on the demography of tropical birds is clearly important for avian conservation.
However, because of the difficulty of finding large numbers of well-hidden nests in tropical forests (10, 11), rigorously assessing the impact of habitat fragmentation on avian nest survivorship has been challenging. Previous studies comparing avian nest survivorship between fragmented and intact forest in the tropics have either used artificial nests and eggs (12, 13), which unfortunately often poorly mimic the fate of real nests and eggs (14–16); or if real nests have been found, have lumped species together in the analysis because of small sample sizes (17), which can be misleading because nest survivorship can vary locally among tropical bird species (18–21). Similarly, the few studies in the tropics that have examined edge effects on nest survival using real nests and eggs have been either single-species studies (22) or have pooled nests across species (17). Thus, our understanding of the influence of landscape structure and nest location on avian nest survival across tropical bird communities is quite limited.
Here we examine in a multispecies analysis the impact of habitat fragmentation on avian nest survival within an Afrotropical understory bird community. In this analysis, we compare daily nest survival and nest success between continuous and fragmented forest among species and nest types. We then examine the influence of landscape structure and nest location on nest survivorship across species and nest types. We include the largest sample of species and nests yet included in such an analysis from the tropics.
Results
Patterns of Nest Location and Failure.
During the 2004 breeding season, over which we compare daily nest survival and nest success between fragmented and continuous forest, we located 472 active nests—nests containing at least one egg or nestling—of 18 species. There were sufficient sample sizes for 8 of these 18 species, representing three nest types (cup, pouch, and dome) and totaling 328 nests. The nesting biology for these eight species is summarized in SI Appendix, Table S1.
Between the 2003 and 2008 breeding seasons, over which we examine environmental predictors of nest survival, we located and monitored 1,315 active nests representing 27 understory bird species (SI Appendix, Table S2). Samples sizes were sufficient to assess the influence of landscape structure and nest location on daily nest survival for 13 of these 27 species, totaling 1,272 nests; and four nest types representing 17 species and 1,299 nests.
In our study system, nest predation was the leading cause of nest failure. Between the 2003 and 2008 breeding seasons, we attributed 97.4% of all nest failures to nest predation.
Nest Survival in Fragmented vs. Continuous Forest.
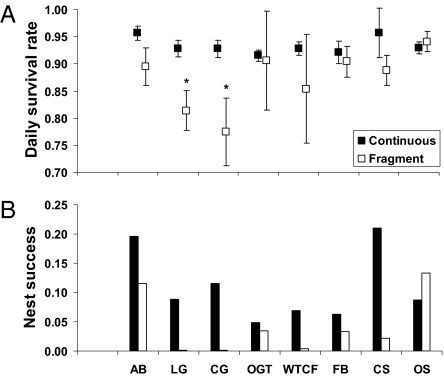
Mean daily survival rate of nests (Fig. 1A) and mean nest success (Fig. 1B) for understory bird species varied significantly between fragmented and continuous forest (paired t test, t = 2.68, df = 7, P < 0.031; paired t-test, t = 2.66, df = 7, P < 0.032). Most notably, mean nest success for seven of eight common species was lower in fragmented than in continuous forest, with the odds ratio for nest failure for these seven species ranging from 1.9 to 196.8. Within fragmented forest, mean nest success ranged from <1.0% to 13.4%, in comparison with 4.9% to 20.9% in continuous forest (Fig. 1B).
Fig. 1.
Comparison between fragmented and continuous forest of (A) mean (±1 SE) daily survival rate of nests for eight understory bird species; (B) mean nest success for the same species. AB, African broadbill; LG, little greenbul; CG, Cabanis's greenbul; OGT, orange ground thrush; WTCF, white-tailed crested flycatcher; FB, forest batis; CS, collared sunbird; OS, olive sunbird. An asterisk indicates that the 95% confidence interval for a fragmentation effect on daily survival rate for the species does not overlap zero.
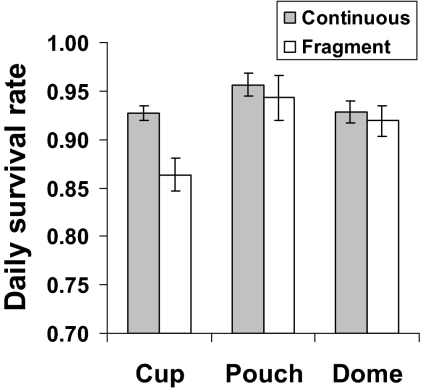
Variation also existed in the daily survival rate among nest types (cup, pouch, and dome) between fragmented and continuous forest (Fig. 2), with species that construct cup nests being significantly more vulnerable (z = 3.41, P < 0.001) in fragmented than in continuous forest. Indeed, the odds of failure for cup-shaped nests was twice as high in fragmented than in continuous forest (odds ratio, 2.003).
Fig. 2.
Comparison between fragmented and continuous forest of mean (±1 SE) daily survival rate of nests among nest types (cup, pouch, and dome).
Influence of Landscape Structure and Nest Location on Daily Nest Survival of Species.
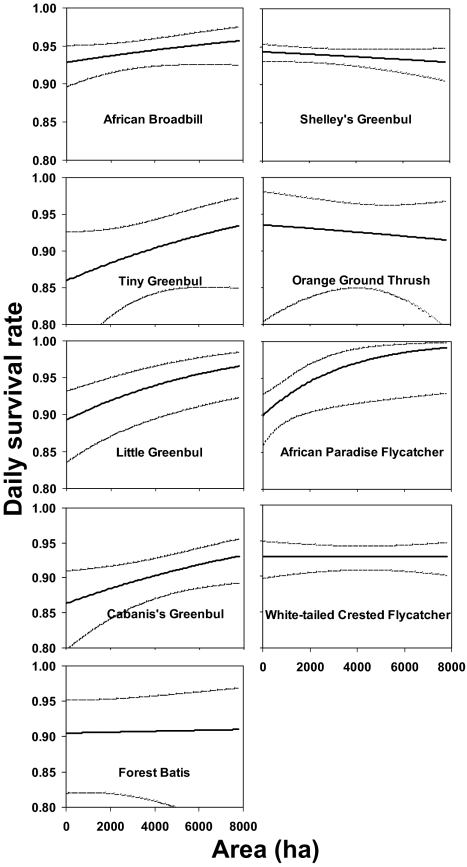
Across all breeding seasons and study sites, the influence of landscape structure and nest location on daily nest survival varied among species. Among 13 common understory bird species, area, distance of nest to edge, and nest height had a dominant influence on daily nest survival (SI Appendix, Table S3). For nine species, area was included in one or more of the top-ranked models (ΔAICc ≤2; defined in Materials and Methods) and was positively associated with daily nest survivorship for seven of nine species (Fig. 3).
Fig. 3.
Relation between daily survival rate (DSR) of nests and area for nine understory bird species. Solid lines represent the estimated DSR obtained by backtransforming the appropriate logit-linear model with all covariates except area set to their mean value. Dashed lines represent upper and lower 95% confidence intervals.
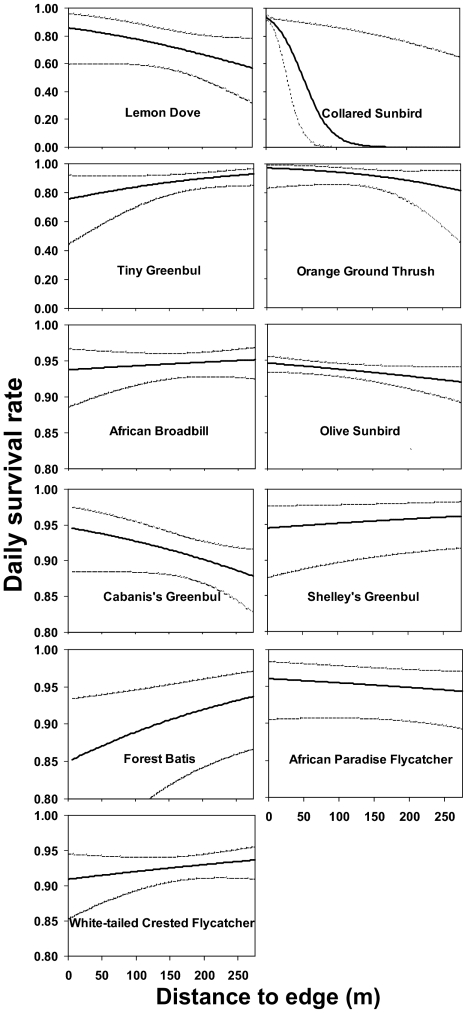
Distance of nest to forest edge also had an important influence on daily nest survival for 11 of 13 common species (SI Appendix, Table S3). However, the influence of distance of nest to edge on daily nest survival varied among species. For 6 of 11 species, distance of nest to edge was negatively associated with daily nest survival and for 5 species was positively associated with daily nest survival (Fig. 4).
Fig. 4.
Relation between daily survival rate (DSR) of nests and distance of nest to edge for 11 understory bird species. Solid lines represent the estimated DSR obtained by backtransforming the appropriate logit-linear model with all covariates except distance of nest to edge set to their mean value. Dashed lines represent upper and lower 95% confidence intervals.
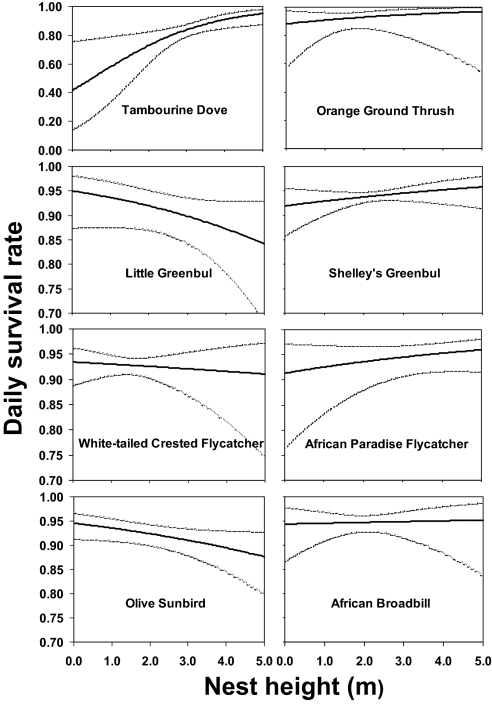
For 8 of 13 common species, nest height was also included in one or more top-ranked models (ΔAICc ≤2) and was positively associated with daily nest survival for 5 species and negatively associated with nest survival for 3 species (Fig. 5 and SI Appendix, Table S3). Forest disturbance, although less important as a predictor of daily nest survival than area, distance to edge, or nest height, was negatively associated with daily nest survival for two species and had a mixed impact on nest survival for one species, indicating that daily nest survival for the former species was lower in slightly and moderately disturbed forest than in primary forest and for the latter species was lower in slightly disturbed but higher in moderately disturbed than in primary forest (SI Appendix, Table S3).
Fig. 5.
Relation between daily survival rate (DSR) of nests and nest height for eight understory bird species. Solid lines represent the estimated DSR obtained by backtransforming the appropriate logit-linear model with all covariates except nest height set to their mean value. Dashed lines represent upper and lower 95% confidence intervals.
Influence of Landscape Structure and Nest Location on Daily Nest Survival of Nest Types.
Across all breeding seasons and study sites, area, distance of nest to edge, and nest height also had a dominant influence on nest survival of nest types. However, the influence of these of environmental factors on daily nest survival varied among nest types (SI Appendix, Table S4).
Nest height was included in one or more top-ranked models (ΔAICc ≤2) for all four common nest types (plate, cup, dome, and pouch). For cup and dome nests, nest height was negatively associated with nest survival, whereas for pouch and plate nests, nest height was positively related to daily nest survival (SI Appendix, Fig. S1).
Forest area and distance to edge were also included as predictor variables in one or more top- ranked models (ΔAICc ≤2) for both cup and pouch nests and were positively associated with nest survival (SI Appendix, Fig. S1). Finally, for dome nests survival was higher in primary forest than in slightly or moderately disturbed forest; and for cup nests variation among breeding seasons was also an important predictor of daily nest survival (SI Appendix, Table S4).
Variation in Nest Survival Among Species and Nest Types.
Across all study sites and breeding seasons, daily survival rate for nests varied among the 13 common understory bird species. Daily survival rates for nests ranged among species from 0.708 to 0.941, with an overall mean daily survival rate of 0.903 (SD ±0.064) across species, sites, and breeding seasons (Table 1). Among nest types, open nests (plate and cup) had significantly lower daily nest survival than enclosed nests (dome and pouch) (z = 2.74, P < 0.007) across all study sites, with the odds of daily nest failure of open nests 23% higher than enclosed nests (odds ratio, 1.23). Among the four most common nest types, daily survival rate for plate < cup < dome < pouch (Table 2).
Table 1.
Comparison of daily survival rate (±1 SE) of nests of 13 common understory bird species across all study sites and breeding seasons
| Species | Daily survival rate | n |
| Tambourine dove | 0.843 ± 0.021 | 52 |
| Lemon dove | 0.708 ± 0.069 | 17 |
| African broadbill | 0.941 ± 0.009 | 57 |
| Little greenbul | 0.929 ± 0.006 | 174 |
| Shelley's greenbul | 0.940 ± 0.005 | 214 |
| Cabanis's greenbul | 0.905 ± 0.014 | 52 |
| Tiny greenbul | 0.900 ± 0.025 | 19 |
| Orange ground thrush | 0.924 ± 0.027 | 9 |
| White-tailed crested flycatcher | 0.928 ± 0.008 | 120 |
| African paradise flycatcher | 0.916 ± 0.008 | 142 |
| Forest batis | 0.939 ± 0.009 | 73 |
| Collared sunbird | 0.922 ± 0.010 | 76 |
| Olive sunbird | 0.940 ± 0.004 | 265 |
Calculated daily survival rate of nests assumed constant survival (S).
Table 2.
Comparison of daily survival rate (±1 SE) of four common nest types and combined open and enclosed nest types across all study sites, breeding seasons, and 17 understory bird species
| Nest type | Species (n) | Nests (n) | Daily survival rate | Open/enclosed nest type daily survival rate |
| Plate | 2 | 69 | 0.826 ± 0.020 | 0.925 ± 0.003 |
| Cup | 12 | 832 | 0.929 ± 0.003 | |
| Dome | 2 | 341 | 0.936 ± 0.004 | 0.938 ± 0.004 |
| Pouch | 1 | 57 | 0.947 ± 0.008 |
Calculated daily survival rate of nest types assumed constant survival (S).
Discussion
Our results indicate that habitat fragmentation reduces daily nest survival and nest success for Afrotropical understory birds in a montane forest and that open cup nests are particularly vulnerable in forest fragments. Our results also reveal that within an Afrotropical understory bird community the relative influence of landscape structure and nest location on nest survival varies among species and nest types, with area, distance of nest to edge, and nest height having a dominant influence. Finally, our results demonstrate, on the basis of the largest sample of species and nests yet included in such an analysis, that multiple environmental factors contribute to reduced nest survival within a tropical understory bird community in a fragmented landscape.
In our study system the predominant cause of nest failure was nest predation, which is consistent with patterns of nest failure observed elsewhere in the tropics (18–20) and in temperate regions (23, 24). Over the course of the study we observed a diverse community of predators, comprising raptors, snakes, rodents, and ants, preying on eggs and young. Although the composition and response of the predator community to habitat fragmentation will ultimately influence spatial patterns of avian nest survival (3), our understanding of the impact of fragmentation on nest predator communities is limited (25, 26) and particularly so in the tropics.
It has been suggested that landscape structure and nest location may mediate nest predation risk via changes in predator abundance and/or behavior (27–29). Thus, variation in nest survival among species and nest types may result from different nest predators or abundance of nest predators preying on different species and nest types (27). Alternatively, landscape structure and nest location may mediate predation risk through alteration of predator searching behavior and/or the influence of habitat structure in impeding predator searches (24, 30). Indeed, there is supporting evidence from temperate regions that predation pressure can vary among major nest predator types as a function of area (30, 31) and distance to edge (31–33). There is also evidence from temperate regions that habitat structure of nest sites can mediate predation risk via changes in predator searching behavior (29).
Although area and edge effects can often be confounded (34), this problem was addressed by considering these covariates separately and together in a candidate model set (SI Appendix, Table S5). For 9 of 13 common species and two of four nest types, area was identified as an important predictor of daily nest survival (SI Appendix, Tables S3 and S4). For nearly all species and nest types area was positively related to daily nest survival (Fig. 3 and SI Appendix, Fig. S1). Increased nest predation in habitat remnants has been attributed to increased abundance of nest predators due to immigration from the surrounding matrix and/or the loss of top predators (35, 36). Although our knowledge about predator abundance and distribution in our study system is most complete for rodents, previous small mammal surveys have revealed that neither aggregate rodent abundance nor abundance of Graphiurus murinus and Rattus rattus, known nest predators, varies significantly with fragment area (37). Thus, patterns of increased nest predation in small fragments are inconsistent with a hypothesis of increased rodent abundance. A recent review of studies examining the impact of fragmentation on nest predators in temperate regions found that avian predators and snakes were likely to be more abundant, more active, or more species-rich in small patches and along edges than rodents (26), and thus we suspect that increased rates of nest predation in small fragments are probably a result of increased abundance and/or activity of nonrodent nest predators in small fragments in our study system.
Increased nest predation along habitat edges due to increased predator abundance and/or activity along edges has long been hypothesized as an important contributory factor to elevated nest mortality in habitat remnants (38). For 11 of 13 common species, distance of nest to edge was an important predictor of nest survival (SI Appendix, Table S3). However, the nature of the relation varied considerably among species, with distance of nest to edge being negatively associated with nest survival for six species and positively associated with nest survival for five species (Fig. 4 and SI Appendix,Table S3). An inverse relation between nest survival and distance to edge was recently reported from another Afrotropical montane forest (22). In the Taita Hills in Kenya, nest survival for the white-starred forest robin (Pogonocichla stellata) was found to be higher in the interior than along the edge in forest remnants. Researchers there hypothesized that an inverse relation may be a result of decreased rodent abundance along fragment edges (22). However, this is not the case in the East Usambara Mountains. In our study system, neither aggregate rodent abundance nor abundance of Graphiurus murinus, a known nest predator, varies significantly between the edge and interior in forest remnants (37). Results from the East Usambara Mountains also indicate that effects of edge on nest survival can vary considerably among species and that interpreting general patterns of nest survival on the basis of single species or even several species warrants caution.
A possible alternative determinant of increased nest survival along forest edges for selective bird species in our study system may be edge avoidance by nonrodent nest predators. In our study system forest raptors, which are important nest predators, are largely restricted to the forest interior. The raptors we have observed most frequently preying on understory bird nests are the African goshawk (Accipiter tachiro) and harrier hawk (Polyboroides radiatus). Since 1987 we have been surveying understory birds at these same study sites using mist nets that are erected in a line and begin at and run perpendicular to the edge and extend up to 0.5 km. On occasion we will capture raptors. The raptor we have most frequently captured over this period is the African goshawk, and the mean distance we have captured it from the forest edge is 133 m. Thus, we believe that edge avoidance by important nest predators could be a determinant of increased nest survival along forest edges in our study system among selected understory bird species; however, knowledge about species-specific predator identity, abundance, and distribution is clearly required to rigorously assess this hypothesis.
The influence of nest height on nest survival also varied considerably among species, with nest height being both positively and negatively associated with nest survival (Fig. 5 and SI Appendix, Fig. S1). Although in temperate forests nest survival has been shown to be lower among shrub-nesting than ground-nesting or canopy-nesting birds (27), all of the species we have included in our analysis are shrub/subcanopy nesters, and thus our results should not be confounded by the inclusion of ground- and canopy-nesting birds. We suspect the varying influence of nest height on nest survival among species is most likely a result of different predators or suites of predators preying on different species and/or nest types. In temperate forests different nest predators have been shown to specialize on ground vs. off-ground nests (25) and open vs. enclosed nests (32). However, once again knowledge about species-specific predator identity, abundance, and distribution is required to evaluate this hypothesis.
Finally, observed variation in nest survival among nest types in the East Usambara Mountains is consistent with patterns reported from other tropical (18, 39), subtropical (40), and temperate (41) regions, with open nests having lower survival than enclosed nests. Across all study sites and breeding seasons, the odds of daily nest failure of open nests were 1.23 times higher than for enclosed nests. Open nests may be more vulnerable than enclosed nests to nest predation because eggs and young are more visible to nest predators that use visual cues (32, 39, 42). Although few studies have previously estimated daily nest survival rates for multiple tropical understory bird species, the observed mean (±1 SD) daily survival rate of nests for 13 common bird species across six breeding seasons in the East Usambara Mountains 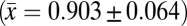 is higher than that reported for two species of manakin over three breeding seasons in lowland Ecuador
is higher than that reported for two species of manakin over three breeding seasons in lowland Ecuador 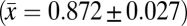 (17), yet is lower than that documented for 10 understory bird species over two breeding seasons in Panama
(17), yet is lower than that documented for 10 understory bird species over two breeding seasons in Panama 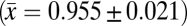 (19).
(19).
In summary, our results showed that fragmentation of an Afromontane forest reduced avian nest survivorship and that multiple environmental factors within a fragmented landscape contributed to reduced avian nest survival. Our results also revealed that the influence of landscape structure and nest location on nest survival varied considerably among species and nest types. By incorporating multiple species and nest types in our analysis, we were able to demonstrate that fragmentation of a tropical landscape had community-wide although often disparate impacts on nest survival of species and nest types. Finally our results highlighted the importance of better knowledge about the effects of habitat fragmentation on the abundance, distribution, and foraging behaviors of important nest predator types and the conservation importance of large continuous forest for enhancing nest survival for Afrotropical understory birds.
Materials and Methods
Study Site.
Our study system (SI Appendix, Fig. S2) consisted of seven forest fragments ranging in size from 0.2 to 521 ha and an adjacent large block of continuous forest of 7,571 ha. The fragments and continuous forest were located within or immediately adjacent to the Amani Nature Reserve on the Amani plateau in the East Usambara Mountains in northeast Tanzania (5°6'S, 38°36'E) at an elevation of 920–1,150 m. The East Usambara Mountains are part of the Eastern Arc Mountains, a global biodiversity hotspot (i.e., a site that contains unusually high numbers of endemic species and has experienced a >70% loss in original habitat) (43). The distance between the fragments and continuous forest was 110 m to 4,100 m, and fragment age ranged from 41 to 73 y. All of the fragments and continuous forest sites have an abrupt edge and are surrounded either entirely by tea, or a combination of tea, Eucalyptus, and fallow and cultivated agricultural land (SI Appendix, Fig. S2).
We selected three study sites within the largest fragment and five study sites within continuous forest that varied in level of disturbance so as to reflect as closely as possible variation in habitat disturbance across the smaller fragments. Each of the 14 study sites in our study system was classified as either primary forest, slightly disturbed forest, or moderately disturbed forest. Previous analysis of habitat structure at these study sites based on a principal components analysis of 10 vegetation features revealed that shrub, tree, and Maesopsis eminii (an exotic invasive tree species) density and ground cover were the most important habitat variables that separated these three broad levels of forest disturbance across our study system (44).
The area of the small fragments was calculated by mapping their perimeters with a global positioning system and the largest fragment and continuous forest from aerial photographs.
Nest Monitoring.
Across the study system understory bird nests, ≤4.5 m in height, were located and monitored over six consecutive breeding seasons extending from late September through early February between 2003 and 2009. The mean interval between nest observations across all species between the 2003 and 2008 breeding seasons was 3.9 d (SD ±1.2). Nest searches were conducted in all study sites between the forest edge and ≈275 m interior, with the exception of the smallest fragments, where searches encompassed the entire fragment. Nests whose contents could not be observed from the ground or by climbing an adjacent tree were checked using a mirror mounted on the end of a pole. The seasonal timing of nest observations did not vary among sites or within and between fragmented and continuous forest, with nests being continuously monitored at all sites between the end of September and early February.
After a nest was located, field technicians identified the species and recorded the number of eggs and young, the fate of the nest, the distance of nest to edge, nest height above ground, and the presence of any nest predator. To facilitate relocation of nests, a small marker flag was placed >5 m from a nest.
In cases where a nest failed, an effort was made to determine the cause of failure. We assumed that predation was the cause of nest failure if the nest disappeared, was torn part, or if the entire contents of the nest—all eggs or nestlings too young to fledge—were missing. The other recorded causes of nest failure were desertion and falling branches and trees. We considered a nest successful if it fledged at least one young.
In comparing daily nest survival and nest success between fragmented and continuous forest we confined the analysis to data gathered during the 2004 breeding season (October 2004 to February 2005) because nests within continuous forest were monitored only during this field season, unlike nests within fragments that were monitored annually between the 2003 and 2008 breeding seasons.
Nest Type.
We classified nests as cup, pouch, dome, plate, or cavity. However, because of small sample sizes, we restricted our analysis during the 2004 breeding season to cup, pouch, and dome nests; and during the 2003–2008 breeding seasons to cup, pouch, dome, and plate nests and to species for which we recorded at least five active nests (SI Appendix, Table S2).
Nest Survival Analysis.
Daily survival rates of nests were estimated using the likelihood-based model of Dinsmore et al. (45) with a logit link to accommodate individual covariates. We selected the Dinsmore et al. model because of its generality. Other commonly used models such as Mayfield (46, 47) and Schaffer (48) are special cases of this model. We also selected the Dinsmore et al. model because of the availability of powerful software [i.e., program MARK (49)] for modeling individual covariates and obtaining point and error estimates.
We selected a priori a set of 14 models (SI Appendix, Table S5) that we believed could potentially explain variation in nest survival, with each model representing a different hypothesis. We used information theoretic methods to quantify the strength of evidence for alternative models for the influence of area (AREA), distance of nest to edge (DISTEDGE), nest height (HEIGHT), habitat disturbance (DISTURB), and breeding season (BRSEASON). Habitat disturbance and breeding season were included as categorical variables in candidate models.
We compared alternative models using Akaikie's information criteria, corrected for small sample size (AICc) and computed model weights (wi) (50). The difference in AICc values (ΔAICc) and the AICc value of the best model (AICc min) along with model weights (wi) provided a measure of the relative strength of evidence for each model. Models with ΔAICc ≤2 were considered to have substantial support, with the exception of those that differed from the best model by one additional parameter and that had essentially the same values as the best model (50, 51). Because these latter models provide no net reduction in AIC (51), we excluded them from consideration.
Ninety-five percent confidence intervals for parameters (β) on the logit scale were computed as β ± 1.96 (SE). For real parameters (e.g., survival, S) confidence intervals were computed by program MARK using the appropriate logit-linear model and the δ method (52), then transformed to the real scale using the inverse-logit function.
Nest success was defined as daily nest survival rate exponentiated by the combined length in days of the incubation and nestling periods (SI Appendix, Table S1). We present means ± SE.
Supplementary Material
Acknowledgments
We thank V. Mkongewa, M. Munissi, A. Mkongewa, and D. Munissi for conducting the nest surveys; A. Chalfoun, S. Skagen, T. O'Shea, and two anonymous reviewers for their comments; and the Tanzania Commission for Science and Technology, Tanzania Wildlife Research Institute, and the Amani Nature Reserve Authority for permission to conduct research. The work was supported by the Critical Ecosystem Partnership Fund, Earthwatch, and US Geological Survey, Fort Collins Science Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104955108/-/DCSupplemental.
References
- 1.Willis EO. Populations and local extinctions of birds on Barro Colorado Island, Panama. Ecol Monogr. 1974;44:153–169. [Google Scholar]
- 2.Terborgh J. Preservation of natural diversity: The problem of extinction prone species. Bioscience. 1974;24:715–722. [Google Scholar]
- 3.Andrén H. In: Mosaic Landscapes and Ecological Processes. Hansson L, Fahrig L, Merriam G, editors. London: Chapman and Hall; 1995. pp. 225–255. [Google Scholar]
- 4.Kurki S, Nikula A, Helle P, Lindén H. Landscape fragmentation and forest composition effects on grouse breeding success in boreal forests. Ecology. 2000;81:1985–1997. [Google Scholar]
- 5.Flaspohler DJ, Temple SA, Rosenfield RN. Species-specific edge effects on nest success and breeding bird density in a forested landscape. Ecol Appl. 2001;11:32–46. [Google Scholar]
- 6.Tewksbury JJ, et al. Tests of landscape influence: Nest predation and brood parasitism in fragmented ecosystems. Ecology. 2006;87:759–768. doi: 10.1890/04-1790. [DOI] [PubMed] [Google Scholar]
- 7.Tscharntke T, et al. Landscape constraints on functional diversity of birds and insects in tropical agroecosystems. Ecology. 2008;89:944–951. doi: 10.1890/07-0455.1. [DOI] [PubMed] [Google Scholar]
- 8.Orme CDL, et al. Global hotspots of species richness are not congruent with endemism or threat. Nature. 2005;436:1016–1019. doi: 10.1038/nature03850. [DOI] [PubMed] [Google Scholar]
- 9.Achard F, et al. Determination of deforestation rates of the world's humid tropical forests. Science. 2002;297:999–1002. doi: 10.1126/science.1070656. [DOI] [PubMed] [Google Scholar]
- 10.Stratford JA, Robinson WD. Gulliver travels to the fragmented tropics: Geographic variation in mechanisms of avian extinction. Front Ecol Environ. 2005;3:91–98. [Google Scholar]
- 11.Robinson WD. Avian reproductive failure in tropical forest fragments. Anim Conserv. 2009;12:276–278. [Google Scholar]
- 12.Loiselle BA, Hoppes WG. Nest predation in insular and mainland lowland rainforest in Panama. Condor. 1983;85:93–95. [Google Scholar]
- 13.Sieving KE. Nest predation and differential insular extinction among selected forest birds of central Panama. Ecology. 1992;73:2310–2328. [Google Scholar]
- 14.Weidinger K. How well do predation rates on artificial nests estimate predation on natural passerine nests? Ibis. 2001;143:632–641. [Google Scholar]
- 15.Faaborg J. Truly artificial nest studies. Conserv Biol. 2004;18:369–370. [Google Scholar]
- 16.Moore RP, Robinson WD. Artificial bird nests, external validity, and bias in ecological field studies. Ecology. 2004;85:1562–1567. [Google Scholar]
- 17.Young BE, Sherry TW, Sigel BJ, Woltmann S. Nesting success of Costa Rican lowland rain forest birds in response to edge and isolation effects. Biotropica. 2008;40:615–622. [Google Scholar]
- 18.Skutch AF. A breeding bird census and nesting success in Central America. Ibis. 1966;108:1–16. [Google Scholar]
- 19.Robinson WD, Robinson TR, Robinson SK, Brawn JD. Nesting success of understory forest birds in central Panama. J Avian Biol. 2000;31:151–164. [Google Scholar]
- 20.Ryder TB, et al. Nest survival for two species of manakins (Pipridae) in lowland Ecuador. J Avian Biol. 2008;39:355–358. [Google Scholar]
- 21.Stanley TR, Newmark WD. Estimating length of avian incubation and nestling stages for Afrotropical forest birds from interval-censored nest records. Auk. 2010;127:79–85. [Google Scholar]
- 22.Spanhoven T, Lehouck V, Boets P, Lens L. Forest fragmentation relaxes natural nest predation in an Afromontane forest. Anim Conserv. 2009;12:267–275. [Google Scholar]
- 23.Ricklefs RE. An analysis of nesting mortality in birds. Smithson Contrib Zool. 1969;9:1–48. [Google Scholar]
- 24.Martin TE. Nest predation and nest sites: New perspectives and old patterns. Bioscience. 1993;43:523–532. [Google Scholar]
- 25.Söderström B, Pärt T, Rydén J. Different nest predator faunas and nest predation risk on ground and shrub nests at forest ecotones: An experiment and a review. Oecologia. 1998;117:108–118. doi: 10.1007/s004420050638. [DOI] [PubMed] [Google Scholar]
- 26.Chalfoun AD, Thompson FR, III, Ratnaswamy MJ. Nest predators and fragmentation: A review and meta-analysis. Conserv Biol. 2002;16:306–318. [Google Scholar]
- 27.Martin TE. Nest predation among vegetation layers and habitat types: Revising the dogmas. Am Nat. 1993;141:897–913. doi: 10.1086/285515. [DOI] [PubMed] [Google Scholar]
- 28.Fontaine JJ, Martin TE. Habitat selection responses of parents to offspring predation risk: An experimental test. Am Nat. 2006;168:811–818. doi: 10.1086/508297. [DOI] [PubMed] [Google Scholar]
- 29.Chalfoun AD, Martin TE. Habitat structure mediates predation risk for sedentary prey: Experimental tests of alternative hypotheses. J Anim Ecol. 2009;78:497–503. doi: 10.1111/j.1365-2656.2008.01506.x. [DOI] [PubMed] [Google Scholar]
- 30.Nour N, Matthysen E, Dhondt AA. Artificial nest predation and habitat fragmentation: Different trends in bird and mammal predators. Ecography. 1993;16:111–116. [Google Scholar]
- 31.Hannon SJ, Cotterill SE. Nest predation in aspen woodlots in an agricultural area in Alberta: The enemy from within. Auk. 1998;115:16–25. [Google Scholar]
- 32.Møller AP. Nest site selection across field-woodland ecotones: The effect of nest predation. Oikos. 1989;56:240–246. [Google Scholar]
- 33.Benson TJ, Brown JD, Bednarz JC. Identifying predators clarifies predictors of nest success in a temperate passerine. J Anim Ecol. 2010;79:225–234. doi: 10.1111/j.1365-2656.2009.01604.x. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher RJ, Jr, Ries L, Battin J, Chalfoun AD. The role of habitat area and edge in fragmented landscapes: Definitively distinct or inevitably intertwined? Can J Zool. 2007;85:1017–1030. [Google Scholar]
- 35.Wilcove DS. Nest predation in forest tracts and the decline of migratory songbirds. Ecology. 1985;66:1211–1214. [Google Scholar]
- 36.Crooks KR, Soulé ME. Mesopredator release and avifaunal extinctions in a fragmented system. Nature. 1999;400:563–566. [Google Scholar]
- 37.Hanson TR, Newmark WD, Stanley WT. Forest fragmentation and predation on artificial nests in the Usambara Mountains, Tanzania. Afr J Ecol. 2007;45:499–507. [Google Scholar]
- 38.Lahti DC. The “edge effect on nest predation” hypothesis after twenty years. Biol Conserv. 2001;99:365–374. [Google Scholar]
- 39.Oniki Y. Is nesting success of birds low in the tropics? Biotropica. 1979;11:60–69. [Google Scholar]
- 40.Auer SA, Bassar RD, Fontaine JJ, Martin TE. Breeding biology of passerines in a subtropical montane forest in northwestern Argentina. Condor. 2007;109:321–333. [Google Scholar]
- 41.Nice MM. Nesting success in altricial birds. Auk. 1957;74:305–321. [Google Scholar]
- 42.Collias NE, Collias EC. Nest Building and Bird Behavior. Princeton: Princeton Univ Press; 1984. [Google Scholar]
- 43.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 44.Newmark WD. A 16-year study of forest disturbance and understory bird community structure and composition in Tanzania. Conserv Biol. 2006;20:122–134. doi: 10.1111/j.1523-1739.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 45.Dinsmore SJ, White GC, Knopf FL. Advanced techniques for modeling avian nest survival. Ecology. 2002;83:3476–3488. [Google Scholar]
- 46.Mayfield HF. Nesting success calculated from exposure. Wilson Bull. 1961;73:255–261. [Google Scholar]
- 47.Mayfield HF. Suggestions for calculating nest success. Wilson Bull. 1975;87:456–466. [Google Scholar]
- 48.Shaffer TL. A unified approach to analyzing nest success. Auk. 2004;121:526–540. [Google Scholar]
- 49.White GC, Burnham KP. Program MARK: Survival estimation from populations of marked animals. Bird Study. 1999;46(Suppl):120–138. [Google Scholar]
- 50.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York: Springer; 2002. [Google Scholar]
- 51.Arnold TD. Uninformative parameters and model selection using Akaike's information criterion. J Wildl Manage. 2010;74:1175–1178. [Google Scholar]
- 52.Powell LA. Approximating variance of demographic parameters using the delta method: A reference for avian biologists. Condor. 2007;109:949–954. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.