Abstract
The Wnt/β-catenin signaling system plays essential roles in embryonic development and in the self-renewal and maintenance of adult stem cells. R-spondins (RSPOs) are a group of secreted proteins that enhance Wnt/β-catenin signaling and have pleiotropic functions in development and stem cell growth. LGR5, an orphan receptor of the G protein-coupled receptor (GPCR) superfamily, is specifically expressed in stem cells of the intestinal crypt and hair follicle. Knockout of LGR5 in the mouse results in neonatal lethality. LGR4, a receptor closely related to LGR5, also has essential roles in development, as its knockout leads to reduced viability and retarded growth. Overexpression of both receptors has been reported in several types of cancer. Here we demonstrate that LGR4 and LGR5 bind the R-spondins with high affinity and mediate the potentiation of Wnt/β-catenin signaling by enhancing Wnt-induced LRP6 phosphorylation. Interestingly, neither receptor is coupled to heterotrimeric G proteins or to β-arrestin when stimulated by the R-spondins, indicating a unique mechanism of action. The findings provide a basis for stem cell-specific effects of Wnt/β-catenin signaling and for the broad range of functions LGR4, LGR5, and the R-spondins have in normal and malignant growth.
Keywords: cell signaling, stem cell control, gastrointestinal growth, colon cancer
Adult stem cells are specialized, undifferentiated cells that are capable of self-renewal and of generating all cell types of the tissue in which they reside. They are generally identified and traced by one marker, or a set of markers, that is specifically expressed in these cells. These markers are likely to constitute some of the important processes that ultimately define stem cells, and thus hold key information to the understanding of stem cell biology. Nevertheless, for the majority of these markers, the functions remain unknown. LGR5 (leucine-rich repeat containing G protein-coupled receptor 5) has been identified and validated as a marker of the crypt basal columnar stem cells along the gastrointestinal tract and of the bulge stem cells in the hair follicle (1–2). This receptor, also known as HG38, GPR49, and FEX, was first reported by us as an orphan receptor (HG38) with homology to the glycoprotein hormone receptor subfamily of the class A rhodopsin-like seven transmembrane (7-TM) domain, G protein-coupled receptors (GPCRs) (3–5). LGR5 is closely related to two other receptors, LGR4 and LGR6 (∼50% identity between each other), and together the trio (LGR4–6) forms a structurally distinct group of 7-TM receptors that have a substantially large N-terminal extracellular domain (ECD) composed of 17 leucine-rich repeats (6). Knockout of LGR5 in the mouse leads to total neonatal lethality accompanied by ankyloglossia and gastrointestinal distension (7). Loss of LGR4 results in reduced viability with developmental defects in many organs, including the kidney (8, 9), testis (10, 11), eye (12), bone (13), skin (14), and gall bladder (15). LGR4 and LGR5 are also overexpressed in several types of cancer and can promote the growth/metastasis of tumor cells (16–18). Despite the critical roles of LGR4 and LGR5 in normal and cancer development and stem cell-specific expression, their endogenous ligands, signaling mechanisms, and potential functions in stem cells remain a mystery.
The R-spondin (RSPO) protein family is a group of four secreted proteins (RSPO1–4) that were isolated as strong potentiators of Wnt/β-catenin signaling (19–21). These proteins share 40–60% identity between each other and a similar structure with a cysteine-rich furin-like domain preceding a thrombospondin-like domain (22, 23). RSPO1–4 can stimulate the proliferation of intestinal crypt stem cells both in vivo and in vitro through enhancement of Wnt/β-catenin signaling (20, 23, 24). Although it has been postulated that RSPOs bind to and activate the Wnt coreceptor LRP6 (21, 25), there have been conflicting reports on the direct interaction between RSPOs and LRP6 (19, 26). Here we show that RSPOs potentiate Wnt/β-catenin signaling by actually functioning as ligands of LGR4 and LGR5.
Results
RSPO1–4 Bind to and Cointernalize with LGR4 and LGR5.
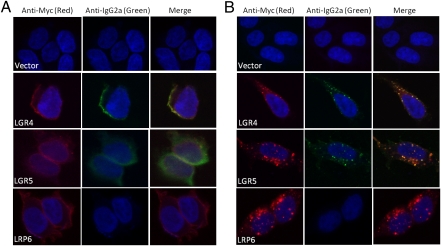
We set out to identify endogenous ligands of LGR4 and LGR5 using a variety of strategies. Initial studies using cell lines overexpressing LGR4 or LGR5 failed to identify any receptor-associated constitutive activity in classical GPCR assays, including stimulation/inhibition of cAMP production, Ca2+ mobilization, and β-arrestin translocation. We also selected a panel of secreted proteins as potential ligands on the basis of various rationales (Table S1) and tested them extensively in G protein signaling or β-arrestin translocation, but failed to identify any positive hits. We then turned our attention to the R-spondins as candidate ligands on the basis of their strong mitogenic effect, specifically on LGR5+ cells (20, 24) and the lack of any unequivocally identified receptors. As no functional assay was available for LGR4 and LGR5, we tested whether RSPO1 could directly bind to LGR4 and LGR5. HEK293 cell lines stably expressing either LGR4 or LGR5 with a Myc tag at the N terminus were established. A fusion gene construct (mRSPO1-Fc), encoding the mature form of mouse RSPO1 and the Fc fragment of mouse IgG2a that was validated to produce biologically active RSPO1 (24), was transfected into HEK293 cells to produce RSPO1 as a secreted protein. The Fc fragment serves as a highly sensitive tag for binding and localization detection. When mRSPO1-Fc was incubated with cells expressing LGR4 or LGR5 at 4 °C (to prevent internalization), a strong signal indicative of binding (green) was observed on the cell surface. Coimmunostaining with an anti–receptor-tag antibody (red) showed colocalization (yellow) of LGR4 and LGR5 with mRSPO1-Fc (Fig. 1A). In contrast, no binding was observed in control (vector-transfected) cells (Fig. 1A). When the binding was carried out at 37 °C in live cells, both receptors (red) and mRSPO1-Fc (green) were found in intracellular bodies (Fig. 1B). Superimposing of the two images revealed near complete colocalization of mRSPO1-Fc with each receptor (Fig. 1B), indicating that mRSPO1 was cointernalized with LGR4 and LGR5. Interestingly, intracellular staining of LGR4 and LGR5 was also observed in the absence of mRSPO1-Fc (Fig. S1B), which may be caused by either constitutive internalization or endogenous expression of multiple RSPOs in HEK293 cells (19) (Fig. S3A). No nonspecific Fc-associated staining (green) was observed in the absence of mRSPO1-Fc at either 4 °C or 37 °C (Fig. S1). HEK293 cell lines stably overexpressing LRP6 were also tested for binding of mRSPO1-Fc. Although the cells exhibited strong expression of LRP6, no mRSPO1-Fc binding was detected at either 4 °C or 37 °C (Fig. 1 A and B and Fig. S1C), indicating no direct interaction between mRSPO1-Fc and LRP6.
Fig. 1.
Binding of mRSPO1-Fc to LGR4 and LGR5 by confocal immunofluorescence analysis. HEK293 cells stably expressing vector, Myc-LGR4, Myc-LGR5, or HA-LRP6 were incubated with mRSPO1-Fc at 4 °C (A), or at 37 °C (B). The cells were then costained with fluorescence-labeled anti-tag antibodies (Cy3–anti-Myc, Alexa594–anti-HA, both mouse IgG1 subtype) for receptor detection (red) and Alexa Fluor 488-labeled anti-IgG2a for mRSPO1-Fc detection (green). Nuclei were counterstained with ToPro-3 (blue).
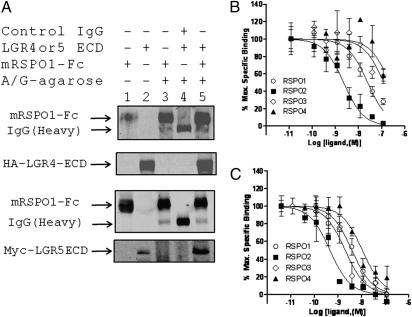
Next, we confirmed the binding of mRSPO1-Fc to LGR5 by FACS analysis. Cells overexpressing LGR5 displayed increased fluorescence intensity as a population compared with vector control cells in the presence of mRSPO1-Fc (Fig. S2A). In contrast, no shift in fluorescence intensity was observed between populations of LRP6 cells and control cells (Fig. S2A). Expression of LGR5 and LRP6 in these cells was confirmed by FACS using anti–receptor-tag antibodies (Fig. S2 B and C). On the basis of the structural similarity of LGR4 and LGR5 to the glycoprotein hormone receptors, the extracellular domains of LGR4 and LGR5 are expected to be soluble and bind the cognate ligand. We confirmed that the ECDs of LGR4 and LGR5 coprecipitated with mRPSO1-Fc, but not with control IgG (Fig. 2A). We then developed a fluorescence-based whole-cell competition binding assay to determine whether purified, recombinant RSPO1–4 could compete with mRSPO1-Fc for binding to LGR4 and LGR5. RSPO1–4 were able to completely displace the binding of mRSPO1-Fc to LGR4 and LGR5 with IC50s in the nanomolar range (Fig. 2 B and C and Table 1), with the exception of the micromolar IC50s for RSPO3 and RSPO4 binding to LGR4 (Table 1). Taken together, the results of our binding analysis indicate that RSPO1–4 can bind to LGR4 and LGR5 with RSPO2 having the highest affinity to both receptors.
Fig. 2.
Binding of RSPO1–4 to LGR4 and LGR5 by coimmunoprecipitation and competition analysis. (A) Coimmunoprecipitation of LGR4ECD and LGR5ECD with mRSPO1-Fc using protein A/G sepharose beads. Pull-down samples (lanes 3–5) were probed with anti-mouse IgG antibody, or with anti-HA (LGR4ECD), or anti-Myc (LGR5ECD) antibody for each ECD. Lanes 1 and 2 are input control. (B and C) Quantitative binding analysis using a whole-cell–based assay. HEK293 cells stably expressing Myc-LGR4 (B) or Myc-LGR5 (C) were incubated with mRSPO1-Fc plus serial dilutions of purified recombinant RSPO1–4. Maximum specific binding is defined by the difference between the data with and without mRSPO1-Fc, which is ∼50% of total binding in general. All error bars are SEM (n = 3–4).
Table 1.
Binding (IC50, nM), and potency (EC50, nM) and maxium effect (Emax, fraction of vector control) of RSPO1–4 in cells overexpressing LGR4 or LGR5
| Vector |
LGR4 |
LGR5 |
|||||||
| Ligand | IC50 | EC50 | Emax | IC50 | EC50 | Emax | IC50 | EC50 | Emax |
| RSPO1 | ND | NC | NC | 25 | 0.02 | NC | 4.0 | 0.008 | NC |
| RSPO2 | ND | 0.2 | 1 | 2.3 | 0.0003 | 0.9 | 0.5 | 0.001 | 1 |
| RSPO3 | ND | 0.1 | 1 | 126 | 0.02 | 0.9 | 2.1 | 0.01 | 1.1 |
| RSPO4 | ND | NC | NC | 228 | 0.09 | NC | 11 | 0.4 | NC |
ND, not determined; NC, not calculated.
LGR4 and LGR5 Potentiate Wnt/β-Catenin Signaling in Response to R-Spondin.
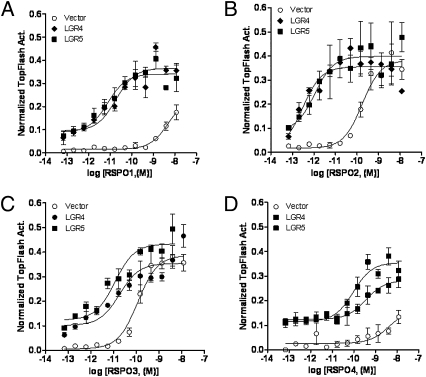
Previously, it was demonstrated that RSPOs potentiate β-catenin/T cell factor (TCF) signaling in a Wnt-dependent fashion (20–22, 25, 26). Using a β-catenin–responsive reporter assay (27), we examined the effect of RSPO treatment on Wnt/β-catenin signaling in HEK293T cells overexpressing LGR4 or LGR5 in the presence of exogenous Wnt3a. Cells transfected with LGR4 or LGR5 displayed a dramatic increase in the potencies of RSPO1–4, ranging from 10- to 1,000-fold, with no significant change in the maximum activity (Emax) of the reporter enzyme compared with vector-transfected cells (Fig. 3 A–D and Table 1). Furthermore, both LGR4- and LGR5-transfected cells showed elevated basal activity relative to vector control cells (Fig. 3 A–D). This could be due to endogenous expression of RSPOs in HEK293T cells (Fig. S3A) and/or constitutive activity of the receptors.
Fig. 3.
Potentiation of Wnt/β-catenin signaling by LGR4 and LGR5 in response to RPSO1–4. HEK293T cells were transiently transfected with LGR4, LGR5, or vector, plus the β-catenin reporter plasmid Super 8× TOPFlash (firefly luciferase) and pRL-SV40 (renilla luciferase) and then stimulated with serial dilutions of purified recombinant RSPO1 (A), RSPO2 (B), RSPO3 (C), or RSPO4 (D) in the presence of Wnt3a conditioned media (CM). Firefly luciferase activity of each well was normalized to that of renilla luciferase activity of the same well. All error bars are SEM (n = 4).
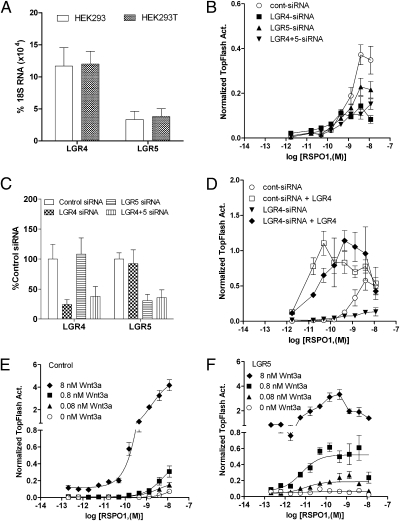
The strong endogenous response of HEK293T cells to RSPOs in the β-catenin reporter assay suggested that one or more of the LGRs are endogenously expressed in these cells. Indeed, quantitative RT-PCR analysis revealed that both receptors are expressed in HEK293 and HEK293T cells with LGR4 yielding the highest expression level (Fig. 4A). After HEK293T cells were transfected with LGR4-siRNA, LGR5-siRNA, or both, to knockdown their expression, we found that the cells transfected with LGR4-siRNA or LGR5-siRNA showed ∼50% or ∼30% reduction, respectively, in response to RSPO1 compared with control siRNA-transfected cells (Fig. 4B). Cells transfected with both LGR4- and LGR5-siRNA showed no further reduction in response (Fig. 4B). The responses to RSPO3 were also reduced to approximately the same extent (Fig. S3B). Quantitative RT-PCR analysis confirmed a commensurable decrease in the mRNA levels of LGR4 and LGR5 (Fig. 4C). The incomplete suppression of endogenous response to RSPO1 and RSPO3 is most likely due to the only partial knockdown of the two receptors. Furthermore, the inhibitory effect of LGR4-siRNA was completely rescued when the cells were cotransfected with LGR4 (Fig. 4D). These data indicate that the endogenous response of HEK293T cells to RSPOs in the reporter enzyme assay is largely mediated by LGR4, consistent with its higher mRNA level and robust response to RSPO1–4 when overexpressed.
Fig. 4.
Effect of LGR4 and LGR5 knockdown and Wnt3a concentration on LGR5-mediated Wnt/β-catenin signaling potentiation. (A) Expression levels of LGR4 and LGR5 in HEK293 and HEK293T cells by quantitative RT-PCR analysis. (B) Effect of LGR4 and LGR5 expression knockdown on RSPO1 response in the presence of Wnt3a CM. (C) Quantitative RT-PCR results of the expression levels of LGR4 and LGR5 in siRNA-transfected cells. (D) Rescue of RSPO1 response in LGR4-siRNA cells by cotransfecting with LGR4. (E and F) Effect of exogenous Wnt3a concentration on RSPO1 response in vector (E) and LGR5 (F) cells. All error bars are SEM (n = 4).
Next, we focused on LGR5 and RSPO1 to investigate the mechanism of action and potential interactions with other players of the Wnt/β-catenin signaling pathway. To characterize the requirement of Wnt3a for RSPO activity, we examined the activity of RSPO1 in vector and LGR5-overexpressing cells at different concentrations of purified recombinant Wnt3a. In vector cells, increasing concentrations of Wnt3a produced a corresponding increase in RSPO1 response (Fig. 4E). In cells overexpressing LGR5, RSPO1 showed a much a higher potency at the same concentration of Wnt3a compared with vector cells (Fig. 4F vs. 4E). Furthermore, the potency of RSPO1 is nearly constant across the various concentrations of Wnt3a in LGR5-overexpressing cells (Fig. 4F, EC50 = 11, 5.7, 6.3, and 9.4 pM for 0, 0.08, 0.8, and 8 nM of Wnt3a, respectively), whereas the Emax is proportional to Wnt3a concentration. We also examined the potential requirement of LRP6 to mediate RSPO1-LGR5 activity. Transfection of LRP6 alone increased Wnt3a-mediated activity by ∼20-fold, but did not change the potency of RSPO1 (Fig. S3C). Cotransfection of LRP6 with LGR5 increased basal activity by another ∼4-fold compared with LRP6-transfected cells (Fig. S3C). The potencies of RSPO1 in LGR5-overexpressing cells and LRP6-LGR5 cells (0.016 and 0.009 nM, respectively) were similar, yet much higher than those observed in LRP6-transfected and control cells. Overexpression of the extracellular domain of LRP6 (LRP6ECD), which behaves as a dominant negative form of LRP6, led to the loss of the effect of RSPO1 (Fig. S3C). Because DKK1 antagonizes Wnt signaling by binding to LRP6 and blocks RSPO activity (26), we also examined its effect on LGR5-mediated RSPO1 activity. Cotransfection of DKK1 completely blocked the activity of RSPO1 in vector cells as well as in LGR5-transfected cells (Fig. S3D). These data, taken together with the activity of RSPO1–4 in LGR4- or LGR5-transfected cells, precisely demonstrate that the potencies of RSPO1–4 are determined by the receptor levels of LGR4 and LGR5, whereas the maximum activity is dependent on the level of Wnt3a and the presence of LRP6. The results of our functional and binding analysis are consistent with a model in which RSPOs directly binds to LGR4 and LGR5 to increase the activity of Wnt/β-catenin signaling.
Potentiation Is Mediated Through Enhanced LRP6 Phosphorylation.
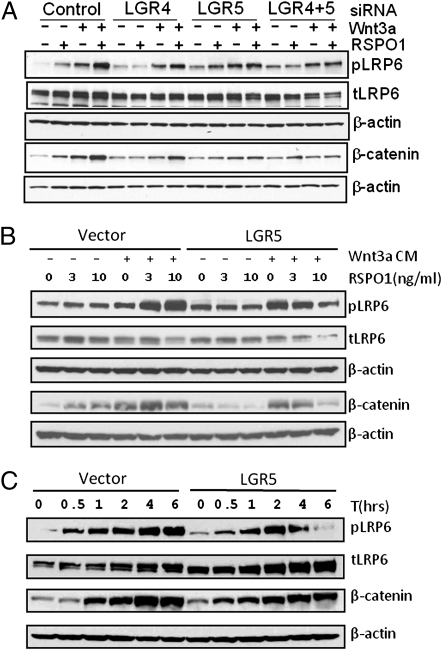
Wnt/β-catenin signaling is characterized by a series of events starting with phosphorylation and internalization of LRP5/6 subsequent to ligand binding, followed by inhibition of GSK3β and an increase in the levels of active β-catenin (28). Previously, it was shown that RSPO1 enhances Wnt3a-induced LRP6 phosphorylation (pLRP6) and active β-catenin accumulation in HEK293 cells (19–21, 25, 26). We confirmed the synergistic effect of RSPO1 and Wnt3a in inducing LRP6 phosphorylation and increasing levels of β-catenin in HEK293 cells (Fig. 5A). Knockdown of the endogenous expression of LGR4 and LGR5 by siRNA led to significant reduction of this effect (Fig. 5A), which, together with the data of the β-catenin reporter assay following expression knockdown, indicate that expression of LGR4 and LGR5 in HEK293T cells is essential for RSPO-enhanced phosphorylation of LRP6 and β-catenin accumulation. We also examined the effect of LGR5 overexpression on pLRP6 and β-catenin accumulation following Wnt3a-RSPO1 treatment. LGR5-overexpressing cells showed increased basal levels of pLRP6 and β-catenin compared with vector cells (Fig. 5B), consistent with increased basal activity in the β-catenin reporter assays (Fig. 3). Surprisingly, treatment of Wnt3a and RSPO1 in LGR5-overexpressing cells led to decreased levels of pLRP6 and β-catenin (Fig. 5B). We then compared the time course of pLRP6 and β-catenin accumulation between vector and LGR5-overexpressing cells following treatment with Wnt3a and RSPO1. In vector cells, the levels of pLRP6 and β-catenin increased with time through 6 h (Fig. 5C). In LGR5-overexpressing cells, pLRP6 also increased with time, but started to decline at 4 h and almost totally disappeared at 6 h. The level of β-catenin failed to reach the maximum level of the vector cells and began to decline at the 6 h time point (Fig. 5C). The results suggest that stimulation of cells overexpressing LGR5 with its ligand and Wnt3a accelerates either LRP6 dephosphorylation or pLRP6 degradation, as well as enhances β-catenin turnover.
Fig. 5.
Effect of LGR4 and LGR5 expression knockdown and LGR5 overexpression on Wnt3a-RSPO1–induced LRP6 phosphorylation and β-catenin accumulation. (A) Effect of LGR4 and LGR5 expression knockdown on endogenous response to Wnt3a-RSPO1 in LRP6 phosphorylation and β-catenin levels. HEK293T cells were transfected with control, LGR4-, or LGR5-siRNA, or both, and 2 d later, the cells were stimulated with RSPO1 (4 nM), or Wnt3a (3 nM), or both for 3 h. Phospho-LRP6 at Ser1490 (pLRP6), total LRP6 (tLRP6), nonmembrane-associated β-catenin (membrane-bound β-catenin was removed with Con A-sepharose beads), and β-actin (loading control) were then probed by immunoblot analysis. (B) Change in pLRP6 and β-catenin levels in response to RSPO1 and Wnt3a treatment in vector and LGR5-overexpressing cells. HEK293 cells stably expressing vector or LGR5 were stimulated with RSPO1 (0, 3, and 10 ng/mL) with or without Wnt3a CM for 3 h, and probed as above. (C) Time course of LRP6 phosphorylation and changes in β-catenin levels following Wnt3a and RSPO1 treatment. The cells were stimulated with RSPO1 (100 ng/mL) and Wnt3a CM for 0–6 h and probed as above.
LGR4 and LGR5 Are Not Coupled to Heterotrimeric G Proteins or to β-Arrestin.
As LGR4 and LGR5 are predicted to be members of the GPCR family on the basis of their homology to the glycoprotein hormone receptors and other rhodopsin-type GPCRs, we investigated whether LGR4 and LGR5 are coupled to heterotrimeric G proteins and/or to β-arrestin. Surprisingly, we did not detect activation of any of the three classic pathways of G proteins [Gαs, Gαq, and Gα(i/O)] in HEK293T cells with or without the overexpression of LGR4 or LGR5 following treatment with various concentrations of RSPOs (Fig. S4 A–E). Cotreatment with Wnt3a did not cause any difference. As some 7-TM receptors are only coupled to β-arrestin (29), we then examined β-arrestin translocation in HEK293T cells cotransfected with β-arrestin2–GFP and LGR4 or LGR5 following treatment with mRSPO1-Fc or purified RSPO1, with or without Wnt3a. No indication of β-arrestin translocation was observed under any circumstances, whereas ligand-receptor colocalization was clearly confirmed (Fig. S5A). As a positive control, robust translocation was observed in cells overexpressing the β2 adrenergic receptor and treated with isoproterenol (Fig. S5B). These data indicate that LGR4 and LGR5, despite having significant homology to the rhodopsin type of GPCRs in the transmembrane regions, are coupled to neither G proteins nor β-arrestin, at least when they are stimulated by the R-spondins.
Discussion
Through a candidate ligand approach, we have uncovered that the R-spondins are high-affinity ligands of LGR4 and LGR5 using a series of binding and functional analyses. The results of the β-catenin reporter assays consistently showed that the potencies of RSPO1–4 are determined by the level of LGR4 and LGR5 expression, whereas the maximum activity is determined by levels of Wnt3a and LRP6. Overexpression of LGR5 led to elevated basal levels of β-catenin reporter activity as well as increased levels of LRP6 phosphorylation and β-catenin, whereas knockdown of endogenous LGR4 expression in HEK293T cells leads to decreased response to RSPOs. The data, together with previously published results (19, 22, 26), strongly suggest a model in which activation of LGR4 and LGR5 by RSPOs leads to an increase in Wnt-dependent LRP6 phosphorylation and consequently enhanced β-catenin activity. However, the exact mechanism of how the activation of LGR4 and LGR5 by RSPOs leads to enhanced LRP6 phosphorylation remains to be understood. We found no evidence for the involvement of heterotrimeric G proteins or β-arrestin. On the other hand, we invariably observed that receptors of LGR4 and LGR5 are highly internalized into large intracellular bodies, with or without being bound by RSPO. Interestingly, it is well established that Wnt/β-catenin signaling requires internalization of the Wnt coreceptors and the sequestration of glycogen synthase kinase 3 inside multivesicular endosomes (30, 31). Therefore, one possible scenario is that the RSPO-LGR complex enhances the internalization of the frizzled-Wnt-LRP6 signalosome into multivesicular endosomes, leading to enhanced LRP6 phosphorylation. However, an intriguing observation is that the level of β-catenin in LGR5-overexpressing cells following R-spondin stimulation exhibits a reduction in the overall accumulation at later time points compared with control cells (Fig. 5 B and C). This is opposed to the dramatic increase of β-catenin reporter activity in LGR5-overexpressing cells following R-spondin addition. Of the potential explanations for this disconnect, one is that LGR5 potentiates β-catenin signaling through enhancing the activity of one or more of the cofactors of β-catenin/TCF while accelerating its desensitization. Another one is that R-spondin stimulation in LGR5-overexpressing cells induces a transient increase in β-catenin level that leads to rapid increase in reporter gene transcription and accumulation of reporter enzyme activity, but was not reflected at any of the time points by immunoblot analysis. We are actively testing these and other possibilities as well as conducting a systematic investigation for the signaling mechanisms of LGR4 and LGR5.
Physiological relevance of the R-spondins as ligands of LGR4 and LGR5 is strongly supported by previous observations made in vitro and in vivo. In organoid cultures of LGR5+ stem cells isolated from the intestine, addition of RSPO1 leads to increased β-catenin/TCF signaling only in the LGR5+ stem cells, but not in the LGR5− daughter cells (32). In whole crypt cultures, supplementation with RSPO1 increased proliferation of the LGR5+ cells (24). Overexpression of RSPO1 in vivo resulted in massive, specific proliferation of LGR5+ crypt stem cells (20, 24). Importantly, RSPO1 was found to be expressed in the Paneth cells of the crypt, which surround and form the niche for the LGR5+ stem cells (32, 33). Furthermore, knockout of LGR5 led to dysregulation of Wnt/β-catenin in the intestine characterized by enhanced Wnt/β-catenin signaling without increase in epithelial cell proliferation (34). Because overactivation of Wnt/β-catenin signaling typically results in increased cell proliferation in the intestine (35, 36), the authors hypothesized that LGR5 plays a negative role in Wnt/β-catenin signaling, but is required for cell proliferation (34). Our finding that LGR5 not only enhances Wnt/β-catenin signaling but also appears to accelerate the degradation of pLRP6 and β-catenin suggests that activation of LGR5 in the intestine potentiates the Wnt/β-catenin signaling system in the stem cells to generate signals that are essential for cell proliferation as well as for the down-regulation of Wnt/β-catenin signaling. Overall, the results and conclusions reported here provide an explanation for the specific effect of RSPO1 on LGR5+ cells and the importance of Wnt/β-catenin signaling for the maintenance of the stemness of stem cells. These findings will facilitate the investigation of the signaling mechanisms and physiological functions of LGR4 and LGR5 and RSPOs in development and in the self-renewal and maintenance of stem cells and the understanding of their roles in tumor formation, growth, and metastasis. Modulation of the activities of this ligand-receptor system may offer novel approaches to the development of regenerative medicine and cancer treatment.
Materials and Methods
Detailed descriptions of materials and protocols can be found in the SI Materials and Methods.
Materials.
N-terminal Myc-tagged full-length mouse LGR4, human LGR5, LGR5ECD, N-terminal HA-tagged mouse LGR4ECD, N-terminal HA-tagged LRP6, and LRP6ECD were constructed using standard cloning techniques. All clones were verified by DNA sequencing. The plasmid Super 8× TOPFlash was purchased from Addgene. pRL-SV40 (SV40 promoter-controlled renilla luciferase) was purchased from Promega. Plasmid encoding mRSPO1-Fc was from Dr. Calvin Kuo at Stanford University, La Jolla, CA. All recombinant proteins were purchased from R&D Systems.
Cell Lines and Immunocytofluorescence Confocal Microscopy.
Plasmids of Myc-LGR4 and Myc-LGR5 were transfected into HEK293 using Fugene 6 (Roche), and bulk stable cells were selected and maintained with puromycin at 1 μg/mL. Cells expressing Myc-LGR4 or Myc-LGR5 were incubated with mRSPO1-Fc either at 4 °C for 2 h or 37 °C for 45 min, washed, fixed, permeabilized (only the cells incubated at 37 °C), and costained with goat antimouse IgG2a plus anti-Myc or anti-HA antibody for 1 h at room temperature. The cells were then washed and nuclei were counterstained with TO-PRO-3. Images were recorded and analyzed using confocal laser scanning microscopy (Leica; TCS SP5 microscope) with LAS AF Lite software.
Coprecipitation Analysis and Whole-Cell Binding Analysis.
Immunoprecipitation was carried out as described (25). For whole-cell competition binding analysis, the cells were incubated with mRSPO-Fc at 4 °C for 3–4 h plus various concentrations of competitor, washed, fixed in 4% paraformaldehyde/PBS, washed and incubated with Alexa Fluor 647-labeled goat antimouse IgG (H+L) for 1 h at room temperature, and washed again. Fluorescence intensity was measured using a Tecan M1000 plate reader with excitation at 630 nm and emission at 670 nm. All experiments were performed at least twice with quadruplicate replicates in each experiment. Data were analyzed using the software GraphPad Prism 5.
β-Catenin Reporter Assays.
TopFlash assays were performed as before with slight modifications (25). Briefly, HEK293T cells were transiently transfected with plasmids of vector or receptor, Super 8× TOPFlash firefly luciferase, and pRL-SV40-renilla luciferase reporter at a ratio of 1:1:0.1 (weight) using FuGene HD, treated with various concentrations of RSPOs in Wnt3a conditioned media (CM) (1:4 dilution). Luciferase assay measurements were carried out using the Dual-Glo luciferase assay kit (Promega) according to the manufacturer’s protocol. All experiments were performed at least twice with quadruplicate replicates in each experiment and the data were analyzed using GraphPad Prism 5. The siRNA used in this study were the human LGR4 and LGR5 ON-Targetplus SMARTpool, and Nontargeting pool as a negative control (Dharmacon).
Quantitative RT-PCR and Immunoblot Analysis.
RNA was isolated using the TRIzol method and quantified expression levels of LGR4 and LGR5, and RSPO1–4 were determined from an ssDNA standard curve using the primers listed in Table S2, and expression was normalized to levels of 18S rRNA. Phospho-LRP6 was probed with a phospho-Ser1490–specific antibody (Cell Signaling; 2568) and total LRP6 were probed with an anti-LRP6 polyclonal antibody (Cell Signaling; 3395). β-Actin were also probed as protein-loading control. Immunoblotting of cytosolic (nonmembrane bound) β-catenin were carried out using cell lysates that were treated with ConA-sepharose beads overnight followed by centrifugation to remove cadherin-bound β-catenin and probed with the anti–β-catenin antibody that detects total β-catenin (Cell Signaling; 9562). All immunoblotting procedures were carried out using HRP-conjugated secondary antibodies by following manufacturer’s suggested protocols.
Supplementary Material
Acknowledgments
We thank Drs. Akifumi Ootani and Calvin Kuo for the mRSPO1-Fc plasmid, Dr. Marc Caron for the β-arrestin2-GFP plasmid, Zhengmei Mao for technical assistance with the confocal microscope, and Dr. Xuejun Fan for technical assistance with FACS analysis. This work was supported in part by Grant RP100678 from the Cancer Prevention and Research Institute of Texas.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106083108/-/DCSupplemental.
References
- 1.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 2.Jaks V, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 3.McDonald T, et al. Identification and cloning of an orphan G protein-coupled receptor of the glycoprotein hormone receptor subfamily. Biochem Biophys Res Commun. 1998;247:266–270. doi: 10.1006/bbrc.1998.8774. [DOI] [PubMed] [Google Scholar]
- 4.Hsu SY, Liang SG, Hsueh AJ. Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol Endocrinol. 1998;12:1830–1845. doi: 10.1210/mend.12.12.0211. [DOI] [PubMed] [Google Scholar]
- 5.Hermey G, Methner A, Schaller HC, Hermans-Borgmeyer I. Identification of a novel seven-transmembrane receptor with homology to glycoprotein receptors and its expression in the adult and developing mouse. Biochem Biophys Res Commun. 1999;254:273–279. doi: 10.1006/bbrc.1998.9882. [DOI] [PubMed] [Google Scholar]
- 6.Hsu SY, et al. The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): Identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol. 2000;14:1257–1271. doi: 10.1210/mend.14.8.0510. [DOI] [PubMed] [Google Scholar]
- 7.Morita H, et al. Neonatal lethality of LGR5 null mice is associated with ankyloglossia and gastrointestinal distension. Mol Cell Biol. 2004;24:9736–9743. doi: 10.1128/MCB.24.22.9736-9743.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazerbourg S, et al. Leucine-rich repeat-containing, G protein-coupled receptor 4 null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality. Mol Endocrinol. 2004;18:2241–2254. doi: 10.1210/me.2004-0133. [DOI] [PubMed] [Google Scholar]
- 9.Kato S, et al. Leucine-rich repeat-containing G protein-coupled receptor-4 (LGR4, Gpr48) is essential for renal development in mice. Nephron Exp Nephrol. 2006;104:e63–e75. doi: 10.1159/000093999. [DOI] [PubMed] [Google Scholar]
- 10.Mendive F, et al. Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev Biol. 2006;290:421–434. doi: 10.1016/j.ydbio.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 11.Hoshii T, et al. LGR4 regulates the postnatal development and integrity of male reproductive tracts in mice. Biol Reprod. 2007;76:303–313. doi: 10.1095/biolreprod.106.054619. [DOI] [PubMed] [Google Scholar]
- 12.Weng J, et al. Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proc Natl Acad Sci USA. 2008;105:6081–6086. doi: 10.1073/pnas.0708257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo J, et al. Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development. 2009;136:2747–2756. doi: 10.1242/dev.033571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohri Y, Kato S, Umezawa A, Okuyama R, Nishimori K. Impaired hair placode formation with reduced expression of hair follicle-related genes in mice lacking Lgr4. Dev Dyn. 2008;237:2235–2242. doi: 10.1002/dvdy.21639. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita R, et al. Defective development of the gall bladder and cystic duct in Lgr4- hypomorphic mice. Dev Dyn. 2009;238:993–1000. doi: 10.1002/dvdy.21900. [DOI] [PubMed] [Google Scholar]
- 16.McClanahan T, et al. Identification of overexpression of orphan G protein-coupled receptor GPR49 in human colon and ovarian primary tumors. Cancer Biol Ther. 2006;5:419–426. doi: 10.4161/cbt.5.4.2521. [DOI] [PubMed] [Google Scholar]
- 17.Tanese K, et al. G-protein-coupled receptor GPR49 is up-regulated in basal cell carcinoma and promotes cell proliferation and tumor formation. Am J Pathol. 2008;173:835–843. doi: 10.2353/ajpath.2008.071091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Y, et al. Up-regulation of GPR48 induced by down-regulation of p27Kip1 enhances carcinoma cell invasiveness and metastasis. Cancer Res. 2006;66:11623–11631. doi: 10.1158/0008-5472.CAN-06-2629. [DOI] [PubMed] [Google Scholar]
- 19.Kazanskaya O, et al. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell. 2004;7:525–534. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Kim KA, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 21.Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J Biol Chem. 2006;281:13247–13257. doi: 10.1074/jbc.M508324200. [DOI] [PubMed] [Google Scholar]
- 22.Kim KA, et al. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell. 2008;19:2588–2596. doi: 10.1091/mbc.E08-02-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim KA, et al. R-Spondin proteins: A novel link to beta-catenin activation. Cell Cycle. 2006;5:23–26. doi: 10.4161/cc.5.1.2305. [DOI] [PubMed] [Google Scholar]
- 24.Ootani A, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei Q, et al. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J Biol Chem. 2007;282:15903–15911. doi: 10.1074/jbc.M701927200. [DOI] [PubMed] [Google Scholar]
- 26.Binnerts ME, et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci USA. 2007;104:14700–14705. doi: 10.1073/pnas.0702305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 28.Bilic J, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 29.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 30.Blitzer JT, Nusse R. A critical role for endocytosis in Wnt signaling. BMC Cell Biol. 2006;7:28. doi: 10.1186/1471-2121-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taelman VF, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao J, et al. R-spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterology. 2007;132:1331–1343. doi: 10.1053/j.gastro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Garcia MI, et al. LGR5 deficiency deregulates Wnt signaling and leads to precocious Paneth cell differentiation in the fetal intestine. Dev Biol. 2009;331:58–67. doi: 10.1016/j.ydbio.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Andreu P, et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 2005;132:1443–1451. doi: 10.1242/dev.01700. [DOI] [PubMed] [Google Scholar]
- 36.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.