Abstract
The Aicda gene product, activation-induced cytidine deaminase (AID), initiates somatic hypermutation, class-switch recombination, and gene conversion of Ig genes by the deamination of deoxycytidine, followed by error-prone mismatch- or base-excision DNA repair. These processes are crucial for the generation of genetically diverse, high affinity antibody and robust humoral immunity, but exact significant genetic damage and promote cell death. In mice, physiologically significant AID expression was thought to be restricted to antigen-activated, mature B cells in germinal centers. We now demonstrate that low levels of AID in bone marrow immature and transitional B cells suppress the development of autoreactivity. Aicda−/− mice exhibit significantly increased serum autoantibody and reduced capacity to purge autoreactive immature and transitional B cells. In vitro, AID deficient immature/transitional B cells are significantly more resistant to anti-IgM–induced apoptosis than their normal counterparts. Thus, early AID expression plays a fundamental and unanticipated role in purging self-reactive immature and transitional B cells during their maturation in the bone marrow.
Keywords: Aicda-knockout mice, B-cell development, B-cell tolerance, competitive hematopoietic reconstitution, lymphopoiesis
B lymphocytes exhibit a unique degree of genomic plasticity; not only are B-cell antigen receptors (BCRs) generated by genomic rearrangements of Ig V, D, and J gene segments, but assembled BCR genes are also modified by class-switch recombination (CSR), somatic hypermutation (SHM), and, in birds, gene conversion (1–3). Whereas V(D)J rearrangement occurs during B lymphopoiesis, SHM and CSR are largely confined to secondary lymphoid tissues, in which activated germinal center (GC) B cells express high levels of activation-induced cytidine deaminase (AID) (2). AID initiates Ig SHM, CSR, and gene conversion by the deamination of deoxycytidine, followed by error-prone mismatch- or base-excision DNA repair (3).
Ig CSR and SHM are crucial to the generation of effective humoral immunity to pathogens. In GC, AID elicits SHM, which drives a process of rapid Darwinian selection for B cells that bind their antigen ligands with increasingly high affinity. CSR permits the generation of distinct classes of mutated, high-affinity antibodies that possess different physiologic qualities and functions, including escape/transport from the blood vasculature, transport across epithelial barriers, and the capacity to arm cellular effector cells.
Exceptionally, mammalian AID expression, SHM, and CSR can also be found in activated B cells outside GC, e.g., in extrafollicular, autoimmune plasmablasts (4) and gut B cells (5). Patients with type I hyper-IgM (HIGM1) syndrome cannot form GC, but nonetheless support B-cell populations with mutated Ig genes (6). In addition, low levels of AID message, CSR, and SHM have been observed in mouse and human immature and transitional 1 (T1) B cells (7–10), and evidence for AID expression and activity in human fetal liver (10–12) suggests that programmed AID expression during B-cell development may be a common vertebrate trait (1, 13, 14). However, the significance of AID expression in developing mouse B cells is controversial (7–10, 15).
Here we demonstrate that Aicda−/− stem cells exhibit a significant advantage over normal hematopoietic competitors in the production of B lymphocytes, but not other leukocytic lineages. This lymphopoietic advantage is associated with substantial impairment of central B-cell tolerance; AID-deficient C57BL/6 (B6) mice express high levels of serum autoantibody (autoAb), and the arrested development of autoimmune, 3H9 VDJ knock-in B cells (16) is substantially restored in Aicda−/− animals. This rescue of B-cell development is accompanied by increased retention of 3H9 VDJ alleles and high levels of the DNA autoAb specified by the 3H9 VDJ (16). Significantly, AID-deficient immature and T1 B cells also exhibit increased resistance to anti-IgM–induced apoptosis in vitro. We conclude that AID expression in developing B cells is a significant component of central B-cell tolerance.
Results
Restricted Aicda Expression During Leukopoiesis.
Developmental AID expression is first detected in mouse pro-B cells and increases on further development; peak AID expression is in immature and T1 B cells from bone marrow (BM), is lower in splenic T1 B cells, and decreases precipitously in the splenic transitional 2 (T2) and mature B-cell compartments (Fig. S1) (8, 9). In contrast, AID cDNA is undetectable in totipotent hematopoietic progenitors, and in cells committed to the T lymphocyte or myeloid lineages (Fig. S1).
Even at its zenith in immature and T1 B cells, this early AID expression is only approximately 3% of that in GC B cells (Fig. S1) (9). Although this low level of AID is associated with occasional SHM and CSR (7–10), there is no evidence that this early AID expression is consequential.
Aicda Impairs B-Cell Development.
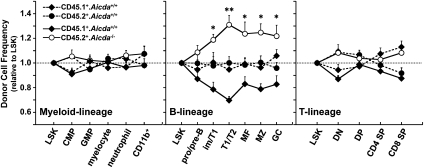
Competitive hematopoietic reconstitution (17) can determine the developmental fitness of stem cells and their progeny. To detect any effects of Aicda on B lymphopoiesis, we injected equal numbers of BM cells from B6.SJL (CD45.1+) and B6 (CD45.2+) or B6.SJL and B6.Aicda−/− (CD45.2+) into sublethally irradiated (B6.SJL × B6)F1 (CD45.1+/CD45.2+) mice. Six weeks after hematopoietic reconstitution, the frequencies of donor and host cells in the myeloid and T- and B-lymphoid compartments (Fig. S2) were determined by flow cytometry (Fig. 1). In mice reconstituted with CD45.1+Aicda+/+ and CD45.2+Aicda+/+ marrow, stem cell [Lin−Sca+Kit+; LSK (18)] chimerism was equivalent (CD45.1+, 47 ± 5.2%; CD45.2+, 51 ± 6.1%), and their progeny equally reconstituted all leukocyte lineages (Fig. 1). In mice reconstituted with CD45.1+Aicda+/+ and CD45.2+Aicda−/− BM, LSK chimerism was also identical (CD45.1, 50 ± 11%; CD45.2, 51 ± 13%), but Aicda−/− progenitors were significantly overrepresented in the BM immature/T1 (P = 0.026), splenic T1/T2 (P = 0.006), and mature (P = 0.045) B-cell compartments (Fig. 1). Indeed, approximately 60% to 70% of the T1/T2 and mature B-cell compartments were derived from Aicda−/− progenitors (Fig. 1). In contrast, CD45.2+Aicda−/− progenitors exhibited no significant advantage in the production of T lymphocytes or myeloid cells (Fig. 1).
Fig. 1.
Developmental effects of Aicda on B-lineage cells. The consequences of Aicda on the generation and maturation of specific hematopoietic cell lineages was determined by competitive hematopoietic reconstitution. Chimeric mice containing approximately 1:1 ratios of competitor stem cell [LSK (18); Fig. S2] populations were generated, and the frequencies of CD45.1+Aicda+/+ (filled diamonds, solid line) and CD45.2+Aicda−/− (open circles, solid line) cells in each hematopoietic compartment (Fig. S2), normalized to the frequency of Aicda+/+ or Aicda−/− LSK cells, are shown. Control reconstitutions with CD45.1+ (diamonds) and CD45.2+ (circles) Aicda+/+ BM cells (broken lines) demonstrate no hematopoietic advantage for either CD45 marker allele. Data are shown as mean ± SEM (n = 7–9).
AutoAb in Aicda−/− Mice.
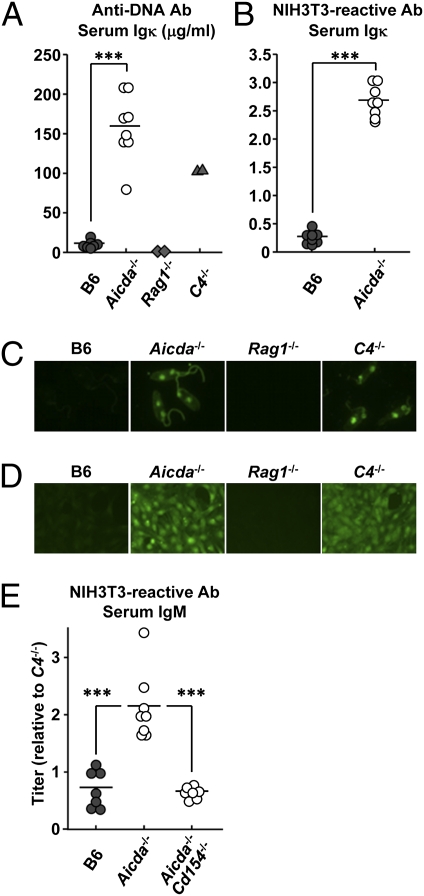
AID deficiency in aging mice (19, 20) and humans (21) has been linked to organ-specific autoAb and autoimmune disease. To determine whether autoAb levels are increased in young Aicda−/− mice, we quantified their serum Ab bearing κ-light chains (Igκ) reactive with DNA or NIH 3T3 cells; serum Igκ Ab titers in congenic B6, autoimmune C4−/− (22), and Ab deficient Rag1−/− mice were also determined as controls (Fig. 2 A and B). By ELISA, serum Igκ titers for DNA or NIH 3T3 cell antigens in 2- to 2.5-mo-old Aicda−/− mice were more than 10-fold higher than age-matched B6 controls, surpassing even that of 5- to 6-mo-old C4−/− mice (Fig. 2 A and B) (22). In immunofluorescence assays, these sera gave similar results (Fig. 2 C and D); whereas serum Igκ from B6 mice was unreactive, serum Igκ from Aicda−/− and C4−/− mice intensely labeled Crithidia luciliae nuclei/kinetoplasts (Fig. 2C) and NIH 3T3 cell nuclear and cytoplasmic antigens (Fig. 2D).
Fig. 2.
Increased serum autoAb in Aicda−/− mice. DNA and cell-reactive autoAb levels in the sera of Aicda−/− mice were compared with congenic B6, autoimmune C4−/− (22), and Ab-deficient Rag1−/− animals. Anti-dsDNA Igκ Ab (A) and self-reactive Igκ Ab (B) (reactive to NIH 3T3 cells) in sera of B6 (●), Aicda−/− (○), C4−/− (▲), and Rag1−/− (◆) mice were determined by ELISA. (C) The presence of anti-DNA autoAb (Igκ) in diluted sera (1:800) of B6 and Aicda−/− mice was compared in a C. luciliae immunofluorescence assay. Representative micrographs are shown; original magnification of ×400. (D) The presence of self-reactive autoAb (Igκ) in diluted sera (1:400) of B6 and Aicda−/− mice was screened on NIH 3T3 cells. Representative photomicrographs are shown; original magnification of ×200. (E) Self-reactive IgM Ab (reactive to NIH 3T3 cells) in sera of B6 (●), Aicda−/− (○), and Aicda−/−Cd154−/− (○) mice were determined by ELISA. Before the assay, serum IgM in each sample was determined and adjusted to 400 μg IgM/mL. In B and E, relative values (normalized to C4−/− serum) are shown. In A, B, and E, points represents individual mice.
The sera of Aicda−/− mice contain only IgM, and it was possible that elevated Igκ autoAb in these mice might represent unspecific binding by highly avid, decavalent IgM. To eliminate this possibility, we determined total serum IgM levels in B6 and congenic Aicda−/− mice, and then diluted each serum to a standard IgM concentration (400 μg/mL). Subsequently, we compared the titers of IgM autoAb in each standardized serum sample in an NIH 3T3 cell ELISA (Fig. 2E). Even when matched in this way, serum IgM from Aicda−/− mice contained approximately three times more autoAb than did B6 controls (Fig. 2E).
Serum AutoAb in Aicda−/− Mice Requires CD154.
In several autoimmune mouse strains, development of autoAb and systemic autoimmunity is correlated with the spontaneous development of GC dependent on CD40–CD154 interaction (23). To determine whether the IgM autoAb of Aicda−/− mice might also be CD154-dependent, we generated doubly deficient Aicda−/−Cd154−/− mice and measured levels of NIH 3T3 cell-reactive IgM Ab in their sera in comparison with congenic Aicda−/− and B6 controls (Fig. 2E). Remarkably, in the absence of CD154, serum IgM autoAb reactive with NIH 3T3 cells decreased in Aicda−/−Cd154−/− mice to the levels present in B6 controls (Fig. 2E). The development of serum IgM autoAb in Aicda−/− mice is T cell-dependent.
Unsurprisingly, the large, spontaneous GCs that are hallmarks of Aicda−/− mice were also lost in Aicda−/−Cd154−/− mice (Fig. S3).
Impaired Central B-Cell Tolerance in Aicda−/− Mice.
Increased serum autoAb in Aicda−/− mice reflects impaired central and/or peripheral tolerance. To determine whether Aicda−/− mice might exhibit defects in peripheral tolerance, we quantified serum BAFF levels and the frequency of CD4+FoxP3+ regulatory T cells in B6 and Aicda−/− mice. In mice and humans, elevated BAFF is associated with impaired peripheral B-cell tolerance (24–26) and regulatory T cells are associated with maintaining B-cell tolerance (27). However, in Aicda−/− mice, serum BAFF concentrations were significantly lower than in B6 controls (39%; P = 0.013; Fig. S4) and the numbers/frequencies of splenic CD4+Foxp3+ T cells in Aicda−/− and B6 mice were similar (P = 0.18; Fig. S4).
To determine whether central tolerance is impaired in Aicda−/− mice, we generated Aicda−/− and Aicda+/+ mice homozygous for the 3H9 VDJ knock-in allele (16). The 3H9 VDJ rearrangement confers self-reactivity in association with approximately 60% of κ-light chains and is strongly linked to dsDNA autoAb (28). In 3H9 knock-in mice, this autoreactivity results in the tolerizing deletion of 30% to 50% of immature B cells (29), VH replacement rearrangements that “edit” the autoreactive 3H9 allele (16), and peripheral B-cell populations expressing light chains that minimize dsDNA reactivity (30). If AID plays a significant role in mediating central B-cell tolerance, the characteristic losses of immature B cells, VH editing, and the suppression of dsDNA autoAb should be mitigated in 3H9.Aicda−/− mice.
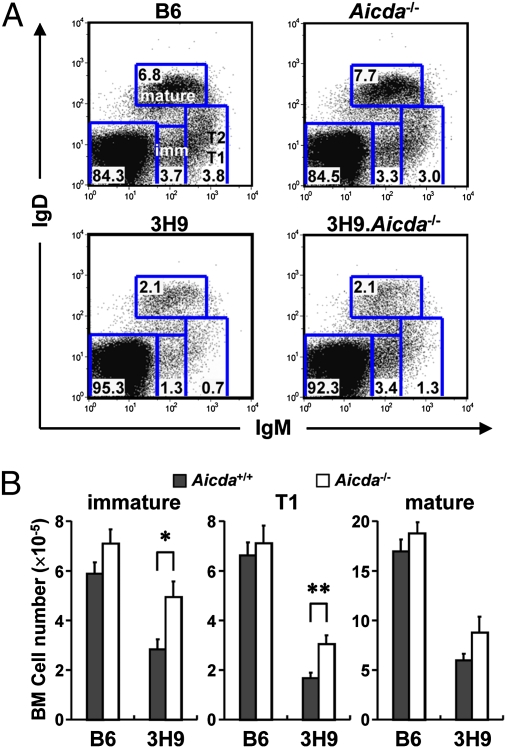
Flow cytometric analysis of BM cells from B6, Aicda−/−, 3H9, and 3H9.Aicda−/− mice illustrate the opposing effects of the 3H9 VDJ knock-in and Aicda on B-cell development (Fig. 3A). Whereas the BM B-cell compartments of Aicda−/− mice are comparable to those of B6 controls, the numbers of immature and T1 B cells in 3H9 mice are reduced significantly (both P < 0.001; Fig. 3 and Fig. S5). In contrast, immature and T1 B-cell numbers in 3H9.Aicda−/− mice are nearly double (P = 0.013 and P = 0.005, respectively) those in congenic 3H9 animals (Fig. 3). The substantial rescue of BM T1 B cells in 3H9.Aicda−/− mice persists as T1 B cells enter the blood and mature to T2 cells in the spleen (Fig. S5).
Fig. 3.
Effect of Aicda on the development of autoreactive B cells. B-cell development in BM of B6, Aicda−/−, 3H9, and 3H9.Aicda−/− mice was compared. (A) Representative flow data for IgM/IgD expression by BM cells of B6, Aicda−/−, 3H9, and 3H9.Aicda−/− mice are shown. Specific developmental compartments [immature (imm), T1 and T2, and mature B (38, 43)] were designated for B6; identical gatings were used for all samples. Numbers within squares represent frequency of cells in those squares. (B) Absolute cell number of the indicated B-cell compartments in of B6 (Aicda+/+, filled bars; Aicda−/− open bars) and 3H9 (Aicda+/+, filled bars; Aicda−/− open bars) mice are shown. Histograms represent mean values ± SEM (n = 7–10).
BCR Specificity and Aicda-Dependent Tolerance.
Basal AID expression in immature/T1 B cells appears to be independent of antigen specificity (9), but the consequence of Aicda activity in these cells could be determined by BCR signaling. If so, surviving immature/T1 B cells in 3H9 mice should be selected for reduced AID expression, lower self-reactivity, or both.
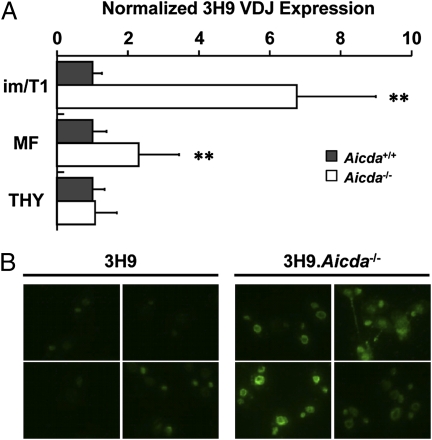
To test this prediction, we quantified AID message (Fig. S1) in live pro-/pre-B and immature/T1 B cells from B6 and 3H9 knock-in mice. Whereas pro-B and pre-B cells from both strains expressed comparable levels of AID transcript (31 vs. 22 cDNA copies/103 cells), in immature/T1 B cells, AID message levels in 3H9 mice were less than 50% of B6 controls (319 vs. 691 cDNA copies/103 cells). Reciprocally, in Aicda−/− mice, selection against autoreactive immature and T1 B cells should be minimized. In 3H9 mice, secondary rearrangements that replace the 3H9 VH gene segment and reduce self-reactivity are common (16). To determine whether this editing process is reduced in the absence of AID selection, we isolated genomic DNA from BM immature/T1 B cells, mature splenic B cells, and thymocytes of 3H9 and 3H9.Aicda−/− mice and quantified the numbers of 3H9 VDJ alleles in each population in reference to the endogenous Cd14 locus (Fig. 4A). As expected, the normalized abundance of 3H9 VDJ gene templates was equivalent (P = 0.801) in thymocytes from 3H9 and 3H9.Aicda−/− mice (Fig. 4A). However, 3H9 VDJ alleles in immature/T1 and mature B cells from 3H9 mice were significantly (P = 0.002 and 0.006, respectively) lower than in 3H9.Aicda−/− mice (Fig. 4A). Indeed, in the restored immature/T1 compartment of 3H9.Aicda−/− mice, 3H9 VDJ templates were sixfold more abundant than in AID-sufficient, 3H9 knock-in animals (Fig. 4A).
Fig. 4.
Effects of Aicda on autoAb production in 3H9 mice. (A) Expression of 3H9 VDJ knock-in alleles (relative to Cd14) in genomic DNA of immature/T1 (im/T1), mature follicular (MF) B cells, and thymocytes (THY) from 3H9 (filled bars) and 3H9.Aicda−/− (open bars) mice were determined by quantitative PCR. The ratio of 3H9/Cd14 in the 3H9 cell compartments was normalized (1.0) for comparison with the analogous ratios for 3H9.Aicda−/− mice. Histograms represent the mean ± SD of individual mice (n = 4–9) in three experiments. (B) The presence of ds DNA autoAb (Igκ) in 3H9.Aicda−/− and 3H9 mice was compared in C. luciliae immunofluorescence assays. Reactivity in diluted sera (1:800) from four 3H9 and four 3H9.Aicda−/− mice were compared individually (42).
3H9 mice produce little dsDNA serum Ab because B cells expressing dsDNA BCR are efficiently tolerized (16). If the effects of AID deficiency extend generally to B-cell tolerance, 3H9.Aicda−/− mice should have high levels of serum Igκ dsDNA Ab. Whereas sera from 3H9 mice reacted weakly or not at all with dsDNA present in C. luciliae, all sera from 3H9.Aicda−/− mice were dsDNA-reactive, and half reacted intensely (Fig. 4B).
VH Mutation and Receptor-Driven Apoptosis in Immature/T1 B Cells.
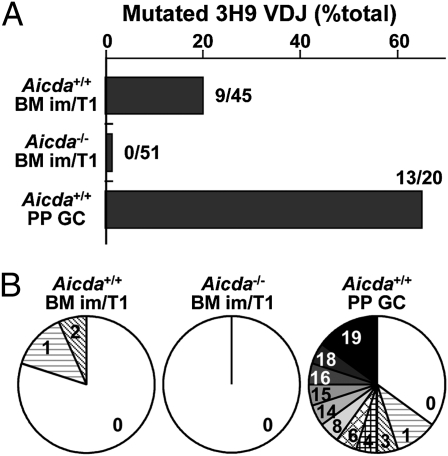
AID activity elicits DNA damage (3). To estimate the genomic effects of AID activity in immature/T1 B cells homozygous for the 3H9 knock-in allele, we sequenced genomic 3H9 VDJ rearrangements from immature/T1 B cells of 3H9 and 3H9.Aicda−/− mice, and from Peyer patch (PP) GC B cells from 3H9 mice. As expected, approximately half (65%; 13 of 20) of 3H9 VDJ rearrangements from 3H9+/+ PP GC B cells were mutated, carrying one to 19 unique point mutations (total, n = 47; Fig. 5). A majority of the mutated 3H9 alleles (nine of 13) shared mutations and could be organized into clonal lineages representing ongoing SHM and proliferation (Fig. S6). In contrast to the absence of mutations in immature/T1 B cells from 3H9.Aicda−/− mice (zero of 51), 20% (nine of 45) of 3H9 VDJ alleles from AID-sufficient congenics carried one or two unique point mutations (total, n = 12; Fig. 5). In all cases, 3H9 mutations were consistent with AID-mediated SHM, and a majority resulted in amino acid replacement (Table S1).
Fig. 5.
SHM in 3H9+/+ immature/T1 B cells. Frequencies of mutated 3H9 VDJ rearrangements (A), and distributions of the numbers of point mutations in each sequence (B) from genomic DNA of BM immature/T1 B cells from 3H9 (n = 45), 3H9.Aicda−/− (n = 51), or PP GC B cells from 3H9 mice (n = 20) are shown.
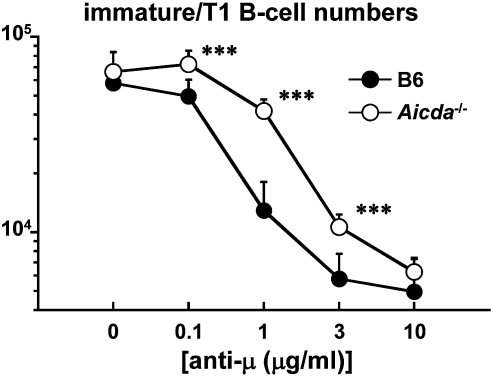
To test directly the role of AID in central B-cell tolerance, we compared susceptibility of AID-sufficient and -deficient immature/T1 B cells to anti-IgM–induced apoptosis. BM cells from WT and Aicda−/− mice were cultured for 24 h with increasing concentrations of anti-IgM Ab (31). Frequency and number of Aicda−/− immature/T1 B cells were comparable to those of congenic controls in the absence of anti-IgM Ab (Fig. 6 and Fig. S7). In contrast, the number of Aicda−/− immature/T1 B cells surpassed that of WT controls after exposure to anti-IgM Ab (Fig. 6). Indeed, viable Aicda−/− immature/T1 B cells were approximately three times more prevalent than Aicda+/+ immature/T1 B cells after stimulation with 1 μg/mL of anti-IgM Ab (Aicda+/+ vs. Aicda−/−, 1.29 × 104 vs. 4.17 × 104; Fig. 6). AID− immature/T1 B cells are relatively resistant to BCR-induced apoptosis.
Fig. 6.
Aicda−/− immature/T1 B cells are resistant to BCR-induced apoptosis. The susceptibility of immature/T1 B cells from Aicda+/+ and Aicda−/− BM to anti-IgM Ab-induced apoptosis was compared in cell culture (31). BM cells from Aicda+/+ and Aicda−/− mice were cultured in triplicate in media containing various concentrations of anti-IgM (anti-μ) Ab for 24 h. The numbers of Aicda+/+ (filled symbols) and Aicda−/− (open symbols) immature/T1 B cells in each treatment are shown. Data shown represent the means ± SD from three independent experiments.
Discussion
Aicda−/− hematopoietic progenitors exhibit increased fitness for the generation of B lymphocytes but not other leukocyte lineages (Fig. 1). In part, this lymphopoietic advantage is the consequence of an unanticipated role for Aicda in central B-cell tolerance: AID activity and BCR signaling converge in immature/T1 B cells to purge self-reactivity (Figs. 2–6).
The significance of AID is apparent in the enhanced transition of Aicda−/− pro-/pre-B to immature/T1 B cells (Fig. 1), the developmental compartments in which early AID expression is maximal (Fig. S1). Coincidently, Aicda−/− immature/T1 B cells are significantly more resistant to tolerization in vivo (Figs. 3 and 4) and BCR-induced apoptosis in vitro (Fig. 6). We conclude that relaxed central tolerance in Aicda−/− mice accounts for most, and perhaps all, of the B-lymphopoietic advantage of Aicda−/− progenitor cells (Fig. 1).
Whereas the increased representation of Aicda−/− immature/T1 B cells continues in mature B-cell compartments after reconstitution with LSK cells permissive for the rearrangement of endogenous Ig loci (Fig. 1), in 3H9.Aicda−/− mice, this selective advantage is reduced or lost after the immature/T1 tolerance checkpoint (Figs. 3 and 4). We conclude that AID-independent tolerance mechanisms vet more mature B cells (32), but that both AID-dependent and -independent tolerance is necessary for optimal control of B-cell autoreactivity.
Indeed, elevated dsDNA autoAb in 3H9.Aicda−/− mice indicates that 3H9 B cells normally lost to AID-dependent tolerance can mature and differentiate despite later, AID-independent tolerance mechanisms (Fig. 4). Serum autoAb (Igκ/IgM) was significantly elevated in Aicda−/− mice, even when IgM concentrations were normalized (Fig. 2). This normalization ensured that our determinations of IgM autoAb levels were not artifacts of unspecific binding.
Like other autoimmune mice (23), the serum autoAb of Aicda−/− mice requires CD154 expression (Fig. 2); spontaneous GC responses were also abrogated in Aicda−/−Cd154−/− mice (Fig. S3), lending support to the notion that self-reactivity is a significant factor in the follicular hypertrophy of AID-deficient mice and humans (19).
To explain the presence of autoAb in young Aicda−/− mice, we made an incomplete search for impairments of B-cell tolerance (Fig. S4). Elevated BAFF is seen in patients with systemic autoimmune disease (26); mice that overexpress BAFF become autoimmune (24) and retain autoreactive T2 B cells (25). However, serum BAFF levels are reduced in Aicda−/− mice (Fig. S4), perhaps because of the increased B cell numbers (33), and we observed no obvious dysregulation of regulatory T cells (Fig. S4). Regardless of mechanism, increased BAFF expression cannot account for elevated serum autoAb of Aicda−/− mice (Fig. 2).
In contrast, impaired central tolerance in Aicda−/− mice could be easily demonstrated by comparing B-cell development in Aicda+/+ and 3H9.Aicda−/− VDJ knock-in mice (Fig. 3 and 4). Normally, autoreactive, immature B cells in 3H9 mice are efficiently suppressed by clonal deletion (29) and receptor editing (16). 3H9.Aicda−/− mice exhibited relaxed central B-cell tolerance as measured by their incomplete deletion of immature/T1 B cells (Fig. 3) and significantly reduced levels of VH editing and elevated dsDNA autoAb (Fig. 4).
We can imagine several nonexclusive mechanisms whereby Aicda might exert its tolerizing effects. For example, Ig SHM (Fig. 5 and Table S1) (7, 8, 10) might abrogate autoreactivity and/or DNA demethylation by AID (34) might slow B-cell development to enhance receptor editing (Fig. 4) indirectly or directly (35). A simple mechanism consistent with our data are that AID removes self-reactive immature/T1 B cells by generating low levels of genomic damage (Fig. 5 and Table S1) that are nonetheless sufficient to elicit apoptosis in immature/T1 B cells activated by self-ligands. Consistent with this idea, Aicda+/+ immature/T1 B cells are more sensitive to BCR-driven apoptosis in vitro than are Aicda−/− immature/T1 B cells (Fig. 6).
The frequency (20%) of mutated 3H9 VDJ rearrangements recovered from 3H9+/+ immature/T1 B cells (Fig. 5) suggests that as many as 40% of actively transcribed 3H9 alleles (3) in immature and T1 B cells carry one or two unique point mutations (Fig. 5 and Table S1). This mutation frequency is much lower (at least ninefold) than that in PP GC B cells (Table S1); we note, however, that, unlike GC B cells (36, 37), immature/T1 B cells are only briefly exposed to AID activity and do not divide (38, 39). Although the VH mutation frequency is extraordinary in 3H9+/+ GC B cells, these cells (and their progeny) accumulate mutations over relatively long time periods—certainly weeks—punctuated by rounds of mutation, selection, and proliferation (37, 40). Consistent with this, we identified two clonal genealogies among the mutated 3H9 genes from PP GC B cells (Fig. S6). In contrast, B cells occupy the immature and T1 compartments much more briefly, perhaps for no more than 4 d (39). This brief exposure to AID activity nonetheless results in a subset of mutated cells that acquire one or two point mutations (Fig. 5). Perhaps surprisingly, during primary responses to proteins conjugated with the (nitrophenyl)acetyl hapten, GC B cells acquire one or two point mutations in expressed VH rearrangements every 2 to 4 d [to day 16 after immunization (37)]. It may be that VH mutation rates in subsets of immature and T1 B cells—autoreactive?—are little different from that in virtually all GC B cells.
Others have shown that AID deficiency can enhance autoimmunity in B6.lpr mice (20) and promote organ-specific responses in germ-free BALB/c mice (19); in both cases, the onset of autoAb and disease became obvious in 6- to 12-mo-old animals. Here, we have demonstrated that AID deficiency leads to impaired central B-cell tolerance in young animals (Figs. 3–6) that results in the production of IgM autoAb in nonautoimmune-prone B6 mice (Figs. 2 and 4B). Significantly, a comparable role for the orthologous AICDA gene in human B-cell tolerance has been discovered by Meyers and colleagues (41).
Despite the clear effects of Aicda on central B-cell tolerance, AID expression by immature/T1 B cells is only 3% of that in GC B cells. Although similarly low levels of AID are critical to the maintenance of totipotency in mouse embryonic stem cells and human fibroblasts (34), it will be crucial to determine how low levels of AID expression purge autoreactivity at the immature/T1 tolerance checkpoint. Only then can we understand how this ancient and protean gene of adaptive immunity (3) acts not only to increase immunological diversity but also to reduce it.
Materials and Methods
Mice.
Mice used in this study are described in SI Materials and Methods.
Quantification of AID Expression.
Expression of AID mRNA was determined by a quantitative RT-PCR (9) referenced to a synthetic standard as described in SI Materials and Methods.
Flow Cytometry and Competitive Hematopoietic Reconstitution.
(B6.SJL × B6)F1 mice (CD45.1+/CD45.2+) were sublethally irradiated (6.5 Gy) and reconstituted with equal numbers of nonadherent BM cells (5 × 106 each) from B6.SJL (CD45.1+) and B6 (CD45.2+) or Aicda−/− (CD45.2+) mice. Six weeks after reconstitution, chimeric mice were killed and frequencies of CD45.1+CD45.2−, CD45.1−CD45.2+ (donor), and CD45.1+CD45.2+ (host) cells in the myeloid and lymphoid compartments of BM, thymus, spleen, and PP were determined by standard flow cytometric criteria (Fig. S2). Detailed methods are included in SI Materials and Methods.
Quantification of AutoAb.
Anti-dsDNA Igκ Ab and self-reactive Igκ/IgM Ab in sera from B6, Aicda−/−, C4−/−, and Rag1−/− mice were measured by ELISA. The presence of anti- DNA/nuclear Igκ Ab and self-reactive Igκ Ab in serially diluted sera from B6, Aicda−/−, 3H9, and 3H9.Aicda−/− mice were screened on slides coated with C. luciliae and NIH 3T3 cells as described (42). Detailed methods are included in SI Materials and Methods.
Amplification of 3H9 VDJ Rearrangements.
The retention of 3H9 VDJ rearrangements in genomic DNA from B-cell subsets were determined by quantitative PCR. The mutation frequency in 3H9 VDJ rearrangements amplified from genomic DNA of 3H9 and 3H9.Aicda−/− BM immature/T1 B cells was compared by sequencing. Detailed methods are included in SI Materials and Methods.
Cell Culture.
The sensitivity of immature/T1 B cells from B6 and Aicda−/− BM was determined in vitro (31). BM cells (1 × 106) from B6 and Aicda−/− mice were cultured in triplicate in media containing various concentrations (0, 0.1, 1, 3, 10 μg/mL) of F(ab)′2 anti-IgM (anti-μ) IgG (Jackson Immunoresearch). After 24 h, live cells were counted and labeled with fluorochrome-tagged mAbs, and frequencies/numbers of immature/T1 B cells (B220+CD93+IgD+/−Igκ+ or -Igλ+; Fig. S7) were determined by flow cytometry.
Statistical Analyses of Data.
Statistical significance (at levels of P ≤ 0.05; P ≤ 0.01; or P ≤ 0.001) in paired data was determined by two-tailed Student t test.
Supplementary Material
Acknowledgments
We thank Drs. A. Watanabe, X. Liang, P. Snowden, and Y. Li for expert assistance, and Drs. S. Unniraman and L. McWilliams for advice. This work was supported by National Institutes of Health Grants AI24335 and AI56363 (to G.K.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102571108/-/DCSupplemental.
References
- 1.Reynaud CA, Bertocci B, Dahan A, Weill JC. Formation of the chicken B-cell repertoire: ontogenesis, regulation of Ig gene rearrangement, and diversification by gene conversion. Adv Immunol. 1994;57:353–378. doi: 10.1016/s0065-2776(08)60676-8. [DOI] [PubMed] [Google Scholar]
- 2.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 3.Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 4.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 5.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weller S, et al. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci USA. 2001;98:1166–1170. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao C, et al. T cell-independent somatic hypermutation in murine B cells with an immature phenotype. Immunity. 2004;20:133–144. doi: 10.1016/s1074-7613(04)00019-6. [DOI] [PubMed] [Google Scholar]
- 8.Han JH, et al. Class switch recombination and somatic hypermutation in early mouse B cells are mediated by B cell and Toll-like receptors. Immunity. 2007;27:64–75. doi: 10.1016/j.immuni.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueda Y, Liao D, Yang K, Patel A, Kelsoe G. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J Immunol. 2007;178:3593–3601. doi: 10.4049/jimmunol.178.6.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuraoka M, et al. Activation-induced cytidine deaminase expression and activity in the absence of germinal centers: Insights into hyper-IgM syndrome. J Immunol. 2009;183:3237–3248. doi: 10.4049/jimmunol.0901548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milili M, Fougereau M, Guglielmi P, Schiff C. Early occurrence of immunoglobulin isotype switching in human fetal liver. Mol Immunol. 1991;28:753–761. doi: 10.1016/0161-5890(91)90118-4. [DOI] [PubMed] [Google Scholar]
- 12.Scheeren FA, et al. T cell-independent development and induction of somatic hypermutation in human IgM+ IgD+ CD27+ B cells. J Exp Med. 2008;205:2033–2042. doi: 10.1084/jem.20070447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynaud CA, Garcia C, Hein WR, Weill JC. Hypermutation generating the sheep immunoglobulin repertoire is an antigen-independent process. Cell. 1995;80:115–125. doi: 10.1016/0092-8674(95)90456-5. [DOI] [PubMed] [Google Scholar]
- 14.Mage RG, Lanning D, Knight KL. B cell and antibody repertoire development in rabbits: the requirement of gut-associated lymphoid tissues. Dev Comp Immunol. 2006;30:137–153. doi: 10.1016/j.dci.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Crouch EE, et al. Regulation of AID expression in the immune response. J Exp Med. 2007;204:1145–1156. doi: 10.1084/jem.20061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Nagy Z, Prak EL, Weigert M. Immunoglobulin heavy chain gene replacement: a mechanism of receptor editing. Immunity. 1995;3:747–755. doi: 10.1016/1074-7613(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 17.Harrison DE. Competitive repopulation: a new assay for long-term stem cell functional capacity. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- 18.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci USA. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hase K, et al. Activation-induced cytidine deaminase deficiency causes organ-specific autoimmune disease. PLoS ONE. 2008;3:e3033. doi: 10.1371/journal.pone.0003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Guo L, Tian J, Zheng B, Han S. Deficiency in activation-induced cytidine deaminase promotes systemic autoimmunity in lpr mice on a C57BL/6 background. Clin Exp Immunol. 2010;159:169–175. doi: 10.1111/j.1365-2249.2009.04058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durandy A, Revy P, Imai K, Fischer A. Hyper-immunoglobulin M syndromes caused by intrinsic B-lymphocyte defects. Immunol Rev. 2005;203:67–79. doi: 10.1111/j.0105-2896.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Koralov SB, Kelsoe G. Complement C4 inhibits systemic autoimmunity through a mechanism independent of complement receptors CR1 and CR2. J Exp Med. 2000;192:1339–1352. doi: 10.1084/jem.192.9.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luzina IG, et al. Spontaneous formation of germinal centers in autoimmune mice. J Leukoc Biol. 2001;70:578–584. [PubMed] [Google Scholar]
- 24.Mackay F, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thien M, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Mackay F, Mackay CR. The role of BAFF in B-cell maturation, T-cell activation and autoimmunity. Trends Immunol. 2002;23:113–115. doi: 10.1016/s1471-4906(01)02159-7. [DOI] [PubMed] [Google Scholar]
- 27.Hervé M, et al. CD40 ligand and MHC class II expression are essential for human peripheral B cell tolerance. J Exp Med. 2007;204:1583–1593. doi: 10.1084/jem.20062287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim SM, Weigert M, Basu C, Erikson J, Radic MZ. Light chain contribution to specificity in anti-DNA antibodies. J Immunol. 1995;155:3223–3233. [PubMed] [Google Scholar]
- 29.Chen C, et al. The site and stage of anti-DNA B-cell deletion. Nature. 1995;373:252–255. doi: 10.1038/373252a0. [DOI] [PubMed] [Google Scholar]
- 30.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: An approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hertz M, Nemazee D. BCR ligation induces receptor editing in IgM+IgD- bone marrow B cells in vitro. Immunity. 1997;6:429–436. doi: 10.1016/s1074-7613(00)80286-1. [DOI] [PubMed] [Google Scholar]
- 32.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20:632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Fagarasan S, et al. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 34.Bhutani N, et al. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature. 2010;463:1042–1047. doi: 10.1038/nature08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai AG, et al. Human chromosomal translocations at CpG sites and a theoretical basis for their lineage and stage specificity. Cell. 2008;135:1130–1142. doi: 10.1016/j.cell.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 37.Jacob J, Przylepa J, Miller C, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J Exp Med. 1993;178:1293–1307. doi: 10.1084/jem.178.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allman D, et al. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 39.Lindsley RC, Thomas M, Srivastava B, Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109:2521–2528. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- 40.Victora GD, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyers G, et al. Activation-induced cytidine deaminase (AID) is required for B cell tolerance in humans. Proc Natl Acad Sci USA. 2011;108:11554–11559. doi: 10.1073/pnas.1102600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holl TM, Haynes BF, Kelsoe G. Stromal cell independent B cell development in vitro: Generation and recovery of autoreactive clones. J Immunol Methods. 2010;354:53–67. doi: 10.1016/j.jim.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li YS, Wasserman R, Hayakawa K, Hardy RR. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5:527–535. doi: 10.1016/s1074-7613(00)80268-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.