Abstract
Type 1 diabetes (T1D) is an autoimmune disease characterized by insulitis and islet β-cell loss. Thus, an effective therapy may require β-cell restoration and immune suppression. Currently, there is no treatment that can achieve both goals efficiently. We report here that GABA exerts antidiabetic effects by acting on both the islet β-cells and immune system. Unlike in adult brain or islet α-cells in which GABA exerts hyperpolarizing effects, in islet β-cells, GABA produces membrane depolarization and Ca2+ influx, leading to the activation of PI3-K/Akt–dependent growth and survival pathways. This provides a potential mechanism underlying our in vivo findings that GABA therapy preserves β-cell mass and prevents the development of T1D. Remarkably, in severely diabetic mice, GABA restores β-cell mass and reverses the disease. Furthermore, GABA suppresses insulitis and systemic inflammatory cytokine production. The β-cell regenerative and immunoinhibitory effects of GABA provide insights into the role of GABA in regulating islet cell function and glucose homeostasis, which may find clinical application.
Keywords: insulin, inflammation, regulatory T-cell
Type 1 diabetes (T1D) is an autoimmune disease characterized by the infiltration of the pancreatic islets by T lymphocytes, macrophages, and other immune cells (i.e., insulitis), and consequent loss of β-cells (1–3). At the onset of T1D, more than 70% of β-cells are destroyed (4), whereas the residual β-cells most likely represent the only reservoir for the regeneration of islet β-cell mass (5). There has been a growing interest in identifying endogenous growth factors for β-cell replication. The incretin hormone GLP-1 has demonstrated a β-cell stimulatory capacity and clinical efficacy in T2D treatment (6), but it is only marginally effective in T1D treatment (7), likely because of an insufficient effect of GLP-1 on the suppression of autoimmunity. Indeed, an effective therapy of T1D requires suppression of the autoimmune process and restoration of islet β-cells.
GABA, synthesized from glutamate by glutamic acid decarboxylase (GAD), is a major neurotransmitter in the CNS (8). In the adult brain, GABA induces a fast inhibition in neurons mainly through the GABAA receptor (GABAAR) (9). Activation of GABAAR, a ligand-gated Cl− ion channel, results in membrane hyperpolarization as a consequence of Cl− influx (8). In the developing brain, however, activation of GABAAR induces membrane depolarization, which regulates neuronal cell proliferation and maturation (10–12). GABAARs are also expressed in various immune cells, including T cells, and appear to exert immunoinhibitory effects (13–15).
GABA is produced by pancreatic β-cells (16). GABA released from β-cells can act on GABAAR in the α-cells, causing membrane hyperpolarization and hence suppressing glucagon secretion (17, 18). An impaired insulin-Akt-GABAAR-glucagon secretory pathway in the islet may be an underlying mechanism for unsuppressed glucagon secretion, despite hyperglycemia, in diabetic subjects (18, 19). Remarkably, studies by our group and others have demonstrated that β-cells also express GABAARs (20, 21), forming an autocrine GABA signaling system (20, 21). However, the role of this autocrine GABA signaling in the regulation of β-cell functions remains largely unknown.
It has been previously demonstrated that persistent high glucose or elevated cytoplasmic ATP levels could suppress GABA production and its release from β-cells (22). In view of the critical role of GABA–GABAAR signaling in neuronal cell proliferation and maturation (11), we hypothesized that activation of GABA–GABAAR signaling in pancreatic β-cells would have trophic activities and exert therapeutic effects in diabetic subjects. In this study, we demonstrated that, unlike in α-cells, in which it induces hyperpolarization, GABA produces membrane depolarization in β-cells, which leads to the opening of voltage-dependent calcium channels (VDCCs) and the activation of the Ca2+-dependent PI3K/Akt cell growth and survival signaling pathway. In T1D mouse models, GABA prevents and reverses the disease by promoting β-cell growth and survival. Furthermore, GABA exerts immunoinhibitory effects, which may likely protect the β-cells from autoimmune destruction.
Results
GABA Promotes β-Cell Proliferation and Protects β-Cell from Apoptosis.
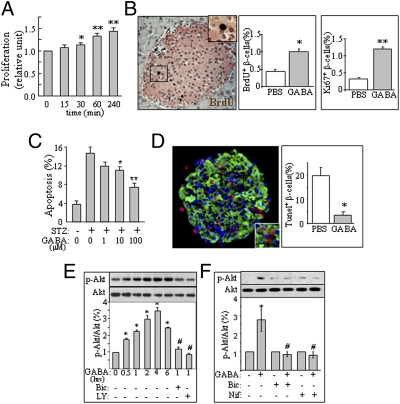
To determine whether GABA has trophic effects in the β-cells, we first performed [3H]thymidine incorporation assay in the INS-1 cell line. We found that GABA treatment time-dependently increased DNA synthesis in these cells (Fig. 1A). We next conducted pancreatic immunohistochemistry to investigate the in vivo effects of GABA on islet β-cells by injecting mice with GABA (i.p., 20 μmol per mouse, 48 h). We found an increase in the number of BrdU+ or Ki67+ islet β-cells in GABA-injected mice (Fig. 1B), suggesting that GABA increases β-cell proliferation in vivo. To determine whether GABA could protect β-cell against apoptosis, we examined apoptosis in INS-1 cells and isolated mouse islets challenged with apoptotic inducers. As shown, GABA significantly reduced streptozotocin (STZ) induced death in the clonal INS-1 cells (Fig. 1C) and in isolated mouse islets challenged with a cytotoxic cytokine mixture (Fig. 1D). These observations suggest that GABA promotes both β-cell replication and survival.
Fig. 1.
GABA activates Ca2+–PI3K/Akt pathway in the β-cells. (A) [3H]thymidine incorporation in INS-1 cells (n = 4). (B) BrdU assay (brown) in the islet β-cells (pink) of CD1 mice injected with GABA. Two i.p. injections of GABA (20 μmol per mouse) were made within 48 h, and BrdU was injected i.p. (100 mg/kg) 6 h before the animal was killed (150–200 islets were examined; n = 5). (C) Apoptosis assay in the INS-1 cells challenged with STZ (15 mM, 24 h) in the presence of different concentrations of GABA (n = 5). (D) TUNEL (red) and insulin (green) dual staining of the isolated islets from CD1 mice, pretreated with or without GABA (100 μM, 16 h), and challenged with a mixture of cytokines (10 ng/mL IL-1β, 50 ng/mL TNF-α, 50 ng/mL IFN-γ) for 20 h (n = 5). (E) Immunoblot of p-Akt and Akt in INS-1 cells treated with GABA (100 μM) for various time intervals, in the presence or absence of bicuculline (Bic, 20 μM) or the PI3K inhibitor LY294002 (LY, 20 μM; n = 8). (F) Immunoblot of p-Akt and Akt in INS-1 cells treated with GABA (100 μM) in the presence or absence of Bic or the Ca2+ channel blocker nifedipine (Nif, 10 nM) for 2 h (n = 4). (*P < 0.05, **P < 0.01 vs. control groups.)
GABA Produces Depolarizing Effects and Initiates Ca2+-PI3-K/Akt Pathway.
We found that GABA stimulated Akt phosphorylation, which was blocked by the GABAAR antagonist bicuculline, PI3K inhibition (Fig. 1E), or the calcium channel blocker nifedipine (Fig. 1F). This suggests that GABAAR-mediated trophic effect is mediated by the Ca2+-dependent PI3K/Akt pathway (10) in β-cells.
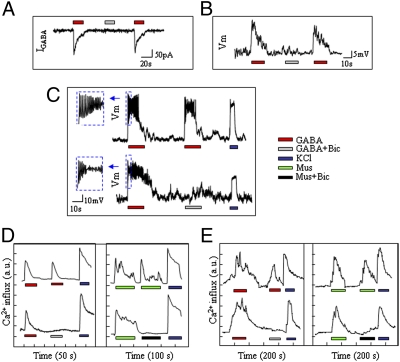
We performed patch-clamp recordings to analyze how GABA elicits Ca2+–PI3K/Akt signaling in the cells. At a holding potential of −60 mV, application of GABA (100 μmol/L) evoked bicuculline-sensitive currents (Fig. 2A); however, under the current-clamp model (Fig. 2 B and C), GABA induced membrane depolarization in INS-1 cells rather than hyperpolarization as seen in the α-cells (17, 18). To address whether GABA-evoked membrane depolarization leads to activation of VDCCs, we measured intracellular Ca2+ mobilization in INS-1 cells (Fig. 2D) and isolated mouse islet β-cells (Fig. 2E) by using a Ca2+ imaging system. We found that both GABA and a GABAAR agonist muscimol (Fig. 2 D and E) stimulated Ca2+ influx in the β-cells. These observations suggested that GABA induced membrane depolarization, subsequent Ca2+ entry, and activation of the PI3K/Akt pathway, which may represent a mechanism underlying its in vivo effects in promoting β-cell growth and survival.
Fig. 2.
GABA produces membrane depolarization in β-cells. Representative traces show GABA-evoked currents (IGABA, A) and GABA-induced depolarization (intracellular sharp recordings) (B) in INS-1 cells. (C) Current-clamp recording (at membrane potential of −60 mV) of INS-1 cells in the presence of GABA (100 μM) with or without Bic (100 μM). Intracellular Ca2+ measurements in INS-1 cells (D) and isolated islet β-cells (E) in the presence of GABA or GABAAR agonist muscimol (10 μM) (E) with or without Bic. (*P < 0.05 and **P < 0.01; n = 3–5.)
GABA Prevents Diabetic Hyperglycemia in T1D Mouse Models.
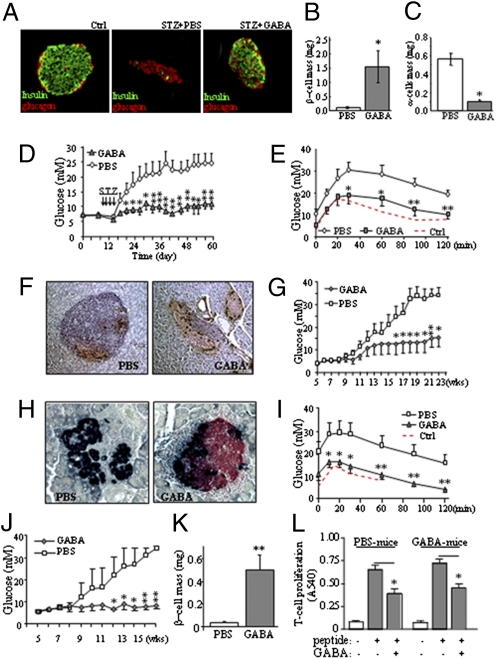
To elucidate whether GABA plays a role in the regulation of glucose homeostasis, we examined its effects in multiple low-dose STZ-induced diabetes (MDSD) (23) in mice. We found that MDSD mice had a severe loss of islet β-cells, with the residual islet containing mostly α-cells (Fig. 3A, Middle). Daily GABA injections initiated 7 d before STZ treatment prevented β-cell loss (Fig. 3A, Right). Thus, β-cell mass was preserved, whereas α-cell mass was reduced (Fig. 3 B and C). Consistently, GABA-treated mice showed higher circulating insulin, lower glucagon (Fig. S1 A–C), nearly normal glycemia (Fig. 3D), and improved metabolic conditions (Fig. S1 D–H), and maintained close to normal glucose tolerance (Fig. 3E), during a period of 53 d after STZ injections. Insulin sensitivity was not altered, but glucagon tolerance was significantly improved (Fig. S1 I and J), indicating that GABA prevented diabetic hyperglycemia in MDSD mice through the preservation of β-cell mass and function.
Fig. 3.
GABA preserves β-cell mass and prevents diabetes in MDSD and NOD mice. (A) Immunohistochemistry of islet β-cells (green) and α-cells (red) in the MDSD mice that received daily saline solution or GABA injections. Analysis of β-cell mass (B) and α-cell mass (C) in GABA- or saline solution-treated MDSD mice (in non–STZ-injected mice, β-cell mass was 1.78 ± 0.25; α-cell mass was 0.22 ± 0.03). (D) Daily i.p. injection of GABA (20 μmol per mouse) prevented STZ-induced (40 mg/kg for 4 d) diabetic hyperglycemia in CD1 mice. (E) i.p. glucose tolerance test (IPGTT) was performed in MDSD mice treated with or without GABA, or in these mice before the STZ injections (Ctrl). (F) Immunostaining for insulin (brown) and glucagon (black) in pancreatic sections of NOD mice (13 wk of age) that received injections of saline solution or GABA; the severity of insulitis was scored on H&E-stained slides. (G) Blood glucose measurement of the NOD mice during the feeding course. (H) Islet immunohistochemistry of insulin (red) and glucagon (black) in the NOD mice at 23 wk of age treated with PBS solution or GABA. (I) IPGTT performed in the NOD mice at 23 wk of age. IPGTT was performed at 7 wk of age before the onset of diabetes as control (Ctrl). (J) Blood glucose measurement of TCR-8.3 NOD mice during the feeding course (n = 10). (K) β-Cell mass measurement of TCR-8.3 NOD mice. (L) Diabetogenic TCR-8.3 NOD CD8+ T cells were cocultured with irradiated antigen-presenting cells as described in Experimental Procedures and stimulated with a peptide mimotope. T-cell proliferation was measured by MTT assay at 72 h. (*P < 0.05 and **P < 0.01; n = 5–8.)
We next investigated the effects of GABA in nonobese diabetic (NOD) mice, a spontaneous autoimmune T1D mouse model (24). We started daily GABA injections in NOD mice before the onset of diabetes. The saline solution-injected mice, at the age of 13 wk, developed severe insulitis, β-cell depletion (∼85% β-cells were destroyed), and hyperglycemia (Fig. 3 F and G). In contrast, the GABA-treated mice showed mostly normal islets (only ∼15% islets had mild insulitis; Fig. 3F), reduced β-cell death and increased β-cell proliferation (Fig. S2 A–C), and relatively constant glucose levels (Fig. 3G). The untreated NOD mice, typically at age 18 wk and thereafter, progressed to severe diabetes and a complete depletion of islet β-cells (Fig. 3H). They required insulin injections to maintain survival. In contrast, GABA-treated mice had preserved β-cell mass (Fig. 3H), no signs of diabetes, and nearly normal glucose tolerance (Fig. 3I).
To assess whether GABA has an inhibitory effect on diabetogenic, islet cell-reactive CD8+ CTLs, we investigated its effects in T-cell receptor transgenic NOD mice (TCR-8.3 NOD). We found that GABA was highly effective at preventing hyperglycemia in these mice (Fig. 3J). It markedly suppressed insulitis (Fig. S3 A and B), preserved β-cell mass (Fig. 3K), enhanced C-peptide levels, and reduced glucagon levels (Fig. S3 C and E).
Because this disease is dependent on diabetogenic CTLs, we examined whether GABA could suppress these cells. Indeed, in vitro, we observed that GABA inhibited the proliferation of the TCR-transgenic CD8+ T cells induced by a peptide mimotope (Fig. 3L). Although this suppression was partial, it was significant.
GABA Reverses Diabetes in T1D Mouse Models.
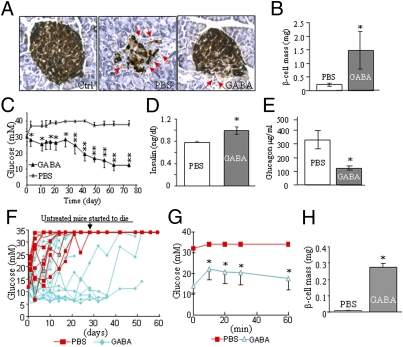
To investigate whether GABA can reverse T1D, we administered it to severely diabetic MDSD mice. Remarkably, GABA treatment reduced lymphocytic islet infiltration, restored the β-cell mass (Fig. 4 A and B), and completely reversed hyperglycemia in these mice (Fig. 4C). This was associated with increased insulin, decreased glucagon levels in the circulation (Fig. 4 D and E), and improved metabolic conditions (Fig. S4 A–D). Similarly, GABA ameliorated diabetes in established hyperglycemic NOD mice (Fig. 4 F and G), which was exemplified by a significant β-cell mass recovery (Fig. 4H) and improved metabolic conditions (Fig. S5 A–D). These data suggest that GABA has stimulatory effects on the regeneration of islet β-cells.
Fig. 4.
GABA restores β-cell mass and reverses diabetes in MDSD and NOD mice. (A) Staining of insulin (brown) and glucagon (black) in pancreatic sections of hyperglycemic MDSD mice (n = 8) receiving daily injections of GABA or saline solution (red arrows indicate infiltrating lymphocytes). (B) β-Cell mass measurement of the MDSD mice. (C) Blood glucose measurement in established diabetic MDSD mice that received daily GABA or saline solution injections during the feeding course (n = 8). Circulating insulin (D) and glucagon (E) levels of the MDSD mice measured by RIA. (F) Blood glucose measurement in the course of administration of GABA or saline solution in hyperglycemic NOD mice (the injections started when the blood glucose level was >12 mM; n = 6–10). (G) IPGTT performed in the NOD mice before the animals were killed (n = 6). (H) β-Cell mass measurement in the NOD mice (n = 5) that received chronic GABA or saline solution injections. (*P < 0.05 and **P < 0.01.)
GABA Suppresses Inflammation and Increases Regulatory T-Cell Numbers.
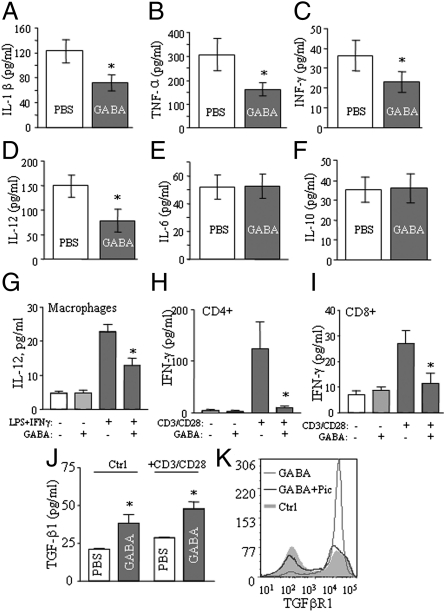
We measured serum levels of several cytokines to assess if GABA has effects on the circulating cytokine profile. Whereas the cytokines examined were present at undetectable or very low levels in the normal mice, they were remarkably elevated in the serum of STZ-treated mice, including IL-1β, TNF-α, IFN-γ, IL-12, IL-6, and IL-10. However, we found that GABA-treated MDSD mice showed significantly decreased circulating inflammatory cytokines, i.e., IL-1β, TNF-α, IFN-γ, and IL-12 (Fig. 5 A–D). Notably, the levels of the anti-inflammatory cytokine IL-10 were not suppressed (Fig. 5F). These results suggest that GABA exerts an anti-inflammatory effect and produces a more favorable cytokine profile, which may be a relevant to the reduced insulitis seen in GABA-treated MDSD (Fig. 4A) and NOD mice (Fig. 3F).
Fig. 5.
GABA exerts anti-inflammatory and immune regulatory effects in mice. Circulating levels of the inflammatory cytokines IL-1β (A), TNF-α (B), IFN-γ (C), IL-12 (D), IL-6 (E), and IL-10 (F) in the MDSD mice by using multiplex bead-based cytokine assay. IL-12 release (determined by ELISA) of LPS+IFN-γ–stimulated splenic adherent cells (macrophage and DCs), with or without GABA (G). IFN-γ release (by ELISA) of cultured splenic CD4+ (H) or CD8+ T cells (I) stimulated with anti-CD3 and anti-CD28 antibodies, with or without GABA. (J) Measurement of TGF-β1 in cultured splenic T cells stimulated with anti-CD3+anti-CD28 antibodies, or not stimulated (Ctrl), in the presence or absence of GABA. (K) Evaluation of TGFβRI expression by flow cytometry in activated CD4+ T cells in the presence or absence of GABA, with or without GABAAR antagonist picrotoxin (Pic). (*P < 0.05; **P < 0.01, n = 5–8.)
The anti-inflammatory effects of GABA were further examined in in vitro assays. As shown, GABA suppressed the production of IL-12 by macrophages, and of IFN-γ by CD4+ and CD8+ T cells (Fig. 5 G–I). Thus, GABA might produce an anti-inflammatory effect by reducing the levels of these cytokines. In view of the immunosuppressive effects of TGF-β1 (25), we also examined this cytokine. GABA increased TGF-β1 production in T-cell cultures, with or without CD3/C28 stimulation (Fig. 5J). It also increased expression of the TGF-βRI (ALK-5) receptor, and this was blocked by the GABAAR antagonist picrotoxin (Fig. 5K). Thus, GABA may also increase this anti-inflammatory cytokine.
Regulatory T cells (Tregs) exert inhibitory effects on immunological responses, and play a major role in the control of T1D (26). We conducted phenotypic analysis of the splenocytes of NOD mice (receiving GABA or saline solution injections for 60 d) by multicolor flow cytometry. Natural Tregs have the CD4+CD25+Foxp3+ phenotype. The numbers of CD4+CD25+Foxp3+ T cells were only modestly increased by GABA (Fig. S6A). Nrp1 is an additional Treg marker we examined. This revealed that GABA increases the total number of CD4+CD25+Foxp3+Nrp1+ Tregs per spleen (Fig. S6 A and B). Interestingly, GABA increased the suppressive activity of sorted CD4+CD25+ Tregs, as determined in a vitro assay of T-cell suppression (Fig. S6C).
Discussion
In the present study, we demonstrated the therapeutic effects of GABA in the prevention and reversal of diabetes in T1D mouse models. The maintenance of β-cell mass is a dynamic process, undergoing both increases and decreases through β-cell replication and apoptosis. Importantly, in diabetic mice, GABA therapy increased β-cell proliferation and decreased β-cell apoptosis, which in turn increased β-cell mass and induced the reversal of hyperglycemia in these mice. In vitro, GABA protected islets against apoptosis induced by inflammatory cytokines. Islet infiltration with immune cells, and associated local release of inflammatory cytokines, induce β-cell death in T1D (1, 2). Our data suggest that GABA exerts anti-inflammatory effects, and is directly inhibitory to T cells and macrophages. This might contribute to the reduction of insulitis and recovery of β-cell mass in diabetic mice.
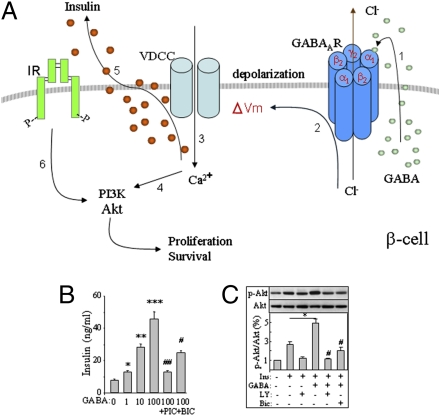
A key finding of the present study is that GABA exerts depolarizing effects and ultimately activates Akt-mediated cell growth and survival signaling pathways in β-cells. Akt plays an important role in β-cell growth and the protection from apoptosis (27, 28). In INS-1 cells and isolated mouse islets, GABA-stimulated Akt activation was abolished by the GABAAR antagonist bicuculline and/or the calcium channel blocker nifedipine. This suggests that GABA caused β-cell depolarization, followed by the opening of VDCC, and then the subsequent activation of the Ca2+/PI3K/Akt signaling pathway (29) (Fig. 6A). This may represent a molecular mechanism underlying its in vivo trophic effects exerted on the islet β-cells.
Fig. 6.
Model shows GABAAR–Ca2+–PI3K/Akt pathway in mediating GABA-induced tropic effects in β-cells (A). (1) GABA activates GABAAR Cl− channel in an autocrine fashion. (2) Cl− efflux leads to membrane depolarization. (3) Activation of VDCCs and subsequent Ca2+ influx. (4) Activation of Ca2+-dependent PI3-K/Akt signaling. (5) Cl−-dependent insulin secretion. (6) Insulin-stimulated activation of PI3K/Akt signaling. The tropic effects mediated by GABABR (47) are not displayed. GABA dose-dependently enhances insulin secretion from INS-1 cells, which is blocked by bicuculline (Bic, 20 μM) and picrotoxin (Pic, 100 μM) (B). GABA enhances insulin-simulated Akt phosphorylation in INS-1 cells, which can be blocked by Bic and LY294002 (LY, 20 μM) (C). (*P < 0.05 and **P < 0.01, n = 5.)
We and others have previously reported that GABA, through GABAAR activation, stimulated insulin secretion in the presence of low or physiological concentration of glucose (20, 21). Therefore, GABA-stimulated insulin secretion (Fig. 6B) may provide additive effects on the activation of Ca2+/PI3K/Akt pathway (Fig. 6A). In fact, the synergistic effects of insulin and GABA on the activation of Akt signaling were apparent in GABA-pretreated INS-1 cells, which showed enhanced levels of Akt phosphorylation in the presence of insulin (Fig. 6C). This may be relevant because insulin plays important autoregulatory roles in β-cell growth, survival, and function (30).
Of note, the activation of GABAAR Cl− channel induces hyperpolarizing effects to suppress α-cell secretion, whereas it exerts depolarizing effects in β-cells (18, 21). The direction of Cl− flow upon opening of GABAAR is dependent on the electrochemical driving force, determined by the resting membrane potential and the intracellular free chloride concentration. In the developing brain, GABA exerts excitatory effects by means of membrane depolarization (31); however, it produces membrane hyperpolarization in the adult brain (9). This shift is initiated by the onset of expression of K+-Cl− cotransporter-2 (KCC2) (12). To date, there is no evidence that KCC2 is localized in the islet cells; however, a functional KCC2 analogue was found in pancreatic α-cells, but not in β-cells (32). This may provide an explanation underlying for the opposite actions of GABA on the islet β- and α-cells.
A recent study by Lee et al. (33) demonstrated that glucagon receptor-KO mice are resistant to STZ-induced diabetes. They found that eliminating glucagon action abrogated the clinical and laboratory manifestations of diabetes in mice, even in the presence of severe insulin deficiency. Indeed, the appropriate ratio of insulin to glucagon is critical in maintaining glycemic stability, particularly in extremes of glucose influx or efflux (34). In accord with this concept, GABA suppresses glucagon and exerts actions beyond stimulating insulin secretion and promoting β-cell growth. Indeed, GABA cooperates with insulin to suppress glucagon secretion as we previously reported (18). This suppression of glucagon production was very likely an additional factor that contributed to remission of T1D in our GABA therapy experiments.
GABA was highly protective in three preclinical T1D models (i.e., MDSD, WT NOD, and transgenic TCR-8.3 NOD mice). These models are characterized by insulitis, although T cells play more prominent pathogenic roles in NOD mice than in MDSD (35). For example, T cell-deficient mice have been reported to have more severe MDSD (35). In contrast, numerous studies have shown that the disease of NOD mice is T cell-dependent (3). However, this does not imply that only T cells participate in the pathogenesis. Indeed, in NOD mice, T cells may interact with macrophages, B cells, or other immune cells, to ultimately produce islet cell injury (2, 3).
In all three models, GABA attenuated insulitis, preserved β-cell mass, and prevented diabetes. CD8+ CTLs appear to have a key role in the initiation of insulitis and β-cell destruction in patients with recent-onset diabetes (36). In NOD mice, CTLs can directly kill islet cells. Notably, in transgenic TCR-8.3 NOD mice, the CTLs react to an islet-cell antigen and induce severe insulitis (37). In vitro, GABA was found to directly suppress the islet-reactive TCR-8.3 transgenic CTLs. Importantly, in vivo, it prevented diabetes in these mice.
Despite the important role of effector T cells in NOD mice, there is increasing evidence that inflammatory mechanisms (usually associated with innate immunity) play a key role in pathogenesis (2). Similarly, inflammation is a prominent feature of MDSD, which contributes to β-cell death (38). In accord with this, anti-inflammatory therapies are protective in both the MDSD and NOD mice (2, 23). Here, we show that the protective effects of GABA in the STZ-treated mice were associated with decreased levels of circulating inflammatory cytokines, i.e., IL-1β, TNF-α, IFN-γ, and IL-12. In contrast, the levels of the anti-inflammatory cytokine IL-10 were not depressed.
TGF-β1 exerts potent immunosuppressive and anti-inflammatory effects (25). Interestingly, in vitro, GABA increased TGF-β1 production by T cells, whether they were unstimulated or activated by CD3/CD28 antibodies. Moreover, it increased the expression TGF-βRI, a key TGF-β signaling mediator, and this appeared to be GABA receptor dependent, as it was blocked by the GABAAR antagonist picrotoxin (Fig. 5K). This suggests that GABA might also inhibit inflammation by stimulation production of TGF-β1.
We considered that GABA might increase the numbers of Tregs or their suppressive function. Interestingly, GABA-treated mice had increased total numbers of splenic CD4+CD25+Foxp3+Nrp1+ Treg cells. There is evidence that Nrp1 increases the suppressive activity of Tregs (39, 40), although its role is still not well understood. The increased numbers of these cells might be related to TGF-β1 stimulation (25). In vitro, sorted CD4+CD25+ Tregs of GABA-treated mice were more suppressive, compared with those of PBS solution-treated mice. These findings point to alterations in Treg activity that might protect against disease in our models. However, their importance remains speculative, and further studies are required.
The effects of GABA on the immune system have not been extensively studied. In humans, GABAAR is expressed by CD4+ and CD8+ T cells, B cells, and certain monocytes (13). Tian et al. (14, 15) reported GABAAR expression by T cells, and that GABA therapy protected NOD mice against T1D, possibly as a result of suppression of Th1 cells. Similarly, Bjurstom et al. (41) reported that GABA, at as low as 100 nM, suppressed CD4+ T cells in murine experimental autoimmune encephalomyelitis. In contrast, Bhat et al. (42) reported functional GABAAR on murine macrophages but not on T cells, and that protection against experimental autoimmune encephalomyelitis was likely the result of an anti-inflammatory effect. The reason for this discrepancy in receptor expression between these studies is not clear, but our results demonstrated that GABA suppresses CD4+ T cells, CD8+ T cells, and macrophages in vitro, and exerts anti-inflammatory effects in vivo.
Remarkably, GABA also reversed established diabetes. This was most notable in STZ-induced disease, whereas disease reversal in NOD mice was less prominent. Indeed, GABA-treated NOD mice often reverted to severe hyperglycemia over time, whereas this did not occur in the MDSD model. In the latter case, there was clear evidence of β-cell regeneration and restoration of its mass. This strongly suggests that GABA acts on β-cells by protecting them from death and by promoting proliferation or restoration in MDSD mice.
Importantly, β-cell restoration was also observed in NOD mice, provided the treatment was initiated early after the onset of diabetes. At the onset of diabetes, the majority of the β-cells are destroyed in the NOD mice (Fig. 3F), but the remaining β-cells may be able to grow and allow β-cell regeneration. The effects of GABA in restoring β-cell mass were clearly evident, as the β-cell mass significantly expanded after GABA therapy. In contrast, the untreated NOD mice displayed complete absence of β-cells soon after the onset of diabetes. In view of this, we speculate that NOD mice that have been diabetic for some time have insufficient residual islet cells to permit recovery, and this question warrants further investigation.
GAD65 has long been considered a major target antigen for autoimmunity in T1D (43). GAD expression in islets has been reported in mice, rats, and humans (44). It was observed that GAD expression levels, and the predominant isoform (GAD65 or GAD67), vary among species (44). GAD67 is more highly expressed in mouse islet cells than GAD65, whereas human islets express primarily GAD65. We have used an anti-GAD antibody that recognizes both GAD65 and GAD67, i.e., we are detecting total GAD. With this method, we readily identified GAD+ islet β-cells in mice, although not all cells were positive (Fig. S7A). We speculate that the depletion of GAD positive β-cells via autoimmune mechanisms causes a decrease in intraislet GABA, reducing its actions and promoting β-cell loss. According to this hypothesis, GABA will prevent autoimmunity, which is consistent with the observation, for example, that GABA therapy in TCR-8.3 NOD mice preserved insulin/GAD double-positive β-cells (Fig. S7B). This notion is also supported by previous findings in transgenic NOD mice. Indeed, Bridgett et al. (45) showed that the islets of NOD mice contain GAD enzymatic activity, as shown by GABA production (16 pmol GABA/μg protein in 90 min). Furthermore, a strain of transgenic NOD mice expressing human GAD65 in their islets produced more GABA (130 pmol GABA/μg protein in 90 min), and had a lower incidence of diabetes. Although not a direct proof of protection by intraislet GABA, this finding is consistent with our hypothesis.
GABA was also shown to exert insulinotropic effects in humans (46). Importantly, GABA does not cross the blood brain barrier and can be administered orally in humans in large amounts (46). Hence, GABA or GABA-mimic drugs may find applications in the prevention and treatment of T1D.
Experimental Procedures
Animal Handling and Tissue Processing.
Male CD1 mice, female NOD/Lt mice, and female TCR-8.3 NOD mice (Jackson Laboratory) were housed under a light/dark cycle of 12 h with free access to food and water. All procedures were approved by the institutional animal care committee. Animal handling and drug injections are described in SI Experimental Procedures.
Immunoblotting, Immunostaining, and α- and β-Cell Mass Measurement.
Immunoblotting, tissue process, and islet cell mass analysis were performed as we described previously (27). Information on the antibodies used is provided in SI Experimental Procedures.
Lymphocytic Stimulation in Vitro Flow Cytometry Analysis.
Spleen cell isolation, in vitro culture, and flow cytometry analysis were performed as described previously (39). The antibodies used and the lymphocytic stimulation in vitro assays were performed as described in SI Experimental Procedures.
Statistical Analysis.
All data are presented as mean ± SEM. Statistical analysis was done by a Student t test or ANOVA with Tukey post-hoc test as appropriate. Significance was assumed at a P value of less than 0.05.
Supplementary Material
Acknowledgments
This work was supported by Canadian Institutes of Health Research (CIHR) grants (to Q.W., H.Z., T.J., and Z.-P.F.), a Juvenile Diabetes Research Foundation grant (to Q.W. and G.J.P.), and a Canadian Diabetes Association grant (to Q.W.). Q.W. is supported by the CIHR New Investigator Program. R.L. is supported by National Natural Science Foundation of China (NSFC)-CIHR program.
Footnotes
Conflict of interest statement: A patent application authored by N.S and Q.W. has been submitted for an invention related to this study.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102715108/-/DCSupplemental.
References
- 1.Atkinson MA, Eisenbarth GS. Type 1 diabetes: New perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 2.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 3.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 4.Pipeleers D, et al. Restoring a functional beta-cell mass in diabetes. Diabetes Obes Metab. 2008;10(suppl 4):54–62. doi: 10.1111/j.1463-1326.2008.00941.x. [DOI] [PubMed] [Google Scholar]
- 5.Meier JJ, et al. Direct evidence of attempted beta cell regeneration in an 89-year-old patient with recent-onset type 1 diabetes. Diabetologia. 2006;49:1838–1844. doi: 10.1007/s00125-006-0308-2. [DOI] [PubMed] [Google Scholar]
- 6.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Hadjiyanni I, Baggio LL, Poussier P, Drucker DJ. Exendin-4 modulates diabetes onset in nonobese diabetic mice. Endocrinology. 2008;149:1338–1349. doi: 10.1210/en.2007-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: Implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13:341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 9.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 10.Fiszman ML, Schousboe A. Role of calcium and kinases on the neurotrophic effect induced by gamma-aminobutyric acid. J Neurosci Res. 2004;76:435–441. doi: 10.1002/jnr.20062. [DOI] [PubMed] [Google Scholar]
- 11.Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig A, Li H, Saarma M, Kaila K, Rivera C. Developmental up-regulation of KCC2 in the absence of GABAergic and glutamatergic transmission. Eur J Neurosci. 2003;18:3199–3206. doi: 10.1111/j.1460-9568.2003.03069.x. [DOI] [PubMed] [Google Scholar]
- 13.Alam S, Laughton DL, Walding A, Wolstenholme AJ. Human peripheral blood mononuclear cells express GABAA receptor subunits. Mol Immunol. 2006;43:1432–1442. doi: 10.1016/j.molimm.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Tian J, et al. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol. 2004;173:5298–5304. doi: 10.4049/jimmunol.173.8.5298. [DOI] [PubMed] [Google Scholar]
- 15.Tian J, Zekzer D, Lu Y, Dang H, Kaufman DL. B cells are crucial for determinant spreading of T cell autoimmunity among beta cell antigens in diabetes-prone nonobese diabetic mice. J Immunol. 2006;176:2654–2661. doi: 10.4049/jimmunol.176.4.2654. [DOI] [PubMed] [Google Scholar]
- 16.Adeghate E, Ponery AS. GABA in the endocrine pancreas: Cellular localization and function in normal and diabetic rats. Tissue Cell. 2002;34:1–6. doi: 10.1054/tice.2002.0217. [DOI] [PubMed] [Google Scholar]
- 17.Rorsman P, et al. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature. 1989;341:233–236. doi: 10.1038/341233a0. [DOI] [PubMed] [Google Scholar]
- 18.Xu E, et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006;3:47–58. doi: 10.1016/j.cmet.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchiyama N, et al. Possible role of alpha-cell insulin resistance in exaggerated glucagon responses to arginine in type 2 diabetes. Diabetes Care. 2007;30:2583–2587. doi: 10.2337/dc07-0066. [DOI] [PubMed] [Google Scholar]
- 20.Braun M, et al. GABA is an autocrine excitatory transmitter in human pancreatic {beta}-cells. Diabetes. 2010;59:1694–1701. doi: 10.2337/db09-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong H, et al. Gamma-aminobutyric acid up- and downregulates insulin secretion from beta cells in concert with changes in glucose concentration. Diabetologia. 2006;49:697–705. doi: 10.1007/s00125-005-0123-1. [DOI] [PubMed] [Google Scholar]
- 22.Winnock F, et al. Correlation between GABA release from rat islet beta-cells and their metabolic state. Am J Physiol Endocrinol Metab. 2002;282:E937–E942. doi: 10.1152/ajpendo.00071.2001. [DOI] [PubMed] [Google Scholar]
- 23.Cockfield SM, Ramassar V, Urmson J, Halloran PF. Multiple low dose streptozotocin induces systemic MHC expression in mice by triggering T cells to release IFN-gamma. J Immunol. 1989;142:1120–1128. [PubMed] [Google Scholar]
- 24.Giarratana N, Penna G, Adorini L. Animal models of spontaneous autoimmune disease: Type 1 diabetes in the nonobese diabetic mouse. Methods Mol Biol. 2007;380:285–311. doi: 10.1007/978-1-59745-395-0_17. [DOI] [PubMed] [Google Scholar]
- 25.Prud'homme GJ. Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest. 2007;87:1077–1091. doi: 10.1038/labinvest.3700669. [DOI] [PubMed] [Google Scholar]
- 26.Tritt M, Sgouroudis E, d'Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes. 2008;57:113–123. doi: 10.2337/db06-1700. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Brubaker PL. Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia. 2002;45:1263–1273. doi: 10.1007/s00125-002-0828-3. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, et al. Glucagon-like peptide-1 regulates proliferation and apoptosis via activation of protein kinase B in pancreatic INS-1 beta cells. Diabetologia. 2004;47:478–487. doi: 10.1007/s00125-004-1327-5. [DOI] [PubMed] [Google Scholar]
- 29.Kanai T, et al. Nav1.7 sodium channel-induced Ca2+ influx decreases tau phosphorylation via glycogen synthase kinase-3beta in adrenal chromaffin cells. Neurochem Int. 2009;54:497–505. doi: 10.1016/j.neuint.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Navarro-Tableros V, Sánchez-Soto MC, García S, Hiriart M. Autocrine regulation of single pancreatic beta-cell survival. Diabetes. 2004;53:2018–2023. doi: 10.2337/diabetes.53.8.2018. [DOI] [PubMed] [Google Scholar]
- 31.Chen G, Trombley PQ, van den Pol AN. Excitatory actions of GABA in developing rat hypothalamic neurones. J Physiol. 1996;494:451–464. doi: 10.1113/jphysiol.1996.sp021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies SL, et al. Expression of K+-Cl- cotransporters in the alpha-cells of rat endocrine pancreas. Biochim Biophys Acta. 2004;1667:7–14. doi: 10.1016/j.bbamem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60:391–397. doi: 10.2337/db10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang MY, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA. 2010;107:4813–4819. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerling IC, Friedman H, Greiner DL, Shultz LD, Leiter EH. Multiple low-dose streptozocin-induced diabetes in NOD-scid/scid mice in the absence of functional lymphocytes. Diabetes. 1994;43:433–440. doi: 10.2337/diab.43.3.433. [DOI] [PubMed] [Google Scholar]
- 36.Richardson SJ, et al. Immunopathology of the human pancreas in type-I diabetes. Semin Immunopathol. 2010;33:9–21. doi: 10.1007/s00281-010-0205-0. [DOI] [PubMed] [Google Scholar]
- 37.Verdaguer J, et al. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med. 1997;186:1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolb-Bachofen V, Epstein S, Kiesel U, Kolb H. Low-dose streptozocin-induced diabetes in mice. Electron microscopy reveals single-cell insulitis before diabetes onset. Diabetes. 1988;37:21–27. doi: 10.2337/diab.37.1.21. [DOI] [PubMed] [Google Scholar]
- 39.Glinka Y, Prud'homme GJ. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J Leukoc Biol. 2008;84:302–310. doi: 10.1189/jlb.0208090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomon BD, Mueller C, Chae WJ, Alabanza LM, Bynoe MS. Neuropilin-1 attenuates autoreactivity in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2011;108:2040–2045. doi: 10.1073/pnas.1008721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjurstöm H, et al. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol. 2008;205:44–50. doi: 10.1016/j.jneuroim.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 42.Bhat R, et al. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci USA. 2010;107:2580–2585. doi: 10.1073/pnas.0915139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinke SA. Finding GAD: Early detection of beta-cell injury. Endocrinology. 2007;148:4568–4571. doi: 10.1210/en.2007-0861. [DOI] [PubMed] [Google Scholar]
- 44.Kim J, et al. Differential expression of GAD65 and GAD67 in human, rat, and mouse pancreatic islets. Diabetes. 1993;42:1799–1808. doi: 10.2337/diab.42.12.1799. [DOI] [PubMed] [Google Scholar]
- 45.Bridgett M, et al. Differential protection in two transgenic lines of NOD/Lt mice hyperexpressing the autoantigen GAD65 in pancreatic beta-cells. Diabetes. 1998;47:1848–1856. doi: 10.2337/diabetes.47.12.1848. [DOI] [PubMed] [Google Scholar]
- 46.Cavagnini F, et al. Effects of gamma aminobutyric acid (GABA) and muscimol on endocrine pancreatic function in man. Metabolism. 1982;31:73–77. [PubMed] [Google Scholar]
- 47.Ligon B, Yang J, Morin SB, Ruberti MF, Steer ML. Regulation of pancreatic islet cell survival and replication by gamma-aminobutyric acid. Diabetologia. 2007;50:764–773. doi: 10.1007/s00125-007-0601-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.