Abstract
PlGF, one of the ligands for VEGFR-1, has been implicated in tumor angiogenesis. However, more recent studies indicate that genetic or pharmacological inhibition of PlGF signaling does not result in reduction of microvascular density in a variety of tumor models. Here we screened 12 human tumor cell lines and identified 3 that are growth inhibited by anti-PlGF antibodies in vivo. We found that efficacy of anti-PlGF treatment strongly correlates with VEGFR-1 expression in tumor cells, but not with antiangiogenesis. In addition, PlGF induced VEGFR-1 signaling and biological responses in tumor cell lines sensitive to anti-PlGF, but not in refractory tumor cell lines or in endothelial cells. Also, genetic ablation of VEGFR-1 signaling in the host did not affect the efficacy of PlGF blockade. Collectively, these findings suggest that the role of PlGF in tumorigenesis largely consists of promoting autocrine/paracrine growth of tumor cells expressing a functional VEGFR-1 rather than stimulation of angiogenesis.
Keywords: stroma, tyrosine kinase, placental growth factor, vascular endothelial growth factor, angiogenesis
The VEGF signaling pathways play important roles in angiogenesis. VEGF-A binds to two tyrosine kinase receptors, VEGFR-1 and VEGFR-2 (1). Although both receptors are expressed in endothelial cells, VEGFR-1 is also expressed in monocyte/macrophages, hematopoietic stem cells, and even some tumor cells (2–4). Most of the biological effects of VEGF-A are mediated by activation of VEGFR-2 (1). VEGFR-1 has a weak tyrosine kinase activity but substantially higher binding affinity for VEGF-A than VEGFR-2 (5). The biological role of VEGFR-1 is highly complex. Although genetic data indicate that signaling downstream of this receptor is not required for developmental angiogenesis (6), a role for VEGFR-1 during tumor-angiogenesis has been recently suggested (7–9). PlGF is a VEGFR-1 specific ligand (10) that was identified 20 years ago (11). Under pathological conditions, PlGF levels are increased in various cell types, including vascular endothelial cell, smooth muscle cells, keratinocytes, hematopoietic cells, retinal pigment epithelial cells, and many different tumor cells (12). Plgf deficient mice are born at normal Mendelian ratios and do not show any obvious vascular defects (13). PlGF overexpression enhanced tumor growth in some models (14, 15), but in others, PlGF paradoxically had an inhibitory effect, likely through formation of VEGF/PlGF heterodimers, which down-regulate VEGFR2 signaling (16, 17).
According to Fisher et al. (7), treatment with an anti-PlGF monoclonal antibody (Mab) reduces microvascular density (MVD) and inhibits primary tumor growth in a variety of murine models. However, in a subsequent study, we reported that blocking PlGF does not result in growth inhibition in any of the tumor models tested (12 murine and 3 human tumor cell lines) (18). Importantly, the antibodies used in these studies were able to block PlGF in vivo (18) as evidenced by their ability to inhibit metastasis of B16F10 cells (7, 19, 20), wound healing (13, 21), and primary tumor growth of a murine cell line overexpressing VEGFR-1. On the other hand, it has been shown that genetic ablation of plgf results in inhibition of tumorigenesis in some models, but not in others (2, 8). Because efficacy in these models was not associated with a reduction in tumor MVD, an alternative mechanism involving vascular normalization has been proposed (8). In addition, it has been recently reported that an anti-human PlGF Mab inhibits growth of DangG and MDA-MB-435 xenografts (8), although the mechanism remained unknown. These observations prompted us to revisit the role of PlGF in human tumor xenograft models. This issue is particularly timely given the ongoing evaluation of anti-PlGF therapy in clinical trials.
Results
Efficacy of Anti-PlGF Antibody Treatment Correlates with VEGFR-1 Expression in Tumor Cells.
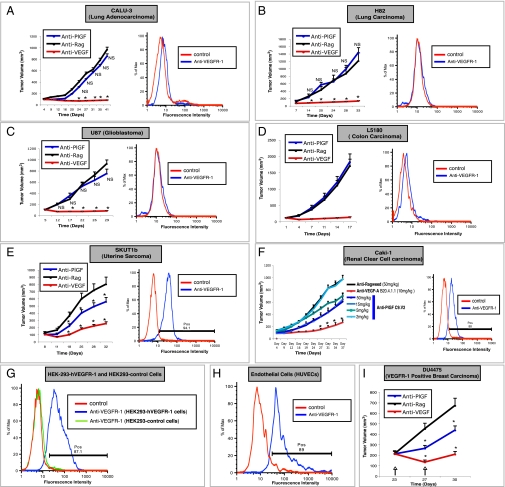
As a first step, we sought to identify cell lines that are growth inhibited by anti-PlGF treatment. To this end, we tested the ability of the validated anti-human and mouse cross-reactive anti-PlGF mAb C9.V2 (18), hereafter referred to as anti-PlGF, to inhibit growth of CALU3, H82, U87, SW480, A549, H1299, L5180, LXFL529, H460, SKUT1b, and CAKI1 tumors (SI Materials and Methods). Consistent with previous findings (18), most models evaluated (9 of 11) did not show any growth inhibition (Fig. 1 A–D, Left, and S1 A–E). However, anti-PlGF treatment significantly reduced the growth of SKUT1b (Fig. 1E, Left) and CAKi1 (Fig. 1F, Left) tumors in a dose-dependent manner. In contrast, all tumor models tested were growth inhibited by anti-VEGF-A treatment (Fig. 1 A–F and Fig. S1 A–E, Left, red line). Together, these data suggest that anti-PlGF mAb treatment does not result in broad inhibition of tumor-angiogenesis and that the effects are tumor model specific. However, PlGF is expressed in both anti-PlGF responsive and refractory tumor models (7, 8, 18) (Fig. S2). We hypothesized that VEGFR-1 expression in tumor cells (3, 4, 22) might be a potential mechanism conferring such model-specific sensitivity to anti-PlGF treatment. In agreement with this hypothesis, we found that VEGFR-1 is expressed in the anti-PlGF sensitive cell lines CAKI1 and SKUT1b (Fig. 1 E and F, Right), but it is undetectable in anti-PlGF resistant tumor cells (Fig. 1 A–D and Fig. S1, Right). Figure 1 G and H shows that VEGFR-1 expression was detected by flow cytometry (SI Materials and Methods) in the positive controls [human umbilical vein endothelial cells (HUVECs) and HEK293-hVEGFR-1] but not in HEK293-empty vector (negative control) cells. Next, we sought to determine whether neutralization of PlGF might be sufficient to inhibit growth of tumors known to be dependent on VEGFR-1 signaling within tumor cells. To this end, we took advantage of DU4475, a VEGFR1-positive breast carcinoma cell line previously shown be sensitive to anti-hVEGFR-1 mAb treatment (4). Figure 1I shows that anti-PlGF mAb treatment inhibits growth of established DU4475 orthotopic tumors. Thus, PlGF blockade can inhibit growth of xenografts dependent on VEGFR-1 signaling and, at least among the models evaluated in this study, efficacy of anti-PlGF antibody treatment strictly correlates with VEGFR-1 expression in tumor cells.
Fig. 1.
Inhibition of tumor growth by Anti-PlGF mAb treatment is restricted to VEGFR-1 positive xenografts. (A–F, Left) Effects of anti-PlGF mAb C9.V2 on primary growth of human tumor xenografts. (F) Dose-dependent inhibition of Caki-1 tumor growth by anti-PlGF C9.V2 Mab. (A–F, Right) Analysis of VEGFR-1 expression in tumor cells. Tumor cells were incubated with biotinylated anti-VEGFR-1 mAb (blue) and or with Streptavidin-PE only as a control (red) as indicated. VEGFR-1 expression was analyzed by flow cytometry. Positive (pos) indicates the calculated percentage of positive cells. (G and H) Flow cytometry VEGFR-1 positive and negative controls. (G) HEK293-VEGFR-1 cells (blue) are VEGFR-1 positive and HEK293-empty vector (green) are VEGFR-1 negative. (H) Endothelial cells (HUVECs) are VEGFR-1 positive (blue). (I) Anti-PlGF inhibits growth of established DU4475 orthotopic breast carcinoma xenografts. Anti-PlGF or anti-Ragweed mAb was given at 15 mg/kg. Anti-VEGF-A mAb was given at 10 mg/kg. All antibody treatments were administrated biweekly. n = 10–15, *P < 0.05 relative to anti-ragweed treatment. Error bars represent SEM.
Efficacy of Anti-PlGF Mabs Is Not Mediated by Antiangiogenesis.
To determine whether efficacy of anti-PlGF mAb treatment is mediated by inhibition of angiogenesis, we quantified MVD (CD31 positive vessels) in sections from DU4475, CAKI1, and SKUT1b tumors at the end-point of the studies (SI Materials and Methods). In contrast to anti-VEGF mAb, anti-PlGF treatment did not cause a significant reduction in tumor vasculature (Fig. S3 A–C). We also wished to evaluate any potential antiangiogenic effects of PlGF Mab in short-term studies. We treated mice bearing exponentially growing tumors of ∼400 mm3 with anti-PlGF, anti-VEGF, or control antibodies for 48 h. CD31 IHC analyses of these tumor tissues showed that anti-PlGF did not cause a reduction in MVD. In contrast, anti-VEGF Mab treatment induced a marked reduction in the number of CD31 positive vessels in SKUT1b tumors (Fig. S3D, Upper). Furthermore, qRT-PCR analyses confirmed that the expression of the transcripts for the pan-vascular markers CD31, VE-cadherin, and MCAM were significantly reduced upon VEGF blockade in SKUT1b. However, anti-PlGF treatment did not decrease the relative mRNA expression levels in any of the vascular markers tested (Fig. S3D, Bottom).
hPlGF Induces Biological Responses in Anti-PlGF Sensitive (VEGFR-1 Positive) Tumor Cells but Not in Endothelial Cells.
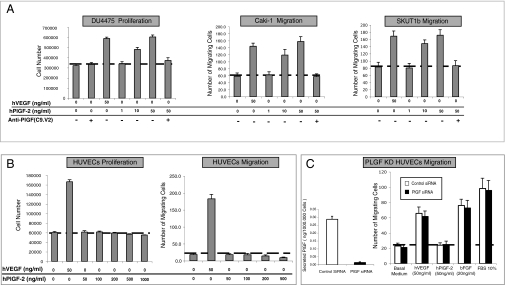
We tested the ability of anti-PlGF sensitive tumor cell lines and endothelial cells to respond to VEGFR-1 stimulation in vitro (SI Materials and Methods). We did not observe any responses to PlGF in anti-PlGF refractory (VEGFR-1 negative) tumor cells (Fig. S4). In contrast, anti-PlGF sensitive tumor cell lines proliferated (DU4475, SKUT1b) and migrated (CAKi1 and SKUT1b cells) in response to hPlGF-2 (or hVEGF-A) in a dose-dependent manner (Figs. 2A and 4D). Figure 2A also shows that anti-PlGF Mab blocked PlGF-induced responses in tumor cells. We also evaluated the responses of endothelial cells (HUVECs) to hPlGF-2 and VEGF-A. In agreement with previous reports, HUVECs responded to VEGF-A but did not show any obvious responses to PlGF in migration (Fig. 2B, Right) and proliferation (Fig. 2B, Left) assays. It has been postulated that endothelial cells do not respond in vitro to exogenous PlGF because they express high levels of endogenous PlGF (13, 23). To test this possibility, we performed PlGF knock-down in HUVECs (SI Materials and Methods). Figure 2C (Left) shows that PlGF knock-down reduces PlGF release by more than 90%. However, HUVECs remained unresponsive to hPlGF-2 but were fully responsive to VEGF-A, bFGF, or FBS (Fig. 2C, Right).
Fig. 2.
Anti-PlGF tumor sensitive tumor-cell lines but not endothelial cells respond to hPlGF-2 stimulation. (A) hPlGF-2 induces dose-dependent biological effects in the anti-PlGF sensitive tumor cell lines DU4475 (Left), CAKI-1 (Center), and SKUT1b (Right), and these effects are blocked by anti-PlGF mAb. (B) hPlGF-2 fails to stimulate HUVEC proliferation (Left) and migration (Right) at all doses tested. (C, Left) Quantification by ELISA of hPlGF released by PlGF knock-down (KD) or control HUVECs. (C, Right) PlGF knock-down (blue bars) and siRNA control HUVECs (green bars) remain unresponsive to hPlGF-2. The figure shows the average values from representative experiments. Doses of ligands are indicated in the figure. Dotted lines represent basal (control) activity. Experiments were repeated at least three times with comparable results. n = 3–5. Error bars represent SD.
Fig. 4.
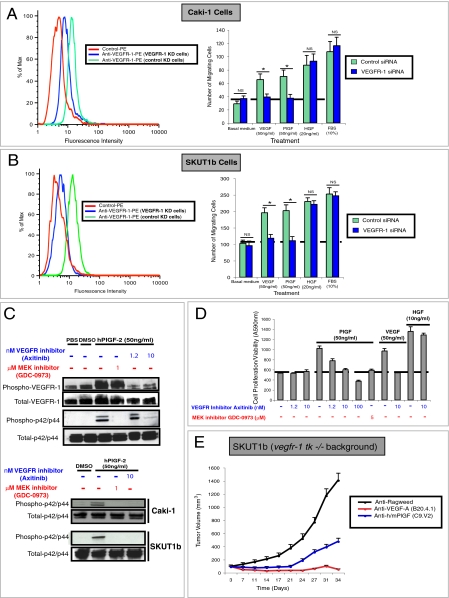
Inhibition of PlGF/VEGFR-1 signaling in tumor but not stromal cells is a major determinant for anti-PlGF efficacy. (A and B, Left) VEGFR-1 siRNA but not control siRNA reduces VEGFR-1 expression by FACS. (A and B, Right) Effects of VEGFR-1 knock-down on migration of Caki-1 and SKUT1b cells in response to PlGF, VEGF, HGF, or 10% FBS. (C, Upper) Effects of axitinib (VEGFR inhibitor) and GDC-0973 on hPlGF-2-induced phosphorylation of VEGFR-1 and p42/p44 in HEK293-VEGFR-1 cells. (C, Lower) Effects of axinitinib and GDC-0973 on hPlGF-2-induced phosphorylation of p42/p44 in HEK-293-CAKI-1 and SKUT1b cells. (D) Effects of axinitinib and GDC-0973 on hPlGF-2-induced proliferation/survival of SKUT1b cells. Dotted lines represent basal levels of migration or proliferation. Experiments were repeated at least three times with comparable results. n = 3–5. Error bars represent SD. (E) Effects of anti-PlGF, anti-VEGF-A, or anti-ragweed mAb on the growth of tumors implanted in vegfr-1 tk−/−, rag2−/− mice. Antibodies were administered as indicated in Fig. 1 and in Materials and Methods. n = 10, relative to anti-ragweed treatment. Error bars represent SEM.
Activation of the Mitogen-Activated Protein Kinase (MAPK) Pathway Is Required for PlGF-Induced Biological Responses in Anti-PlGF Sensitive Tumor Cells.
Previous studies have shown that the (MAPK) and PI3K pathways are activated in response to ligand stimulation in some cell lines overexpressing VEGFR-1 (18, 24).
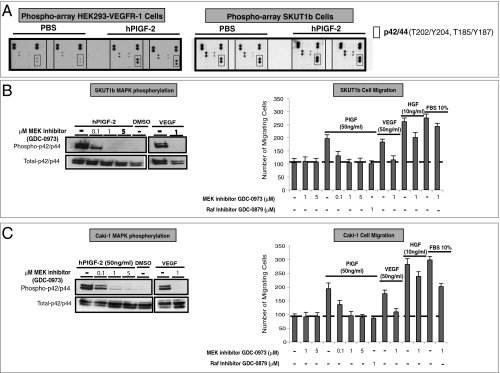
To gain further insights into PlGF/VEGFR-1 signaling in tumor cells, we first performed phospho-kinase antibody array experiments with cell lysates from hPlGF-2 or mock stimulated HEK293-VEGFR-1 cells (SI Materials and Methods). Figure 3A (Left) shows that p42/p44 was activated by PlGF stimulation. No significant differences in phosphorylation of protein kinase B (PKB/AKT) or other proteins included in this array were apparent. Nearly identical results were obtained when lysates from the VEGFR-1 positive uterine sarcoma cell line SKUT1b were analyzed (Fig. 3A, Right). MAPK activation by PlGF was confirmed by Western blot in both SKUT1b (Fig. 3B, Left, and Fig. S5A) and CAKI1 (Fig. 3C, Left, and Fig. S5A, Right). We next used MAPK pathway inhibitors to investigate whether MAPK activation is required for PlGF-induced migration and proliferation. Figure 3 B and C (Left) shows that the MEK inhibitor GDC-0973/XL-518 (US patent 20110086837) (25) efficiently blocks PlGF-induced MAPK phosphorylation without affecting cell viability (Fig. 3 B and C, Right, and Fig. S5C). In addition, GDC-0973 and the RAF inhibitor GDC-0879 (26) (Fig. 3 B and C, right panels), but not Rac, JNK (SP600125), or Rho inhibitors (Fig. S5B), completely suppressed PlGF-responses. However, they only slightly reduced HGF- or FBS-induced CAKi1 and SKUT1b migration and SKUT1b survival/proliferation (Fig. 3 B and C, Right, and Fig. 4D). Interestingly, the dose-dependent inhibition of PlGF-induced MAPK phosphorylation by GDC-0973 parallels the inhibition of migration and proliferation induced by this agent (Fig. 3 B and C).
Fig. 3.
hPlGF-2-induced responses in anti-PlGF sensitive cell lines require MAPK activation. (A) Phospho-antibody array analyses of hPlGF or mock-stimulated HEK293-VEGFR-1 (Left) and SKUT1b (Right) cells. The figure shows only a relevant section of phopho-array membrane. (B, Left) Effects of MEK inhibitor GDC-0973 on PlGF-induced MAPK phosphorylation in SKUT1b cells. (B, Right) Effects of GDC-0973 or RAF inhibitor (GDC-0879) on PlGF-induced SKUT1b cell migration. (C, Left) Effect of MEK inhibitor on PlGF-induced MAPK phosphorylation in CAKI-1 cells. (C, Right) Effects of MEK inhibitor and RAF inhibitor on PlGF-induced Caki-1 cell migration. Dotted lines represent basal (control) activity. Experiments were repeated at least three times with comparable results. n = 3–5. Error bars represent SD.
Inhibition of PlGF/VEGFR-1 Signaling In Tumor but Not Stromal Cells Is a Major Determinant for Anti-PlGF Efficacy.
To confirm the role of VEGFR-1 in PlGF-induced responses in anti-PlGF sensitive tumor cell lines, we knocked-down VEGFR-1 in CAKI1 and SKUT1b cells using siRNA oligonucleotides (SI Materials and Methods). Figure 4 A and B (Left) shows that VEGFR-1 siRNA but not control siRNA markedly decreases VEGFR-1 expression in both cell lines. VEGFR-1 knock-down also suppressed the ability of these cells to migrate in response to PlGF or VEGF-A but did not affect their ability to respond to HGF or 10% FBS (Fig. 4 A and B, Right). Consistent with these findings, VEGFR-1 depletion with a different siRNA oligonucleotide sequence (VEGFR-1 SiRNA no. 2; Fig. S6A) also specifically inhibited VEGF- and PlGF-induced responses. We found that although PlGF strongly induced tyrosine phosphorylation in HEK293 cells overexpressing hVEGFR-1 (Fig. 4C), it barely affected VEGFR-1 phosphorylation in CAKI1 or SKUT1b (Fig. S5A). This result was not unexpected, because ligand-dependent tyrosine phosphorylation of VEGFR-1 is known to be very low (or undetectable) in cells endogenously expressing this receptor (27–29). To test the potential relevance of tyrosine phosphorylation in the activation of PlGF/VEGFR-1 downstream signaling, we used the VEGFR tyrosine kinase inhibitor axitinib (30). Figure 4C shows that the MEK inhibitor GDC-0973 specifically inhibits MAPK but not VEGFR-1 phosphorylation in HEK293-VEGFR-1 cells. However, axitinib inhibited both PlGF-induced phosphorylation of VEGFR-1 and downstream MAPK activation in a dose-dependent manner. Similar to anti-PlGF mAb (18) (Fig. 2A and Fig. S6C) and MEK inhibitors (Fig. 3 B and C and Fig. S6B), axitinib inhibited hPlGF-induced signal transduction (Fig. 4C), SKUT1b cell survival/proliferation (Fig. 4D) and migration of CAKI1 and SKUT1b cells (Fig. S6B). These findings indicate that VEGFR-1 expression and phosphorylation are required for PlGF-induced biological responses in anti-PlGF sensitive tumor cells.
It has been postulated that anti-PlGF efficacy, in the absence of MVD changes, is due to normalization of the vasculature as a consequence of reduced infiltration of VEGFR-1 positive tumor-associated macrophages (TAMs) (8). To probe whether tumor growth inhibition by anti-PlGF indeed requires inhibition of VEGFR-1 signaling in TAMs, hematopoietic stem cells, or other stromal cells, we implanted SKUT1B anti-PlGF sensitive tumor cells in vegfr-1 tk −/−, rag2−/− mice (6). Because these mice express a VEGFR-1 mutant that lacks most of its intracellular domain (including the tyrosine kinase domain), PlGF should be unable to activate VEGFR-1 signaling in host (murine) cells. Figure 4E shows that implantation of SKUT1b cells in vegfr-1 tk−/− does not impair the ability of anti-PlGF to inhibit tumor growth. Similarly, Fig. S6D shows that anti-PlGF treatment has comparable effects on Caki-1 tumor growth in rag2−/− or vegfr-1 tk−/− vs. rag2−/−, vegfr-1+/+ mice. These data indicate that anti-PlGF efficacy is mediated by blockade of PlGF/hVEGFR-1 signaling in the tumor cells but not by inhibition of VEGFR-1 signaling in host cells.
Discussion
Anti-PlGF therapy is currently being evaluated in clinical trials. Nevertheless, the significance of PlGF as a therapeutic target remains incompletely understood.
Recent studies suggest that PlGF inhibition reduces tumor growth and angiogenesis by decreasing recruitment of macrophages in tumor tissue (7). However, subsequent reports revealed that inhibition of PlGF-induced signaling does not necessarily inhibit tumor growth, nor does it correlate with pruning of tumor vessels (8). It has been also hypothesized that the efficacy of PlGF inhibition, in the absence of a significant reduction in tumor MVD, is mediated by vascular normalization following reduced TAM infiltration (8, 18). However, this hypothesis does not fully explain the lack of broad antitumor efficacy and the model-dependent efficacy of PlGF inhibition.
Although VEGFR-1 has previously been shown to be expressed in some tumor cells (2–4), the possibility that VEGFR-1 expression may confer sensitivity to PlGF inhibition was not previously investigated. It is interesting to note that of the 12 murine tumor models we recently evaluated (18), inhibition of primary tumor growth by anti-PlGF treatment was restricted to a cell line engineered to overexpress VEGFR-1. Here, we identified three untransfected human tumor cell lines (CAKI1, SKUT1b, and DU4475) sensitive to PlGF neutralization. Remarkably, all anti-PlGF sensitive tumor cell lines identified in the present study were found to be VEGFR-1 positive. Conversely, all anti-PlGF resistant cell lines were VEGFR-1 negative. These data suggest that blockade of PlGF/VEGFR-1 signaling in tumor cells may be required for anti-PlGF mAb efficacy.
Importantly, no decreases in MVD were observed in the sensitive models, suggesting that efficacy is not mediated by antiangiogenesis. Consistent with these findings, in vitro experiments indicate that anti-PlGF sensitive tumor cells lines, unlike anti-PlGF refractory tumor cells or endothelial cells, respond to PlGF stimulation via VEGFR-1 signaling activation. The divergent ability of VEGFR-1 positive tumor cells and vascular endothelial cells to respond to VEGFR-1 ligand stimulation is puzzling. However, it is consistent with previous reports (10, 31) and also with genetic data indicating that, at least during embryonic angiogenesis, endothelial VEGFR-1 acts mainly as a nonsignaling decoy (6, 10). Although it has been proposed that the lack of responsiveness of endothelial cells to PlGF reflects VEGFR-1 occupation due to high levels of endogenous PlGF, our PlGF knock-down experiment argues against this possibility. Further studies are required to elucidate the mechanisms underlying such cell type-dependent responses.
Implantation of anti-PlGF responsive tumors in vegfr-1 tk−/−, rag2−/− mice did not affect the efficacy of anti-PlGF treatment, indicating that VEGFR-1 signaling in stromal cells is not required for the protumor effects of PlGF. Although the data presented here indicate that inhibition of PlGF/VEGFR-1 signaling in tumor cells is a major mechanism underlying anti-PlGF efficacy, alternative mechanisms may be important in other models. In this context, it is tempting to speculate that the efficacy of anti-PlGF (8) or anti-VEGFR-1 mAbs (32) in hepatocellular carcinoma models involves, at least in part, inhibition of release of paracrine growth factors from sinusoidal endothelial cells (e.g., hepatocyte growth factor or IL-6), which has been previously shown to be regulated by endothelial VEGFR-1 (33).
We believe that our findings not only underscore an important and potentially clinically relevant mechanism of action of PlGF Mab, but may also help reconcile conflicting data in the literature. Indeed, the recently reported ability of an anti-human PlGF Mab (8) to reduce MDA-MB-435 tumor growth very likely reflects the presence of a previously described functional VEGFR-1 in these cells (4).
It is presently unclear whether the apparent higher incidence of anti-PlGF efficacy in human xenografts (3 of 15 models tested) compared with the lack of growth inhibition in all 12 murine tumor models truly represents a higher incidence of VEGFR-1 expression/activity in human tumors. In addition, the signaling data we present suggests that the VEGFR-1 pathway contributes to PlGF-induced effects in tumor cells mainly through MAPK activation. Thus, VEGFR-1 expression/activity may provide a selective growth advantage to tumors that are highly dependent on Ras/Raf/MAPK signaling. In this context it is interesting that VEGFR-1 signaling within tumor cells previously has been shown to modulate growth and survival of several Ras/MAPK pathway-driven mouse tumor models and cell lines (3, 34). Growing evidence also supports a possible role for VEGFR-1 signaling in certain human cancers. In vitro studies suggested a role for VEGFR-1 signaling in survival of colorectal and pancreatic cancer cell lines during epithelial to mesenchymal transition (22, 35–37). Also, VEGFR-1 signaling is required for growth of patient-derived malignant melanoma-initiating human cells in mice (38), and anti-hVEGFR-1 mAb treatment increases the survival of mice injected with acute lymphoblastic leukemia cells (39) and also inhibits tumor growth of VEGFR-1 positive breast carcinoma and melanoma xenografts (4). Furthermore, expression of VEGFR-1 in tumor cells has been observed in human biopsies (3, 40, 41). Finally, mutations in VEGFR-1 have been found in human cancers, including ∼10% of melanomas (42).
In conclusion, we show that, among the models we tested, efficacy of anti-PlGF mAb treatment is limited to VEGFR-1 expressing tumors, because it requires inhibition of PlGF/VEGFR-1 signaling within tumor cells. These findings may be relevant in the context of ongoing clinical evaluation of anti-PlGF (43), anti-VEGFR-1 (44) Mabs, VEGF-Trap (45), and other VEGFR inhibitor therapies. It is tempting to speculate that VEGFR-1 expression/activity may be a biomarker to select patients and indications likely to benefit from anti-PlGF therapies.
Materials and Methods
Animals and Cell Lines.
Female Beige nude and BALB/c nude mice were obtained from Charles River. RAG2−/− mice were from Jackson Laboratories. flt1 tk−/− mice were generated as described (6). flt-1 tk, rag-2 double ko mice were generated by crossing Flt-1 tk −/− with with rag2−/− mice.
Tumor cell lines were obtained from the ATCC. Tumor cells were maintained in RPMI-1640 containing 10% FBS (Sigma, Sigma-Aldrich), penicillin (100 units/mL), streptomycin (100 μg/mL), and l-glutamine (2 mmol/L). Hek293 cells were cultured in DMEM supplemented with 10% FBS (Sigma, Sigma-Aldrich), l-glutamine (2 mmol/L), and puromycin (1 μg/mL). Primary HUVEC were purchased from Lonza and maintained in EGM-2 medium (Lonza). Only low-passage HUVECs were used in our experiments. All cells were cultured at 37 °C in a humidified incubator containing 5% CO2. Hek293-hVEGFR-1 and HEK293-control cell lines were generated by transfection followed by puromycin selection.
Supplementary Material
Acknowledgments
We thank the Genentech animal facility and the protein purification and antibody technology groups. We also thank H. Koeppen for histopathological analysis and L. Gilmour, R. Neupane, and C.P. Poon from the FACS lab for excellent support.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109029108/-/DCSupplemental.
References
- 1.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 2.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenberger BM, et al. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–279. doi: 10.1016/j.cell.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, et al. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer. 2006;119:1519–1529. doi: 10.1002/ijc.21865. [DOI] [PubMed] [Google Scholar]
- 5.Sawano A, Takahashi T, Yamaguchi S, Aonuma M, Shibuya M. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differ. 1996;7:213–221. [PubMed] [Google Scholar]
- 6.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer C, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 8.Van de Veire S, et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell. 2010;141:178–190. doi: 10.1016/j.cell.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 9.Muramatsu M, Yamamoto S, Osawa T, Shibuya M. Vascular endothelial growth factor receptor-1 signaling promotes mobilization of macrophage lineage cells from bone marrow and stimulates solid tumor growth. Cancer Res. 2010;70:8211–8221. doi: 10.1158/0008-5472.CAN-10-0202. [DOI] [PubMed] [Google Scholar]
- 10.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- 11.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci USA. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2:re1. doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 14.Hiratsuka S, et al. Involvement of Flt-1 tyrosine kinase (vascular endothelial growth factor receptor-1) in pathological angiogenesis. Cancer Res. 2001;61:1207–1213. [PubMed] [Google Scholar]
- 15.Marcellini M, et al. Increased melanoma growth and metastasis spreading in mice overexpressing placenta growth factor. Am J Pathol. 2006;169:643–654. doi: 10.2353/ajpath.2006.051041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schomber T, et al. Placental growth factor-1 attenuates vascular endothelial growth factor-A-dependent tumor angiogenesis during beta cell carcinogenesis. Cancer Res. 2007;67:10840–10848. doi: 10.1158/0008-5472.CAN-07-1034. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson A, et al. Placenta growth factor-1 antagonizes VEGF-induced angiogenesis and tumor growth by the formation of functionally inactive PlGF-1/VEGF heterodimers. Cancer Cell. 2002;1:99–108. doi: 10.1016/s1535-6108(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 18.Bais C, et al. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell. 2010;141:166–177. doi: 10.1016/j.cell.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 19.Hiratsuka S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cianfarani F, et al. Placenta growth factor in diabetic wound healing: altered expression and therapeutic potential. Am J Pathol. 2006;169:1167–1182. doi: 10.2353/ajpath.2006.051314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bates RC, et al. Flt-1-dependent survival characterizes the epithelial-mesenchymal transition of colonic organoids. Curr Biol. 2003;13:1721–1727. doi: 10.1016/j.cub.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Autiero M, Luttun A, Tjwa M, Carmeliet P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost. 2003;1:1356–1370. doi: 10.1046/j.1538-7836.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, et al. RACK1 regulates VEGF/Flt1-mediated cell migration via activation of a PI3-K/Akt pathway. J Biol Chem. doi: 10.1074/jbc.M110.165605. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frémin C, Meloche S. From basic research to clinical development of MEK1/2 inhibitors for cancer therapy. J Hematol Oncol. 2010;3:8. doi: 10.1186/1756-8722-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoeflich KP, et al. Antitumor efficacy of the novel RAF inhibitor GDC-0879 is predicted by BRAFV600E mutational status and sustained extracellular signal-regulated kinase/mitogen-activated protein kinase pathway suppression. Cancer Res. 2009;69:3042–3051. doi: 10.1158/0008-5472.CAN-08-3563. [DOI] [PubMed] [Google Scholar]
- 27.Seetharam L, et al. A unique signal transduction from FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene. 1995;10:135–147. [PubMed] [Google Scholar]
- 28.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 29.Gille H, et al. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276:3222–3230. doi: 10.1074/jbc.M002016200. [DOI] [PubMed] [Google Scholar]
- 30.Kindler HL, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 31.Cao Y, et al. Heterodimers of placenta growth factor/vascular endothelial growth factor. Endothelial activity, tumor cell expression, and high affinity binding to Flk-1/KDR. J Biol Chem. 1996;271:3154–3162. doi: 10.1074/jbc.271.6.3154. [DOI] [PubMed] [Google Scholar]
- 32.Yoshiji H, et al. Halting the interaction between vascular endothelial growth factor and its receptors attenuates liver carcinogenesis in mice. Hepatology. 2004;39:1517–1524. doi: 10.1002/hep.20218. [DOI] [PubMed] [Google Scholar]
- 33.LeCouter J, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 34.Bianco R, et al. Vascular endothelial growth factor receptor-1 contributes to resistance to anti-epidermal growth factor receptor drugs in human cancer cells. Clin Cancer Res. 2008;14:5069–5080. doi: 10.1158/1078-0432.CCR-07-4905. [DOI] [PubMed] [Google Scholar]
- 35.Fan F, et al. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene. 2005;24:2647–2653. doi: 10.1038/sj.onc.1208246. [DOI] [PubMed] [Google Scholar]
- 36.Lesslie DP, et al. Vascular endothelial growth factor receptor-1 mediates migration of human colorectal carcinoma cells by activation of Src family kinases. Br J Cancer. 2006;94:1710–1717. doi: 10.1038/sj.bjc.6603143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wey JS, et al. Vascular endothelial growth factor receptor-1 promotes migration and invasion in pancreatic carcinoma cell lines. Cancer. 2005;104:427–438. doi: 10.1002/cncr.21145. [DOI] [PubMed] [Google Scholar]
- 38.Frank NY, et al. VEGFR-1 expressed by malignant melanoma initiating cells is required for tumor growth. Cancer Res. doi: 10.1158/0008-5472.CAN-10-1660. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fragoso R, et al. VEGFR-1 (FLT-1) activation modulates acute lymphoblastic leukemia localization and survival within the bone marrow, determining the onset of extramedullary disease. Blood. 2006;107:1608–1616. doi: 10.1182/blood-2005-06-2530. [DOI] [PubMed] [Google Scholar]
- 40.Chung GG, et al. Vascular endothelial growth factor, FLT-1, and FLK-1 analysis in a pancreatic cancer tissue microarray. Cancer. 2006;106:1677–1684. doi: 10.1002/cncr.21783. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh S, et al. High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Hum Pathol. 2008;39:1835–1843. doi: 10.1016/j.humpath.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prickett TD, et al. Analysis of the tyrosine kinome in melanoma reveals recurrent mutations in ERBB4. Nat Genet. 2009;41:1127–1132. doi: 10.1038/ng.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lassen U, et al. A phase I dose escalation study of TB-403, a monoclonal antibody directed against PlGF, in patients with solid tumors. Mol Cancer Ther. 2009;8(12 Suppl):A111. doi: 10.1038/bjc.2011.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz JD, Rowinsky EK, Youssoufian H, Pytowski B, Wu Y. Vascular endothelial growth factor receptor-1 in human cancer: concise review and rationale for development of IMC-18F1 (Human antibody targeting vascular endothelial growth factor receptor-1) Cancer. 2010;116(4 Suppl):1027–1032. doi: 10.1002/cncr.24789. [DOI] [PubMed] [Google Scholar]
- 45.Lockhart AC, et al. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol. 2010;28:207–214. doi: 10.1200/JCO.2009.22.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.