Abstract
The activation of innate immune responses by nucleic acids is central to the generation of host responses against pathogens; however, nucleic acids can also trigger the development and/or exacerbation of pathogenic responses such as autoimmunity. We previously demonstrated that the selective activation of nucleic acid-sensing cytosolic and Toll-like receptors is contingent on the promiscuous sensing of nucleic acids by high-mobility group box proteins (HMGBs). From this, we reasoned that nonimmunogenic nucleotides with high-affinity HMGB binding may function as suppressing agents for HMGB-mediated diseases, particularly those initiated and/or exacerbated by nucleic acids. Here we characterize an array of HMGB-binding, nonimmunogenic oligodeoxynucleotides (ni-ODNs). Interestingly, we find that binding affinity is rather independent of nucleotide sequence, but is instead dependent on length and structure of the deoxyribose backbone. We further show that these ni-ODNs can strongly suppress the activation of innate immune responses induced by both classes of nucleic acid-sensing receptors. We also provide evidence for the suppressive effect of an ni-ODN, termed ISM ODN, on the induction of adaptive immune responses and in mouse models of sepsis and autoimmunity. We discuss our findings in relation to the critical role of HMGBs in initiating immune responses and the possible use of these ni-ODNs in therapeutic interventions.
Keywords: pattern recognition receptors, retinoic acid-inducible gene I-like receptor, experimental autoimmune encephalomyelitis
The innate immune system is integral to the protection of the host against invading pathogens by providing immediate defense and the subsequent activation of the adaptive immune system. Pattern recognition receptors (PRRs) are germ line-encoded receptors that recognize conserved pathogen-associated molecular patterns (PAMPs) and potently activate cells of the innate immune system (1, 2). The protective role of PRRs against infectious microbial pathogens via the induction of adaptive immunity has been unequivocally demonstrated; however, the activation of these receptors may also result in eliciting harmful immune responses such as life-threatening inflammation and autoimmunity (3, 4). During microbial infection or tissue damage, DNA and RNA potently activate the innate and subsequent adaptive immune responses. In mammals, the transmembrane PRR Toll-like receptor (TLR)3, TLR7, and TLR9, respectively, recognize double-stranded RNA, single-stranded and short double-stranded RNAs, and hypomethylated DNA, whereas retinoic acid-inducible gene I (RIG-I)-like receptors, namely RIG-I and melanoma differentiation-associated gene 5 (MDA5), are best known as RNA-sensing receptors in the cytosol, but more recently have been shown to also participate in the cytosolic DNA-sensing system (5–8). In addition, cytosolic DNA-sensing receptors, which include DNA-dependent activator of IFN regulatory factors (IRFs) (DAI), absent in melanoma 2 (AIM2), among others, have been identified (9, 10). The hallmark of the innate immune responses activated by these receptors is the induction of type I IFNs, proinflammatory cytokines, and chemokines, except for AIM2, which induces the inflammasome (6, 10).
We showed previously that high-mobility group box proteins (HMGB1, 2, and 3) are essential for triggering all nucleic acid receptor-mediated innate immune responses (11). Indeed, HMGBs bind to various immunogenic nucleic acids in vitro, and cells in which the expression of HMGBs is suppressed exhibit a profound defect in their ability to evoke innate immune responses, indicating a hierarchy in the nucleic acid-mediated activation of immune responses wherein the selective activation of nucleic acid-sensing receptors is contingent on the more promiscuous sensing of nucleic acids by HMGBs. In addition, HMGB1 is secreted by innate immune cells in response to PAMPs and released by injured or dying cells, and transmits signals to the cell interior via the activation of receptors that include TLR4, thereby occupying a crucial role in the pathogenesis of both sterile and infectious inflammations (12–14). In this context, numerous antagonists that neutralize HMGB1 in preclinical disease models have supported the role of HMGB1 in regulating innate and adaptive immune responses in health and during inflammatory diseases such as arthritis, sterile ischemia/reperfusion injury, cancer, and infection (15, 16). Thus, selectively targeting HMGB1 and its family members may be of clinical use and could provide key insights into the pathogenesis of various inflammation-associated diseases.
In view of our previous findings that HMGBs bind nucleic acids promiscuously, we searched for nonimmunogenic oligodeoxynucleotides (ni-ODNs) showing high-affinity HMGB binding with the rationale that such nucleotides may effectively suppress HMGB-associated diseases initiated and/or exacerbated by nucleic acids. Here we introduce an array of ni-ODNs that can strongly bind to HMGBs, which we find depends on their length and phosphorothioate deoxyribose backbone. We also show an inhibitory effect of an ni-ODN in the induction of adaptive immune responses as well as in animal disease models associated with HMGBs, and discuss the significance of our findings from a therapeutic point of view.
Results
Generation of ni-ODNs with High-Affinity Binding to HMGBs.
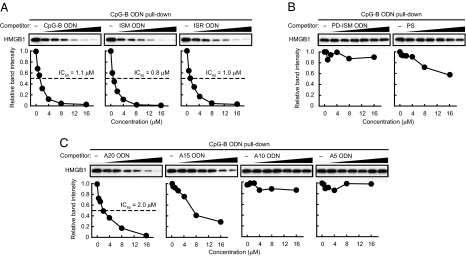
On the basis of our previous finding that the TLR9 agonist CpG-B ODN shows a markedly high affinity with HMGBs (11), we rationalized that analogs without the CpG motif may also show similar if not higher affinities with HMGBs but, unlike CpG-B ODN, which activates TLR9 (5), will be nonimmunogenic (17). Thus, we generated ODNs carrying GpG or GpC in lieu of the CpG dinucleotide sequence of CpG-B ODN (Fig. S1A), respectively termed ISM ODN and ISR ODN, and then examined their interaction with HMGBs with these ODNs by competitive pull-down assay: In this assay, inhibition of the precipitation of a recombinant HMGB protein by biotin-conjugated CpG-B ODN was monitored by increasing the amounts of unconjugated ODN of interest (11). As expected, the precipitation of HMGB1 was inhibited by unconjugated CpG-B ODN in a dose-dependent manner, whereas ISM and ISR ODNs also showed inhibition in this assay (Fig. 1A). Among the three ODNs examined, the half-maximal inhibitory concentration (IC50) was lowest for ISM ODN, suggesting that ISM ODN has the highest affinity with HMGB1 (Fig. 1A). Unlike CpG-B ODN, ISM and ISR ODNs were inert in evoking innate immune responses on the basis of cytokine induction in dendritic cells (DCs) (Fig. S1B).
Fig. 1.
Binding of HMGB1 to ODNs. Pull-down assay was performed using recombinant HMGB1 and biotin-conjugated CpG-B ODN in the presence of increasing amounts of unconjugated CpG-B, ISM, and ISR ODNs (A), PS and PD-ISM ODN (B), and A20, A15, A10, and A5 ODNs (C) (0.5, 1, 2, 4, 8, or 16 μM). The relative band intensity of precipitated HMGB1 is depicted graphically.
Because these ODNs all have a phosphorothioate backbone instead of the usual phosphodiester backbone (11), we next asked whether the nature of the sugar backbone would affect ODN affinity with HMGB1 by generating an ISM ODN with a natural phosphodiester bond, termed PD-ISM ODN. When PD-ISM ODN was subjected to the pull-down assay, the inhibition of HMGB1–CpG-B ODN interaction was not observed, indicating that the phosphorothioate backbone is a critical element for ODN binding to HMGB1 (Fig. 1B). However, because the inhibition of HMGB1 precipitation by a base-free phosphorothioate deoxyribose homopolymer, termed PS (18), is far weaker than the inhibition by CpG-B ODN and ISM and ISR ODNs of identical length, it is clear that the bases also contribute to HMGB1 binding (Fig. 1B).
The above observations prompted us to examine whether the base sequence and length of ODNs affect their binding to HMGB1 by generating various lengths of poly(dA) with the phosphorothioate backbone (Fig. S1C). As shown in Fig. 1C, 20-mer poly(dA) (A20 ODN) inhibited the binding of HMGB1 to CpG-B ODN, but the inhibition was much weaker for 15-mer poly(dA) (A15 ODN), whereas 10- and 5-mer poly(dA) (A10 and A5 ODNs, respectively) failed to inhibit the CpG-B ODN–HMGB1 interaction entirely. Essentially the same observations were made when poly(dC) ODNs (C20, C15, C10, and C5 ODNs) were similarly examined (Fig. S1D). These observations thus identify three critical elements of ODNs for their high-affinity binding to HMGB1, namely ODN phosphorothioate backbone and length and, albeit to a lesser extent, base sequence. Finally, we also found that HMGB1, HMGB2, and HMGB3 were all bound directly to ISM ODN (Fig. S1E). Collectively, these results are consistent with our previous finding of promiscuous binding of HMGBs (11) and demonstrate that even ni-ODNs can bind HMGBs with high affinity, provided that they have the characteristics defined above.
Suppressive Effect of ISM ODN on Nucleic Acid-Mediated Immune Responses.
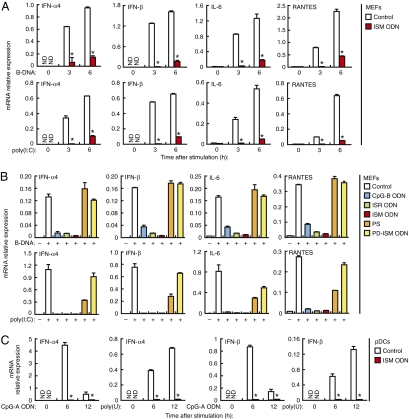
We next examined whether ISM ODN, which shows the highest binding affinity with HMGBs, suppresses nucleic acid-mediated innate immune responses. As shown in Fig. 2A, mouse embryonic fibroblasts (MEFs) pretreated with ISM ODN and subsequently stimulated with poly(dA-dT)·poly(dT-dA) (B-DNA) (19) or poly(I:C) showed markedly suppressed mRNA induction of type I IFNs, IL-6, and RANTES genes compared with mock-treated MEFs; as expected, ISM ODN strongly interferes with the binding of HMGB1 to B-DNA or poly(I:C) (Fig. S2A). On the other hand, the mRNA induction of the same genes by lipopolysaccharide (LPS) stimulation remained unaffected (Fig. S2B). To examine the correlation between HMGB binding affinity and the suppressive effect of the above ODNs, we compared ISM ODN with CpG-B ODN, ISR ODN, PS, and PD-ISM ODN for their suppressive effect on cytosolic nucleic acid stimulations in MEFs. As shown in Fig. 2B, mRNA induction of the cytokine genes was also strongly suppressed by CpG-B or ISR ODNs, albeit less effectively than ISM ODN. On the other hand, the suppressive effect was very weak, if at all, for PS and PD-ISM ODN. Essentially the same observation was made for the induction of IFN-β and IL-6 secretion by ELISA (Fig. S2 C and D). In addition, A20 or C20 ODNs effectively suppressed IFN-β mRNA induction by B-DNA, whereas the effect diminished rather markedly as ODN length decreased (Fig. S2E). Thus, the suppressive effect of each ODN correlates with its binding affinity with HMGB1.
Fig. 2.
Suppressive effect of ISM ODN on nucleic acid-mediated innate immune responses. (A and B) MEFs were left untreated (control) or pretreated with the indicated ODN (1 μM) for 1 h, and then stimulated with B-DNA (5 μg/mL) or poly(I:C) (5 μg/mL) for 3 h (A and B) or 6 h (A). (C) Bone marrow-derived pDCs were left untreated (control) or pretreated with ISM ODN for 1 h, and then stimulated with CpG-A ODN (1 μM) or poly(U) (5 μg/mL) for 6 or 12 h. Cytokine mRNA expression levels of the indicated genes were measured by quantitative RT-PCR. *P < 0.01 compared with control. Data in all panels are presented as means and SD (n = 3). ND, not detected.
Because HMGBs are essential for both cytosolic and TLR-dependent sensing of nucleic acids (11), we next determined whether TLR responses to nucleic acids are also suppressed by ISM ODN. First, bone marrow cells were cultured in vitro with the Flt3 ligand to enrich plasmacytoid DCs (pDCs), which produce type I IFN en masse upon TLR7 or TLR9 stimulation (20), and the effect of ISM ODN was then examined. As shown in Fig. 2C, the mRNA induction by CpG-A ODN or poly(U), which respectively activate TLR9 and TLR7, was strongly suppressed when these cells were pretreated with ISM ODN. Consistent with this, a strong suppression by ISM ODN was also observed on the secretion of IFN-α and IFN-β by these cells (Fig. S2F). Similarly, when conventional DCs (cDCs), which were obtained by in vitro culture of bone marrow cells with GM-CSF, were examined for CpG-B ODN-TLR9-mediated IL-6 and TNF-α production, the ISM ODN pretreatment of the cells resulted in a strong suppression, whereas the LPS-TLR4-mediated production of the same cytokines remained unaffected (Fig. S2G). Taken together, these findings all support our initial rationalization that ni-ODNs with high affinity for HMGBs can selectively suppress nucleic acid-activated immune responses via cytosolic receptors or TLRs.
Effect of ISM ODN on Cellular Delivery of and Receptor Signaling by Nucleic Acids.
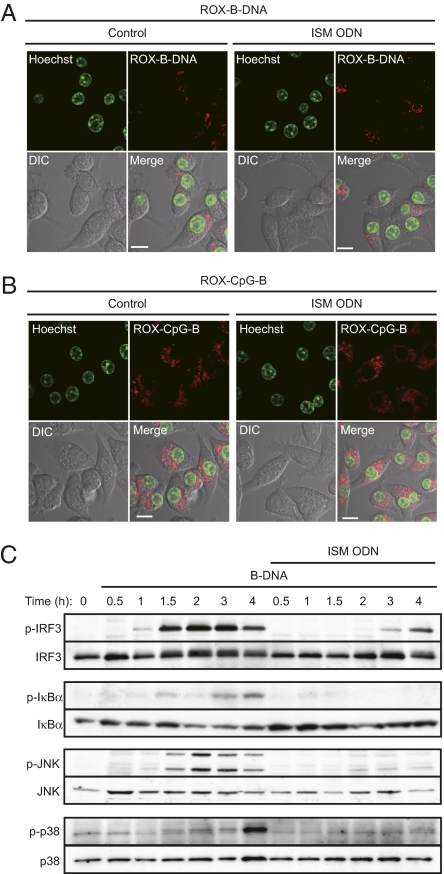
To address where these ODNs exert their suppressive effect on nucleic acid-mediated innate immune responses, we first examined whether ISM ODN affects the cellular uptake of B-DNA by fluorescence microscopy. When a macrophage-like cell line, RAW264.7, was pretreated or mock-treated with ISM ODN and then transfected with rhodamine-labeled B-DNA, the B-DNA uptake in the cells was equally observed in the cytosolic fraction as spot-like shapes, suggesting that ISM ODN pretreatment does not interfere with the B-DNA uptake into the cells (Fig. 3A and Fig. S3A). Similarly, the uptake of CpG-B ODN remained unaffected by ISM ODN pretreatment (Fig. 3B and Fig. S3B). Interestingly, when rhodamine-conjugated ISM ODN was delivered into the cells, it merged with FITC-labeled dextran, an endosome/lysosome marker, as well as with LysoTracker, a lysosome marker (Fig. S3C); this pattern is essentially similar to that of the TLR9 agonist CpG-B ODN, as demonstrated in previous studies (21, 22), and suggests that the endosomal/lysosomal trafficking of these ODNs occurs similarly. On the other hand, rhodamine-labeled B-DNA merged with dextran but not with LysoTracker (Fig. S3D). Thus, taken together with previous reports showing that HMGB1 is also expressed in endosomes (11, 23), these findings suggest that it is the endosomal compartment where ISM ODN exerts its suppressive effect on B-DNA–mediated innate immune signaling by competing with this and other immunogenic nucleic acids for binding with HMGBs (Discussion).
Fig. 3.
Suppression of nucleic acid-mediated signaling pathways by ISM ODN. (A and B) RAW264.7 cells were left untreated (control) (Left) or treated with ISM ODN (1 μM) (Right) for 1 h, and then stimulated with rhodamine-conjugated B-DNA (1 μg/mL) (ROX-B-DNA; red) (A) or rhodamine-conjugated CpG-B ODN (1 μM) (ROX-CpG-B; red) (B) for 1 h. Fluorescence images were observed by confocal microscope. The nucleus was stained with Hoechst 33342 (green). (Scale bars, 10 μm.) (C) MEFs were left untreated or pretreated with ISM ODN for 1 h, and then stimulated with 10 μg/mL B-DNA for the indicated time periods. The phosphorylation of IRF3, IκB-α, JNK, and p38 was assessed by immunoblot analysis using the indicated antibodies.
To further consolidate the notion that ISM ODN indeed suppresses innate immune signaling, we examined the induction of phosphorylation of IRF3, IκB-α, JNK, and p38 MAPK following cytosolic B-DNA stimulation in MEFs with or without ISM ODN pretreatment. As shown in Fig. 3C, the phosphorylation of all was inhibited by ISM ODN pretreatment, which was similarly observed in cells stimulated by poly(I:C) (Fig. S3E). Because the sequence of ISM ODN is identical to that of the TLR9 agonist CpG-B ODN aside from one nucleotide, we wished to confirm that the inhibitory effect of ISM ODN is not mediated by TLR9 by any means. When wild-type and Tlr9-deficient cDCs were cytosolically stimulated with B-DNA or poly(I:C), we observed that the induction of IL-6 and TNF-α mRNAs was equally suppressed by ISM ODN in cells of both genetic backgrounds, indicating that ISM ODN bound to HMGBs exerts its function independently of TLR9 (Fig. S3F). Taken together with these findings, we surmise that ISM ODN does not interfere with the uptake of nucleic acids but rather inhibits the subsequent ligand binding to the PRRs, thereby blocking downstream signal transduction events (Discussion).
ISM ODN Suppresses Nucleic Acid-Mediated Immune Responses in Vivo.
Nucleic acids potently enhance adaptive immune responses, be they protective or harmful to the host, via the activation of innate immune receptors (24). To investigate the effect of ISM ODN on nucleic acid-mediated adaptive immune responses in vivo, mice were immunized with chicken ovalbumin (OVA) protein using B-DNA or CpG-A ODN as an adjuvant (22) and either with or without pretreatment with ISM ODN, and the induction of CD8α+ cytotoxic T lymphocytes (CTLs) was examined by flow cytometry. As shown in Fig. 4A, the induction of OVA-specific CTL responses was strongly suppressed by the pretreatment of mice with ISM ODN. In addition, when OVA-specific IgG1 production was monitored in the sera from mice immunized with OVA and B-DNA, it was found to be suppressed and almost undetectable in ISM ODN-pretreated mice (Fig. S4A). Thus, ISM ODN also serves as a potent suppressor of nucleic acid-mediated adaptive immune responses.
Fig. 4.
Effect of ISM ODN on nucleic acid-mediated immune responses and disease models in vivo. (A) Mice were immunized with 0.5 mg of OVA and DNA (10 μg of B-DNA or 50 μg of CpG-A ODN) in the presence or absence of 1 mg of ISM ODN. Six days later, splenocytes were isolated and subjected to three-color FACS analysis using an anti-CD8α antibody, anti-CD44 antibody, and OVA-specific MHC class I tetramer. The data shown were gated on CD8α-positive events. The numbers indicate the percentage of tetramer-positive cells relative to the total number of CD8α+ T cells. (B) Mean EAE scores ± SD for mice treated with PBS (control) or ISM ODN (n = 4 per group) are shown. (C) Mice were i.p. injected with 1.25 mg of LPS, with or without (control) 0.1 mg of ISM ODN 1 h before LPS injection. The survival rate of each group was monitored for 72 h (n = 10).
Therapeutic Effects of ISM ODN in Immunological Disorder Models.
The above findings prompted us to examine whether ISM ODN suppresses inflammation or autoimmune disorders in which the contribution of nucleic acids and/or HMGB1 has been implicated. There is ample evidence that autoantibodies bound to self-DNA or -RNA, or DNA derived from dead cells of inflammatory lesions, can activate nucleic acid-sensing receptors and subsequent immune responses (4, 24–26). A typical example is experimental autoimmune encephalomyelitis (EAE), an animal autoimmune demyelinating disease model of human multiple sclerosis, wherein signaling by nucleic acid-sensing TLRs contributes to the development and pathology of the disease (27–29). Thus, we examined whether ISM ODN can suppress EAE development in mice immunized with myelin oligodendrocyte glycoprotein peptide emulsified in complete Freund's adjuvant. Eight days after immunization, these mice were injected i.v. with ISM ODN or were mock-injected. Remarkably, EAE development, which was observed in the mock-injected mice, was strongly suppressed in the ISM ODN-injected mice (Fig. 4B).
We next examined the effect of ISM ODN on an established LPS-induced septic shock model for which an active role for HMGB1 has been reported (30). Interestingly, when mice were i.p. injected with a lethal dose of LPS, those pretreated with ISM ODN showed a significantly high survival rate: As shown in Fig. 4C, whereas all mice without ISM ODN pretreatment died within 48 h, 70% of those mice with ODN pretreatment survived even 72 h after LPS injection. It is worth noting that, unlike the control mice, the induction of serum aspartate transaminase (AST) and alanine transaminase (ALT), which are indicators of liver injury, remained strongly suppressed in ISM ODN-pretreated mice 16 h after LPS injection, suggesting that ISM ODN itself has little or no toxicity (Fig. S4B). To further examine how ISM ODN suppresses LPS-induced lethality in mice, we analyzed the levels of serum proinflammatory cytokines after LPS injection. As shown in Fig. S4C, IL-6 and TNF-α production levels in sera were found to be the same between ISM ODN-pretreated and mock-pretreated mice 2 h after LPS injection; however, cytokine levels were markedly reduced in the ODN-pretreated mice at later time points. These findings suggest that the early-phase induction of these cytokines via the LPS-TLR4 signaling pathway is not affected by ISM ODN, but rather it is the later-phase induction that is mediated by HMGB1 which is inhibited by the ODN. Indeed, HMGB1 is induced by LPS injection at this later phase (i.e., 8–32 h after injection) (30). It has also been shown that HMGB1, which activates TLR4 and possibly other receptors, is required for a lethal inflammation (12, 13, 16). Thus, these observations raise the interesting possibility that ISM ODN not only suppresses nucleic acid-mediated immune responses but may also affect the activity of HMGB1 as a proinflammatory cytokine (Discussion).
Discussion
Nucleic acid-mediated immune responses have become an attractive area of study, given their connection to protective and pathological immunities. In view of our previous finding that HMGBs function as universal sentinels for the innate immune responses activated by nucleic acids (11), we aimed here to demonstrate proof of concept for targeting HMGBs as a means for therapeutic intervention for immunological disorders. To do so, we reasoned that ni-ODNs with a high affinity for HMGBs would interfere with the activation of subsequent nucleic acid-mediated immune responses. Indeed, ISM ODN was found to bind HMGBs with high affinity (Fig. 1 and Figs. S1 D and E and S2A) and to suppress immune responses induced by nucleic acid-sensing cytosolic receptors or TLRs (Fig. 2 and Fig. S2 C, D, and F). Because intracellular uptake/delivery of the immunogenic nucleic acids remained unaffected in the presence of ISM ODN (Fig. 3 A and B) yet downstream signaling events were suppressed (Fig. 3C and Fig. S3E), it is likely that ISM ODN competes with immunogenic nucleic acids for HMGB binding. On the basis of our observations (Fig. S3 C and D) and on previous reports showing that HMGB1 is expressed also in endosomes (11, 23), this suppression of HMGB1 function by ISM ODN likely occurs in endosomal vesicles. This view is consistent with our previous notion that the association of ligands with the endosomal membrane and their binding to innate receptors are the common events for the activation of nucleic acid-mediated immune responses (11, 23, 31).
We surmise further that HMGBs function as essential and common components of nucleic acid ligands, without which nucleic acids cannot efficiently bind and activate their cognate receptors, and that the ni-ODNs described here likely interfere with this critical interaction through their binding to HMGBs. Indeed, our data show a correlation between the binding affinity of ODNs with HMGB1 and the magnitude of the suppressive effect of ODNs. Of the structural elements of ODNs that determine binding affinity with HMGBs, our data indicated that a length of more than 15 nucleotides and a phosphorothioate backbone are most critical (Fig. 1 B and C and Fig. S1D). In this regard, previous reports have shown that one HMGB1 molecule covers 15–18 bp of DNA (32, 33), which corroborates our findings that affinity markedly decreased as the ODNs became shorter than 20 nucleotides (Fig. 1C and Fig. S1D). Our results also show the importance of phosphorothioate modification of a normal phosphodiester backbone for the high-affinity interaction of ni-ODNs with HMGBs (Fig. 1B); it is interesting to note that this backbone structure per se also shows binding affinity with other proteins including TLR9 (18, 34). However, it is clear that the nucleotide bases attached to the backbone are also critical for ODN–HMGB interaction. In an attempt to suppress the activation of TLR7- or TLR9-mediated immune responses, relatively short (15- to 25-mer, in most cases) partially or entirely phosphorothioated ODNs have been studied (35, 36). In addition, several classes of “inhibitory ODNs” have been developed, and some of them are reported to ameliorate disease symptoms of animal models of autoimmune diseases and septic shock (36); however, it has been shown that the affinity of inhibitory ODNs with the receptors does not necessarily correlate with their inhibitory activity (37). In light of our present findings, these ODNs may also exert their inhibitory activities on TLR signaling through their interaction with HMGBs.
Nucleic acid-mediated immune responses have been implicated in the pathogenesis of inflammatory and autoimmune diseases (4, 24). Our present study indicates that ISM ODN may serve as an effective compound for the treatment of autoimmunity and sepsis (Fig. 4 B and C). Notably, ISM ODN treatment did not affect the growth of primary MEFs in vitro (Fig. S3G) and did not change AST and ALT levels in sera (Fig. S4B), suggesting that ISM ODN treatment has little, if any, toxic effect. Because it has been suggested that TLR9 signaling is related to the exacerbation of EAE symptoms (28, 29), we surmise that ISM ODN may block pathological TLR9 signaling, thereby attenuating the progression of the disease. Moreover, we show ISM ODN suppression of LPS-mediated endotoxin shock, which provides yet another interesting possibility for ODN-based therapy for sepsis and numerous other inflammatory diseases involving HMGB1 (16). Although further study will be required to clarify how ISM ODN inhibits LPS-mediated endotoxin shock, the following possibilities may be considered. It is possible that ISM ODN inhibits the function of HMGB1 as an inflammatory cytokine when released into the extracellular environment by active secretion from stimulated monocytes/macrophages (30) or by passive diffusion from necrotic cells, functioning as a potent proinflammatory cytokine (12) via the activation of TLR4 and other receptors (13). Thus, ODN interaction with secreted HMGB1 may cause the functional inactivation of HMGB1 as an inflammatory cytokine, possibly through the induction of a conformational change. On the other hand, because ISM ODN shows no effect on LPS-mediated response in vitro (Fig. S2 B and G), HMGB1 may need to interact with an additional molecule for its inflammatory cytokine action in vivo. Because LPS injection into mice induces massive death of hepatocytes and other cells (38), one possibility is the suppression of inflammatory responses by the necrotic cell-derived DNA that may activate the innate immune receptors via interaction with HMGBs. This notion is supported by our observation that IL-6 and TNF-α production induced by necrotic cells is inhibited by treatment with ISM ODN (Fig. S4D). Another intriguing possibility is that HMGB1 secreted from cells associates with nucleic acids and that the resulting complex may be the active form, whose function is inhibited by the ODN.
Because of its inflammatory activity, HMGB1 has been targeted for therapy in the treatment of numerous inflammatory diseases. For instance, a previous study has demonstrated that injection of an anti-HMGB1 antibody protects mice from LPS-induced lethal shock (30). In addition, because the protein is often overexpressed in cancer cells, there is much hope for therapeutic strategies based on targeting HMGB1 (39). Thus, ISM ODN or more effective derivatives may lead to the development of alternate therapeutic interventions for these diseases. Further evaluation of the efficacy and safety of ISM ODN and other ODNs, compared with other strategies targeting HMGB1, will be critical for pursuing such possibilities.
Materials and Methods
Poly(dA-dT)·poly(dT-dA) (B-DNA), poly(U), and LPS were purchased from Sigma. Poly(I:C) was purchased from GE Healthcare Biosciences. ODNs for CpG-B (17), CpG-A (GGtgcatcgatgcaGGGGGg) (40), ISM, ISR, PD-ISM (tccatgaggttcctgatgct), poly(dA), and poly(dC) (uppercase, phosphorothioate backbone; lowercase, phosphodiester backbone) with or without biotin, rhodamine, or FITC were purchased from FASMAC. PS (18) was used as described previously (11). Complex formation of B-DNA, poly(I:C), or poly(U) with Lipofectamine 2000 (Invitrogen) and that of CpG-A ODN with N-[1-(2,3-dioleoyloxy)propyl]-N, N, N-trimethylammonium methyl-sulfate (DOTAP) (Roche Applied Science) was prepared as described previously (11, 22). Additional information is available in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported in part by a Grant-In-Aid for Scientific Research on Innovative Areas and by the Global Center of Excellence Program “Integrative Life Science Based on the Study of Biosignaling Mechanisms” from the Ministry of Education, Culture, Sports, and Science, Japan. T.B. is a research fellow of the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108535108/-/DCSupplemental.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 3.Pisetsky DS. The role of innate immunity in the induction of autoimmunity. Autoimmun Rev. 2008;8:69–72. doi: 10.1016/j.autrev.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 7.Yoneyama M, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 8.Choi MK, et al. A selective contribution of the RIG-I-like receptor pathway to type I interferon responses activated by cytosolic DNA. Proc Natl Acad Sci USA. 2009;106:17870–17875. doi: 10.1073/pnas.0909545106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 10.Schroder K, Muruve DA, Tschopp J. Innate immunity: Cytoplasmic DNA sensing by the AIM2 inflammasome. Curr Biol. 2009;19:R262–R265. doi: 10.1016/j.cub.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Yanai H, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 12.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi ME. HMGB1 loves company. J Leukoc Biol. 2009;86:573–576. doi: 10.1189/jlb.1008585. [DOI] [PubMed] [Google Scholar]
- 14.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 15.Apetoh L, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 16.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieg AM, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 18.Haas T, et al. The DNA sugar backbone 2′ deoxyribose determines Toll-like receptor 9 activation. Immunity. 2008;28:315–323. doi: 10.1016/j.immuni.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Ishii KJ, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 20.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 21.Latz E, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 22.Honda K, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 23.Ivanov S, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lövgren T, Eloranta ML, Båve U, Alm GV, Rönnblom L. Induction of interferon-α production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 26.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: The role of Toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 27.Marta M, Andersson A, Isaksson M, Kämpe O, Lobell A. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur J Immunol. 2008;38:565–575. doi: 10.1002/eji.200737187. [DOI] [PubMed] [Google Scholar]
- 28.Prinz M, et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest. 2006;116:456–464. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segal BM, Chang JT, Shevach EM. CpG oligonucleotides are potent adjuvants for the activation of autoreactive encephalitogenic T cells in vivo. J Immunol. 2000;164:5683–5688. doi: 10.4049/jimmunol.164.11.5683. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 31.Tian J, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 32.Saito K, Kikuchi T, Shirakawa H, Yoshida M. The stabilized structural array of two HMG1/2-boxes endowed by a linker sequence between them is requisite for the effective binding of HMG1 with DNA. J Biochem. 1999;125:399–405. doi: 10.1093/oxfordjournals.jbchem.a022300. [DOI] [PubMed] [Google Scholar]
- 33.Stott K, Tang GS, Lee KB, Thomas JO. Structure of a complex of tandem HMG boxes and DNA. J Mol Biol. 2006;360:90–104. doi: 10.1016/j.jmb.2006.04.059. [DOI] [PubMed] [Google Scholar]
- 34.Peck ML, Herschlag D. Effects of oligonucleotide length and atomic composition on stimulation of the ATPase activity of translation initiation factor elF4A. RNA. 1999;5:1210–1221. doi: 10.1017/s1355838299990817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halpern MD, Pisetsky DS. In vitro inhibition of murine IFN γ production by phosphorothioate deoxyguanosine oligomers. Immunopharmacology. 1995;29:47–52. doi: 10.1016/0162-3109(95)00043-s. [DOI] [PubMed] [Google Scholar]
- 36.Lenert PS. Classification, mechanisms of action, and therapeutic applications of inhibitory oligonucleotides for Toll-like receptors (TLR) 7 and 9. Mediators Inflamm. 2010;2010:986596. doi: 10.1155/2010/986596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashman RF, Goeken JA, Latz E, Lenert P. Optimal oligonucleotide sequences for TLR9 inhibitory activity in human cells: Lack of correlation with TLR9 binding. Int Immunol. 2011;23:203–214. doi: 10.1093/intimm/dxq473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nolan JP. Endotoxin, reticuloendothelial function, and liver injury. Hepatology. 1981;1:458–465. doi: 10.1002/hep.1840010516. [DOI] [PubMed] [Google Scholar]
- 39.Tang D, Kang R, Zeh HJ, III, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799:131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verthelyi D, Ishii KJ, Gursel M, Takeshita F, Klinman DM. Human peripheral blood cells differentially recognize and respond to two distinct CPG motifs. J Immunol. 2001;166:2372–2377. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.