Mitotic spindle, the structure essential for equal partitioning of the chromosomes into daughter cells, is of pivotal importance for the maintenance of genomic stability during cell division (1). Microtubules (MTs) that constitute the mitotic spindle are polymers of α- and β-tubulin dimers. Generation of MT starts with a process called MT nucleation in which tubulin dimers polymerize (2). An MT is typically nucleated at an MT organizing center (MTOC) that includes centrosomes and kinetochores/chromatin (2, 3). Emerging evidence indicates that an MT is capable of functioning as an additional MTOC independent of the centrosome. The initial evidence that MT arrays could originate from preexisting MTs came from the observation that EB1-GFP, an ectopically expressed MT plus end binding protein, was localized to acentrosomal spindles (4). The notion was additionally supported by several subsequent studies (3, 5, 6). Growing evidence suggests that MT-dependent MT nucleation has its unique features that are not shared by MT nucleation processes occurring at centrosome or kinetochore/chromatin (3, 5, 6).
At the molecular level, MT nucleation is a complex process that involves multiple proteins. A critical step in MT nucleation is the recruitment of the γ-tubulin ring complexes (γ-TuRC) to MTOC mediated by a protein named Nedd1 (2, 7). This step brings γ-tubulin to MTOC, thereby initiating MT nucleation. Recent studies have identified an important regulator, termed Augmin, that is crucial for MT-based MT nucleation (6, 8). Augmin, a protein complex consisting of eight protein components, binds to an MT, which in turn, recruits the Nedd1-γ-TuRC complex to MT (6). Therefore, Augmin functions to bridge the interaction between the Nedd1-γ-TuRC complex and MT. Intriguingly, although it is also located at the centrosome and seems to be critical for the centrosome integrity (8, 9), Augmin is not essential for centrosome-dependent MT nucleation (8).
Despite recent progress, questions remain regarding how Augmin itself is regulated at the molecular level and how Augmin interacts with MT. A work by Johmura et al. (10) in PNAS uncovers some molecular processes concerning the role of Augmin and its molecular regulation, thus providing some fitting answers to the above questions (10). In this study, Johmura et al. (10) discover that Plk1, a mammalian homolog of Drosophila Polo (11, 12), plays a key role in promoting the interaction between MT and the Augmin-Nedd1-γ-TuRC complex. Plk1 is known to be involved in many aspects of cell-cycle progression (11, 12). It is also implicated in centrosome-based MT nucleation (13). This study by Johmura et al. (10) adds a twist to MT nucleation by showing that it is MT-dependent and controlled by Plk1 as well.
Through screening previously characterized centrosomal proteins, Johmura et al. (10) identify Nedd1 as a protein that strongly binds to the Polo-box domain (PBD) of Plk1, a domain critically required for the mitotic functions of Plk1 (14). This finding is consistent with a previous study showing that Plk1 interacts and phosphorylates Nedd1, thereby promoting MT nucleation at the centrosome (13). Additional studies by Johmura et al. (10) reveal that endogenous Plk1 is also significantly localized along the mitotic spindle in addition to spindle poles, kinetochores, and the midbody in mitotic cells. Because Nedd1 has been shown to mediate MT nucleation in all known MTOCs and Plk1 is involved in centrosome-based MT nucleation (7, 13), this observation raises the possibility that Plk1 may also play a role in MT nucleation at the mitotic spindle. Previous studies have shown that Plk1 interacts with Nedd1 mediated by phosphorylated threonine 550 (T550) (13, 15). The work by Johmura et al. (10) attempts to identify additional phosphorylation site(s) of Nedd1 that may also facilitate its interaction with Plk1 and subsequent localization to the spindle. Indeed, Johmura et al. (10) uncover a residue, S460, whose phosphorylation mediates the strong interaction between Nedd1 and Plk1 PBD. Are there any differential roles of phospho-T550 and phospho-S460 in mediating the interaction with Plk1? Johmura et al. (10) show that the fast-migrating form (on denaturing gels), under phosphorylated Nedd1 uses phospho-T550 residues to interact with Plk1, whereas the slow-migrating, hyperphosphorylated form used phospho-S460. Johmura et al. (10) also find that phosphorylation at S411 is responsible for the slow mobility of Nedd1. The kinase that phosphorylates S411 remains controversial, although Cdc2 is a candidate (7, 13). Johmura et al. (10) subsequently identify Cdc2 as the kinase that phosphorylates S460, the same kinase that phosphorylates T550 and possibly, S411 as well (7, 13). Thus, Nedd1 phosphorylation by Cdc2 at S460 apparently functions to recruit Plk1 to the Nedd1-γ-TuRC complex. Interestingly, phosphorylation of S460 is independent of the phosphorylation status of T550 and S411, and S460 phosphorylation is only associated with slow-migrating Nedd1, suggesting that S460 phosphorylation may occur after S411 phosphorylation but has no direct sequential relationship with that of T550.
What is the functional significance of the Plk1-Nedd1 interaction mediated through S460? Johmura et al. (10) set up an elegant system where shRNA-insensitive WT or mutant forms of Nedd1 containing mutations at the phosphorylation sites are stably expressed in HeLa cells. The endogenous Nedd1 is then depleted using shRNA to Nedd1. This approach effectively replaces endogenous Nedd1 with ectopically expressed WT or mutant Nedd1. With this system, Johmura et al. (10) show that the Plk1-Nedd1 interaction mediated through S460 is required for proper targeting of Nedd1 to the spindle. Consequently, replacing endogenous Nedd1 with ectopically expressed Nedd1 containing S460A mutation or the S460A/T550A double mutations results in a significantly elevated number of cells with spindle defects, chromosomal missegregation, prolonged preanaphase, and apoptosis. Mutation of S411, but not T550, to alanine (A) also prevents Nedd1 from its localization to the mitotic spindle. These observations suggest that a sequential phosphorylation of S411 and S460 is essential for targeting Nedd1 to the spindle and that T550 phosphorylation does not seem to be involved in this process.
How does the Plk1-Nedd1 interaction target the Nedd1-γ-TuRC complex to the spindle? Because the Augmin complex, a known regulator of MT-based MT nucleation, interacts with Nedd1 (6, 8) and because Plk1 directly phosphorylates many proteins during mitosis (11), Johmura et al. (10) ask whether Plk1 phosphorylates any component(s) in the Augmin complex. Johmura et al. (10) observe that Hice1, an Augmin component previously shown to directly interact with MT, is strongly phosphorylated by Plk1. 2D gel electrophoresis analysis reveals multiple phosphorylated forms of Hice1 that occur during M phase but not S phase of the cell cycle. Depletion of Plk1 greatly reduces these phosphorylated forms. Furthermore, cells expressing the Nedd1 with S460A mutation in cells depleted of endogenous Nedd1 exhibit dramatically reduced levels of Hice1 phosphorylation. In contrast, the T550A mutation has no effect on the levels of Hice1 phosphorylation. Combined, these results strongly suggest that Plk1 and its interaction with Nedd1 through the S460 residue are required for Hice1 phosphorylation during mitosis. Intriguingly, cells expressing Nedd1 with the S411A mutation have a more severe defect in Hice1 phosphorylation. S411 phosphorylation has been shown to be critical for the Nedd1-Augmin interaction (6, 8). Consistent with earlier studies, Johmura et al. (10) observe that, whereas the Nedd1 S460A mutant protein strongly interacts with Hice1, the S411A mutant fails to do so. Hence, phosphorylation of Nedd1 at S460 is required for its interaction with Plk1 but not Hice1. However, phosphorylation of Nedd1 at S411 is essential for its interaction with Augmin but not Plk1. In other words, the function of distinct phosphorylation events on Nedd1 is to bridge Plk1 and Hice1 so that Plk1 can phosphorylate the latter. It is clear that S411 phosphorylation and the subsequent Nedd1-Augmin interaction precede phosphorylation of Hice1, an Augmin component, by Plk1. It is also obvious that Plk1 is recruited by Nedd1 to the Nedd1-Augmin complex after phosphorylation of Nedd1 by Cdc2 at S460.
What is the functional significance of Hice1 phosphorylation by Plk1? By taking advantage of HeLa cells ectopically expressing RNAi-insensitive, WT Nedd1 or various phosphomutants, Johmura et al. (10) reveal that Hice1 phosphorylation by Plk1 is required for its interaction with MT in a MT cosedimentation assay. Mass spectrometric analyses followed by mutagenesis assays identifies six Plk1-dependent phosphorylation sites in Hice1 protein that are important for mediating the interaction between Hice1 and MT. Is the Hice1-MT interaction mediated by Plk1 phosphorylation important for MT-based MT nucleation? Using MT regrowth assays that measure MT nucleation coupled with time-lapse microscopy, Johmura et al. (10) show that, after deletion of endogenous Hice1 by RNAi, HeLa cells ectopically expressing RNAi-resistant Hice1 mutants with all six Plk1 phosphorylation sites mutated to alanines but not to aspartic acids, displayed a severely compromised MT nucleation and polymerization activity, and resulted in impaired bipolar spindle morphologies and spindle stability. Combined, these results indicate that Plk1 promotes the recruitment of Hice1 to the mitotic spindle by phosphorylation and facilitates MT-dependent MT nucleation within the spindle fibers.
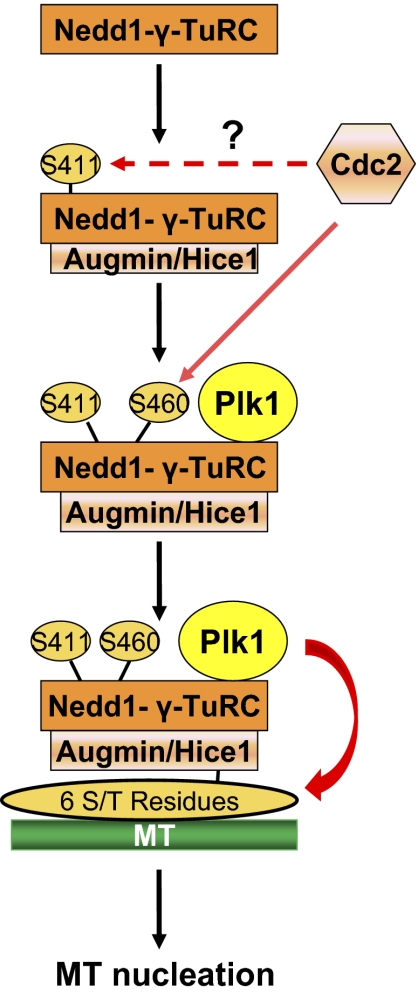
A clear regulatory pathway mediating MT-based MT nucleation has emerged from the study by Johmura et al. (10) (Fig. 1). This pathway entails a three-step phosphorylation process. Step 1 involves phosphorylation of Nedd1 at S411 that facilitates the interaction of the Nedd1-γ-TuRC complex with Augmin. Step 2 involves phosphorylation of Nedd1 at S460 by Cdc2, leading to the recruitment of Plk1 to the Nedd1-γ-TuRC-Augmin complex. Step 3 involves Plk1 phosphorylation of Hice1, a component of Augmin, which leads to the binding of the Nedd1-γ-TuRC-Augmin complex to MT and enhanced MT nucleation from within the spindle.
Fig. 1.
Plk1-dependent MT-based MT nucleation. In MT-based MT nucleation, Nedd1 is first phosphorylated at S411, possibly by Cdc2. This phosphorylation promotes the recruitment of Augmin to the Nedd1-γ-TuRC complex, leading to additional phosphorylation of Nedd1 at S460 by Cdc2. Phosphorylation of S460 creates a docking site for Plk1, resulting in its interaction with the Nedd1-γ-TuRC-Augmin complex. Plk1, in turn, phosphorylates Hice1 (one of eight components within the Augmin complex) at six serine/threonine sites, thereby facilitating the interaction of the Nedd1-γ-TuRC-Augmin complex with MT and subsequent MT nucleation within MT.
The study by Johmura et al. (10) defines the role of Plk1 and Augmin in MT-based MT nucleation and is an important step to further our understanding of the MT nucleation process as well as mitotic spindle formation and stability during cell division. We anticipate that the coming years will see new progresses with regard to the precise roles of mitotic kinases, including Cdc2 and Plk1 in the regulation of microtubule nucleation powered by the centrosome and microtubules. Because mitotic spindle integrity and function are crucial for equal segregation of chromosomes during cell division, this line of study is of great significance to the elucidation of molecular mechanisms by which the cell suppresses chromosomal instability, aneuploidy, and malignant transformation during cell division.
Acknowledgments
This study was supported in part by US Public Service Awards ES019929 (to D.X.), CA090658 (to W.D.), and CA150512 (to W.D.). This work was also supported in part by the National Institute on Environmental Health Sciences Center Fund and Superfund Grants ES000260 and ES010344.
Footnotes
The authors declare no conflict of interest.
See companion article on page 11446.
References
- 1.Gadde S, Heald R. Mechanisms and molecules of the mitotic spindle. Curr Biol. 2004;14:R797–R805. doi: 10.1016/j.cub.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 2.Wiese C, Zheng Y. Microtubule nucleation: Gamma-tubulin and beyond. J Cell Sci. 2006;119:4143–4153. doi: 10.1242/jcs.03226. [DOI] [PubMed] [Google Scholar]
- 3.O'Connell CB, Khodjakov AL. Cooperative mechanisms of mitotic spindle formation. J Cell Sci. 2007;120:1717–1722. doi: 10.1242/jcs.03442. [DOI] [PubMed] [Google Scholar]
- 4.Mahoney NM, Goshima G, Douglass AD, Vale RD. Making microtubules and mitotic spindles in cells without functional centrosomes. Curr Biol. 2006;16:564–569. doi: 10.1016/j.cub.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 5.Murata T, et al. Microtubule-dependent microtubule nucleation based on recruitment of gamma-tubulin in higher plants. Nat Cell Biol. 2005;7:961–968. doi: 10.1038/ncb1306. [DOI] [PubMed] [Google Scholar]
- 6.Uehara R, et al. The augmin complex plays a critical role in spindle microtubule generation for mitotic progression and cytokinesis in human cells. Proc Natl Acad Sci USA. 2009;106:6998–7003. doi: 10.1073/pnas.0901587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lüders J, Patel UK, Stearns T. GCP-WD is a gamma-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat Cell Biol. 2006;8:137–147. doi: 10.1038/ncb1349. [DOI] [PubMed] [Google Scholar]
- 8.Goshima G, Mayer M, Zhang N, Stuurman N, Vale RD. Augmin: A protein complex required for centrosome-independent microtubule generation within the spindle. J Cell Biol. 2008;181:421–429. doi: 10.1083/jcb.200711053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawo S, et al. HAUS, the 8-subunit human Augmin complex, regulates centrosome and spindle integrity. Curr Biol. 2009;19:816–826. doi: 10.1016/j.cub.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 10.Johmura Y, et al. Regulation of microtubule-based microtubule nucleation by mammalian polo-like kinase 1. Proc Natl Acad Sci USA. 2011;108:11446–11451. doi: 10.1073/pnas.1106223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai W. Polo-like kinases, an introduction. Oncogene. 2005;24:214–216. doi: 10.1038/sj.onc.1208270. [DOI] [PubMed] [Google Scholar]
- 12.Archambault V, Glover DM. Polo-like kinases: Conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10:265–275. doi: 10.1038/nrm2653. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, et al. Sequential phosphorylation of Nedd1 by Cdk1 and Plk1 is required for targeting of the gammaTuRC to the centrosome. J Cell Sci. 2009;122:2240–2251. doi: 10.1242/jcs.042747. [DOI] [PubMed] [Google Scholar]
- 14.Park JE, et al. Polo-box domain: A versatile mediator of polo-like kinase function. Cell Mol Life Sci. 2010;67:1957–1970. doi: 10.1007/s00018-010-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haren L, Stearns T, Lüders J. Plk1-dependent recruitment of gamma-tubulin complexes to mitotic centrosomes involves multiple PCM components. PLoS One. 2009;4:e5976. doi: 10.1371/journal.pone.0005976. [DOI] [PMC free article] [PubMed] [Google Scholar]