Abstract
All four stereoisomers (7–10) of ethyl undeca-2,4-dienoate were prepared in ≥98% isomeric purity by Pd-catalyzed alkenylation (Negishi coupling) using ethyl (E)- and (Z)-β-bromoacrylates. Although the stereoisomeric purity of the 2Z,4E-isomer (8) prepared by Suzuki coupling using conventional alkoxide and carbonate bases was ≤ 95%, as reported earlier, the use of CsF or nBu4NF as a promoter base has now been found to give all of 7–10 in ≥98% selectivity. Other widely known methods reveal considerable limitations. Heck alkenylation was satisfactory for the syntheses of the 2E,4E and 2E,4Z isomers of ≥98% purity, but the purity of the 2Z,4E isomer was ≤ 95%. Mutually complementary Horner–Wadsworth–Emmons and Still–Gennari (SG) olefinations are also of considerably limited scopes. Neither 2E,4Z nor 2Z,4Z isomer is readily prepared in ≥90% selectivity. In addition to (2Z,4E)-dienoic esters, some (2Z,4E,6E)- and (2Z,4E,6Z)-trienoic esters have been prepared in ≥98% selectivity by a newly devised Pd-catalyzed alkenylation–SG olefination tandem process. As models for conjugated higher oligoenoic esters, all eight stereoisomers for ethyl trideca-2,4,6-trienoate (23–30) have been prepared in ≥98% overall selectivity.
Keywords: highly selective synthesis, Pd-catalyzed Negishi coupling
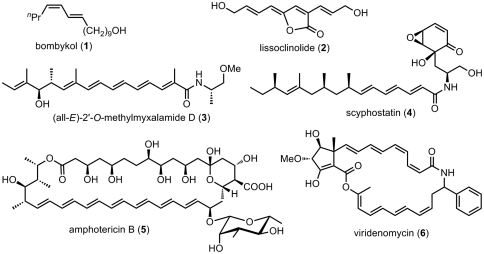
Alkenes represent a large number of natural products of bioactive and medicinally important compounds. Of interest in this study are those containing conjugated dienes and oligoenes (1–11) (Fig. 1). Among numerous known methods for the syntheses of alkenes including conjugated dienes and oligoenes (12, 13), carbonyl olefination reactions, notably Wittig olefination (14) and its variants, such as Horner–Wadsworth–Emmons (HWE hereafter) (15, 16), and Still–Gennari (SG hereafter) (17) as well as Ando (18) modifications have been widely used along with related Si-promoted (Peterson) (19) and S-promoted (Julia and Paris) (20) olefination reactions. As useful as these reactions are, they often lack very high (≥98%) stereoselectivity.
Fig. 1.
Some naturally occurring conjugated di- and oligoenes of biological and medicinal importance.
In search for highly (≥98%) stereoselective alkene synthesis methodology, our attention was initially drawn to alkyne hydroboration, which generally proceeds in > 99% syn-selectivity. Following some promising leads of Brown (21) and Zweifel and co-workers (22–25), we reported in 1973 a couple of highly selective routes to 1,4-disubstituted (E,E)- and (E,Z)-1,3-dienes (1, 3, 26), which appear to represent the earliest syntheses of unsymmetrically substituted conjugated dienes in very high (≥97%) selectivity via organometallic alkenylation.
Despite highly selective and satisfactory results described above, our continued search for a more generally applicable and straightforward route to conjugated di- and oligoenes led to the discovery in 1976 of the Pd-catalyzed alkenyl–alkenyl coupling with alkenylalanes (27) and subsequently with alkenyl derivatives containing Zr (28) and Zn (29), collectively known as Negishi coupling (30, 31). Along with the subsequently developed alkenylboron version (Suzuki coupling) (32–34), the Pd-catalyzed alkenyl–alkenyl coupling (35) has collectively begun establishing itself as the modern “cornerstone” of alkene and oligoene synthesis.
Over the past few decades, alkyne elementometalation reactions (36), i.e., addition of element–metal bonds to alkynes, including hydrometalation, carbometalation, and halometalation, has proven to be critically important in the syntheses of alkene-containing compounds. The ultimate goal of this and related studies in the authors’ group is to be able to synthesize in high yields, efficiently, and selectively (≥98%) all possible types of conjugated di-, tri-, and higher oligoenes. In this study, however, attention is focused on the following three specific aspects: (i) synthesis of all possible stereoisomers of conjugated dienoic and trienoic esters containing (E)- and/or (Z)-1,2-disubstituted alkene units via alkyne elementometalation (36) –Pd-catalyzed cross-coupling of Negishi (Zn, Zr, Al) (27–31) and Suzuki (B) (32–34) versions, (ii) critical comparison of the Pd-catalyzed alkenylation routes with the carbonyl olefination routes represented here by mutually complementary HWE (14–16) and SG (14, 17, 18) olefinations as well as Heck alkenylation (37–39), all of which are known to proceed via π-addition–β-elimination and, (iii) feasibility of synergistic uses of Pd-catalyzed alkenylation and carbonyl olefination.
At this point, some comments on those closely related prior investigations of our own and other workers are in order.
We recently reported highly (≥98%) stereoselective syntheses of conjugated tetra- through heptaenoic esters (40), but this work dealt only with their all-E isomers.
Syntheses of the (2Z,4E) and (2E,4Z) isomers of some conjugated dienes by Negishi (27, 35) and Suzuki (32, 33, 35, 41) alkenylations are known. Whereas Negishi coupling has uniformly led to ≥97–98% stereoselectivity, Suzuki reported stereo scrambling to varying extents (≥5%) for the synthesis of ethyl (2Z,4E)-nona-2,4-dienoate (41). More recently, however, Molander and co-workers (42, 43) reported highly stereoselective synthesis of all four stereoisomers of conjugated dienes via modified Suzuki coupling, although no 2,4-dienoic esters were prepared.
HWE and SG olefinations and even Heck alkenylation generally require esters and related reaction-promoting groups for satisfactory results, whereas the Pd-catalyzed alkenylation is free from this restriction. For critical comparisons of all of these competing processes for alkene syntheses, di- and oligoenoic esters were chosen as synthetic targets.
In a recent attempt to synthesize all eight stereoisomers of 2,4,6-trienoic esters by Khrimian (44), two of the four isomers containing the (Z)-γ,δ-ethenylidene group, i.e., (2E,4Z,6Z) and (2E,4Z,6E) isomers were prepared in ca. 95% stereoselectivity, but the other two isomers were prepared only as the minor (≤ 5%) by-products of the two mentioned above.
In another recent study, ethyl (2Z,4Z)-5-tributylstannylpenta-2,4-dienoate was obtained only as the minor component of a 1/1.8 mixture of the (2Z,4Z) and (2E,4Z) isomers by SG olefination (45). A more favorable but still impure 3.5/1 mixture of the two isomers were obtained by Ando modification (18) and used in the subsequent Stille coupling for a total synthesis of (+)-sorangicin A (46).
It may be stated on the basis of those presented above that no highly (≥98%) selective route to all eight stereoisomers of conjugated trienoic esters has as yet been developed and that its development is very desirable.
Results and Discussion
Highly (≥98%) Selective Synthesis of All Four Possible Stereosiomers of 2,4-Dienoic Esters by Alkyne Elementometalation–Pd-Catalyzed Alkenyl–Alkenyl Cross-Coupling Tandem Processes.
(a) Negishi coupling.
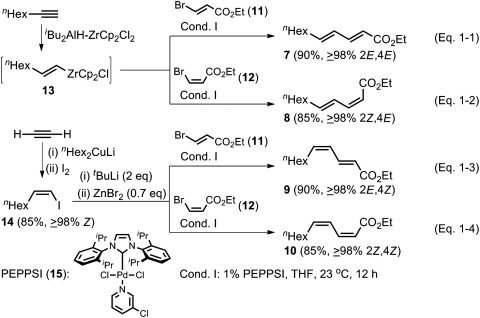
Although highly favorable results were well anticipated, all four stereoisomers of ethyl 2,4-undecadienoate (7–10) were prepared by the Negishi alkenyl–alkenyl coupling primarily to document the yield and stereoselectivity for each of the four possible stereoisomers for providing a set of reference points in critical comparisons of various competing methods.
The experimental results are summarized in Scheme 1. Negishi coupling of (E) and (Z) isomers of ethyl 3-bromoacrylate (11 and 12) (refs. 47 and 48, respectively) with (E)-1-octenylzirconocene chloride (13) generated in situ via hydrozirconation (49) in > 90% yield in THF in the presence of 1 mol % of PEPPSI (15) (pyridine-enhanced precatalyst preparation stabilization and initiation obtained from Aldrich Chemicals Co.) (50) at 23 °C for 12 h provided 7 and 8 of ≥98% isomeric purity in 90 and 85% yields, respectively (Scheme 1, Eqs. 1-1 and 1-2). For the syntheses of the (2E,4Z) and (2Z,4Z) isomers (9 and 10), (Z)-1-octenyl iodide (14) was prepared via carbocupration (51). The desired 9 and 10 were obtained as ≥98% isomerically pure compounds in 90 and 85% yields, respectively (Scheme 1, Eqs. 1-3 and 1-4).
Scheme 1.
Highly selective synthesis of all four possible stereosiomers of 2,4-dienoic esters by alkyne elementometalation–Pd-catalyzed Negishi coupling protocol.
(b) Suzuki coupling.
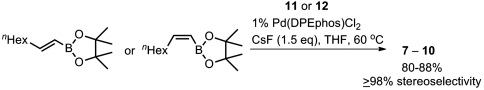
In our recent study on alkyne haloboration-based alkene syntheses (52–54), we have just found that the use of CsF or 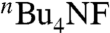 as a base is significantly more effective than commonly used alkoxide and carbonate bases both in promoting Suzuki coupling and suppressing unwanted alkenyl stereo scrambling. We now find the previously undescribed procedure using CsF can suppress stereoisomerization in the synthesis of 7–10 of ≥98% stereoisomeric purity, as summarized in Scheme 2.
as a base is significantly more effective than commonly used alkoxide and carbonate bases both in promoting Suzuki coupling and suppressing unwanted alkenyl stereo scrambling. We now find the previously undescribed procedure using CsF can suppress stereoisomerization in the synthesis of 7–10 of ≥98% stereoisomeric purity, as summarized in Scheme 2.
Scheme 2.
Highly selective synthesis of all four possible stereosiomers of 2,4-dienoic esters by fluoride promoted Suzuki alkenylation.
Critical Analysis of the Scope and Limitations of Heck Alkenylation.
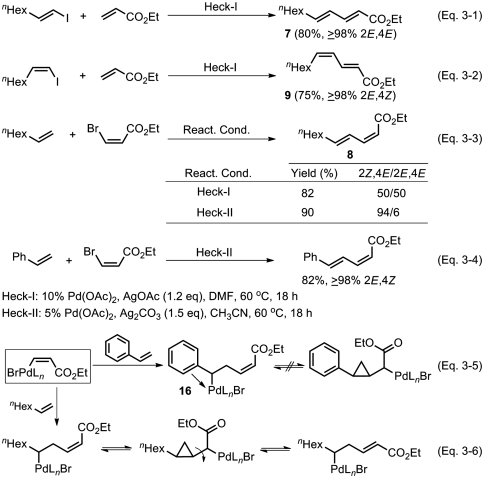
Heck alkenylation (37–39) is fundamentally E selective with respect to nonhalogenated terminal alkenes. Consequently, incorporation of a (Z)-alkenyl group via Heck alkenylation requires the use of stereochemically preset (Z) haloalkenes. It is therefore not practically feasible to prepare conjugated (Z,Z) alkenes by Heck alkenylation. Another main limitation of this reaction is that it is practically limited to those cases where certain types of alkenes, typically some π-conjugated alkenes, including styrenes and α,β-unsaturated carbonyl derivatives, are used (55). With these generalizations in mind, syntheses of 7, 8, and 9 were carried out. As indicated by the results summarized in Scheme 3, both (2E,4E) and (2E,4Z) isomers (7 and 9) can be prepared in good yields and ≥98% stereoselectivity (Scheme 3, Eqs. 3-1 and 3-2), but the synthesis of the (2Z,4E) isomer (8) has been accompanied by stereoisomerization (Scheme 3, Eq. 3-3). We then noted in the literature (56) that the use of styrene as nonhalogenated alkenes is stereoselective, producing the (2Z,4E) isomers in ≥98% stereoselectivity (Scheme 3, Eq. 3-4). Thus, stereoselectivity in these Heck reactions depends not only on reaction conditions but also on alkene structures. The difference between the reactions of styrenes and those of alkyl substituted alkenes may be rationalized by invoking significant π-stabilization of a putative benzylpalladium derivative (16) (Scheme 3, Eq. 3-5), which suppresses stereoisomerization possibly via homoallyl-cyclopropylcarbinyl rearrangement that can readily occur in the absence of such stabilization (Scheme 3, Eq. 3-6).
Scheme 3.
Critical analysis of the scope and limitations of Heck alkenylation.
Critical Analysis of the Current Scope and Limitations of HWE and SG Olefinations for Highly (≥98%) Selective Synthesis of 2,4-Dienoic Esters.
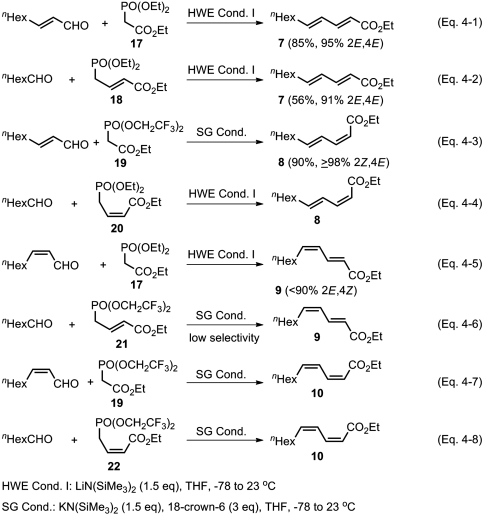
For the synthesis of the four stereoisomers of ethyl undeca-2,4-dienoates (7–10), the following eight HWE and/or SG olefination routes may be considered (Scheme 4).
Scheme 4.
Critical analysis of the current scope and limitations of HWE and SG olefinations for highly selective synthesis of 2,4-dienoic esters.
In reality, however, the following limitations and difficulties are noted in the literature:
(Z)-4-phosphonocrotonic esters (20 and 22) are easily isomerized when they are dealt with bases in HWE or SG conditions. The reactions shown in Scheme 4, Eqs. 4-4 and 4-8, are currently very limited.
Some documented unsatisfactory results (57) indicate that the (Z)-selectivity of SG olefination is practically limited to those cases where (Z)-α,β-alkenyl bonds are formed. Thus, those processes shown in Scheme 4, Eqs. 4-6 and 4-8, must also be considered impractical at present.
As is known, it is cumbersome and difficult to prepare and use (Z)-α,β-unsaturated aldehydes. Thus, for example, ethyl (2E,4Z)-deca-2,4-dienoate (9) was prepared analogously to that shown in Scheme 4, Eq. 4-5, involving fractional distillation, but the overall selectivity was still only 88% 2E,4Z (58).
Although some highly (≥98%) stereoselective examples of HWE reaction are known (15, 16, 57–62), it often displays ≤ 95%, typically 80–90%, stereoselectivity.
With all of these subtle variations and limitations in mind, our attention here is mainly focused on finding and/or confirming hard facts about those 2,4-dienoic ester syntheses shown in Scheme 4, Eqs. 4-1 to 4-3. In contrast with frequently less than highly (≥98%) selective HWE olefination discussed above, SG olefination represented by Scheme 4, Eq. 4-3, appears to be generally ≥98% selective (17). We have indeed confirmed this generalization by converting commercially available (E)-crotonaldehyde (≥98% E, from TCI) and (E)-2-nonenal (97% E, from Aldrich Chemicals Co.) to ≥98% isomerically pure ethyl (2Z,4E)-hexa-2,4-dienoate and ethyl (2Z,4E)-undeca-2,4-dienoate (8), respectively, in nearly quantitative yields by NMR. These favorable results also promise to provide a reliable foundation for highly (≥98%) selective syntheses of various conjugated tri- and higher oligoenoic esters containing the (2Z,4E)-dienoic ester fragment by Pd-catalyzed alkenylation–SG olefination tandem process (vide infra).
Highly (≥98%) Selective Syntheses of All Eight Possible Stereoisomers of 2,4,6-Trienoic Esters.
Despite notable and promising recent efforts (44), all eight possible stereoisomers of conjugated 2,4,6-trienoic esters do not appear to have been prepared in good yields (≥70–80%) and ≥95% selectivity. Accordingly, we decided to accomplish these goals through applications of the knowledge and experience gained in the preceding sections. Specifically, we selected the eight stereoisomers of ethyl trideca-2,4,6-trienoate (23–30) as our target.
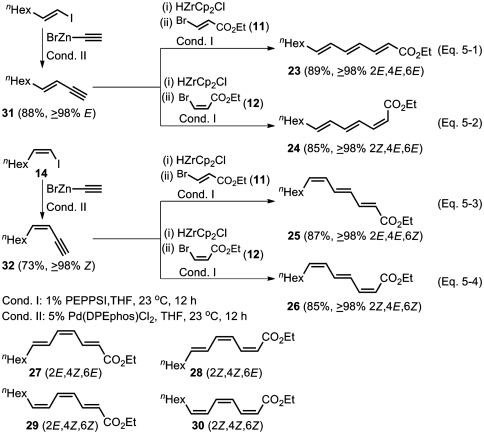
All Four Stereoisomers of Ethyl (4E)-Trideca-2,4,6-Trienoates (23–26).
(a) Alkyne elementometalation–Pd-catalyzed Negishi coupling protocol.
Treatment of (E)-1-octenylzirconocene chloride (13) with iodine in THF provided (E)-1-iodo-1-octene of ≥98% isomeric purity in 90% yield (49), whereas its Z isomer 14 of ≥98% isomeric purity was prepared as described earlier in 85% yield. Pd-catalyzed reactions of (E)- and (Z)-1-iodo-1-octenes with ethynylzinc bromide in THF at 23 °C produced (E)- and (Z)-3-decen-1-ynes (31 and 32) of ≥98% isomeric purity in 88% and 73% yields, respectively. Hydrozirconation (49) of (E)-3-decen-1-yne in THF followed by addition of 11 (1 equivalent) and 1 mol % PEPPSI provided after 12 h at 23 °C the desired ethyl (all-E)-trideca-2,4,6-trienoate (23) (Scheme 5, Eq. 5-1), of ≥98% purity in 89% yield, whereas the use of the (Z)-3-bromoacrylic ester (12) in place of 11 led to the formation of ≥98% pure ethyl (2Z,4E,6E)-trideca-2,4,6-trienoate (24) in 85% yield (Scheme 5, Eq. 5-2). In essentially the same manner, the corresponding (2E,4E,6Z) and (2Z,4E,6Z) isomers (25 and 26) of ≥98% isomeric purity were obtained in 87% and 85% yields, respectively (Scheme 5, Eqs. 5-3 and 5-4).
Scheme 5.
Highly selective syntheses of all four stereoisomers of ethyl (4E)-trideca-2,4,6-trienoates (23–26) by using alkyne elementometalation–Pd-catalyzed Negishi coupling protocol.
(b) Alkyne elementometalation–Pd-catalyzed Negishi coupling–carbonyl olefination protocol.
In view of the exceptionally high (≥98%) and seemingly general stereoselectivity displayed by SG olefination for the synthesis of conjugated (2Z,4E)-di- and oligoenoic esters, it is desirable to further explore the scope and limitations of SG olefination along this line. On the basis of what has been discussed in the preceding sections, combined use of Pd-catalyzed Negishi and/or Suzuki coupling and SG olefination appears particularly attractive. Some prototypical examples of highly (≥98%) selective syntheses of conjugated (2Z,4E)-trienoic esters are presented below.
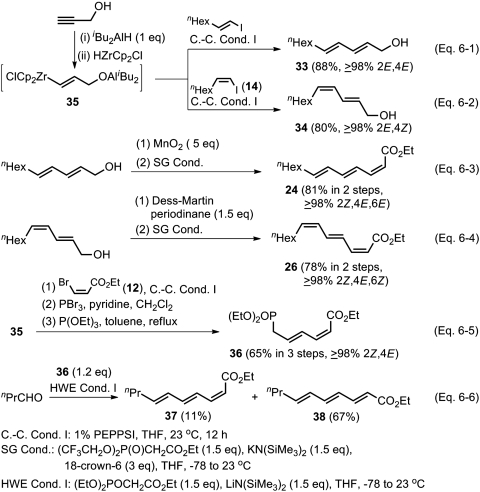
Of the eight stereoisomers of a given conjugated triene, the (2Z,4E,6E) and (2Z,4E,6Z) isomers were thought to be accessible in ≥98% selectivity. To probe the feasibility of this strategy, (2E,4E)- and (2E,4Z)-undeca-2,4-dien-1-ols (33 and 34, respectively) were prepared in one step from (E)- and (Z)-1-iodo-1-octenes by their Negishi coupling catalyzed with 1 mol % of PEPPSI with (1E)-3-diisobutylaluminoxy-1-propenyl-zirconocene chloride (35), generated in situ by successive treatment of propargyl alcohol with 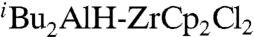 (49) (Scheme 6, Eqs. 6-1 and 6-2). The desired dienols (33 and 34) obtained in ≥98% selectivity and 88% and 80% yields were converted to the corresponding aldehydes in 90% and 91% yields, respectively, by MnO2 or Dess–Martin oxidation. As anticipated, SG olefination of these aldehydes proceeded in excellent yields to produce ≥98% isomerically pure conjugated trienoic esters 24 and 26, respectively, as shown in Scheme 6, Eqs. 6-3 and 6-4. It should be noted that the ≥98% Z geometry of the (Z)-C═C bond of 34 is fully retained in 26. We also attempted earlier to synthesize 37, a close analogue of 24, by an alternate Pd-catalyzed Negishi coupling–HWE olefination route as shown in Scheme 6, Eqs. 6-5 and 6-6. Unfortunately, this reaction led to an extensive stereoisomerization of the α,β-alkenyl group, suggesting that preset (Z)-C═C bond in vinylogous HWE reagents may not be retained in the olefination step.
(49) (Scheme 6, Eqs. 6-1 and 6-2). The desired dienols (33 and 34) obtained in ≥98% selectivity and 88% and 80% yields were converted to the corresponding aldehydes in 90% and 91% yields, respectively, by MnO2 or Dess–Martin oxidation. As anticipated, SG olefination of these aldehydes proceeded in excellent yields to produce ≥98% isomerically pure conjugated trienoic esters 24 and 26, respectively, as shown in Scheme 6, Eqs. 6-3 and 6-4. It should be noted that the ≥98% Z geometry of the (Z)-C═C bond of 34 is fully retained in 26. We also attempted earlier to synthesize 37, a close analogue of 24, by an alternate Pd-catalyzed Negishi coupling–HWE olefination route as shown in Scheme 6, Eqs. 6-5 and 6-6. Unfortunately, this reaction led to an extensive stereoisomerization of the α,β-alkenyl group, suggesting that preset (Z)-C═C bond in vinylogous HWE reagents may not be retained in the olefination step.
Scheme 6.
Highly selective synthesis of 24 and 26 by using alkyne elementomatalation–Pd-catalyzed Negishi coupling–SG olefination protocol.
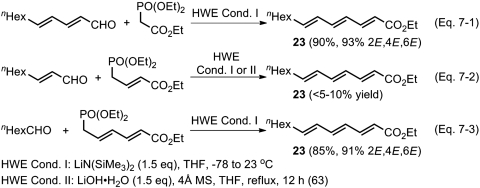
In an early section, we discussed the generally ≤ 98% E selectivity in HWE reaction, the typical E selectivity observed with an alkyl aldehyde being 85–90%. In our recent work on conjugated (all-E)-oligoenoic esters via Pd-catalyzed Negishi coupling–HWE olefination strategy, however, it was confirmed that the use of aryl and α,β-unsaturated aldehydes tended to lead to ≥98% E selectivity (62). Prompted by these results, we examined the synthesis of (all-E)-trienoic ester (23) by three mutually complementary routes. However, the results summarized in Scheme 7 have revealed yet another unexpected difficulty associated with HWE reaction. Those reactions shown in Scheme 7, Eqs. 7-1 and 7-3, proceeded in good yields. In sharp contrast, the reaction shown in Scheme 7, Eq. 7-2 (63), gave the desired product 23 in less than 5–10% yields. This reaction is under further investigation.
Scheme 7.
Critical comparision of the synthesis of (all-E)-trienoic ester (23) by three mutually complementary HWE olefination routes.
All Four Stereoisomers of Ethyl (4Z)-Trideca-2,4,6-Trienoates (27–30).
(a) Alkyne elementometalation–Pd-catalyzed Negishi coupling protocol.
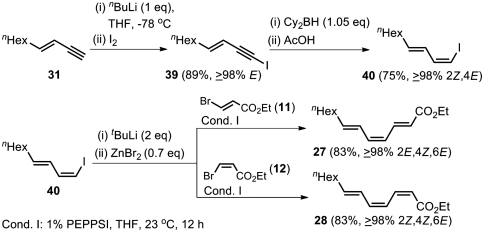
The alkyne elementometalation–Pd-catalyzed Negishi coupling protocol shown in Scheme 5 has provided uniformly satisfactory routes to all four stereoisomers of ethyl (4E)-trideca-2,4,6-trienoates (23–26) in three steps without generating any detectable amounts of stereoisomers or any other isomers. We therefore opted for reporting to the same strategy for the syntheses of 27–30 as well by making use of both (E)- and (Z)-3-decen-1-ynes (31 and 32, respectively) prepared in two steps as shown in Scheme 5. Conversion of 31 into (1Z,3E)-1-iodo-1,3-decadiene (40) was performed in two steps, as shown in Scheme 8, via well-known terminal alkyne iodination (89% yield) and hydroboration with dicyclohexylborane followed by protonolysis with HOAc in THF (75% yield) (64). Once 40 of ≥98% stereoisomeric purity was obtained, its lithiation followed by zincation and Negishi coupling with ethyl (E)- or (Z)-β-bromoacrylate in the presence of 1 mol % of PEPPSI in THF at 23 °C afforded the desired (2E,4Z,6E)-27 or (2Z,4Z,6E)-28 of ≥98% stereoisomeric purity in good yield (Scheme 8).
Scheme 8.
Highly selective synthesis of ethyl (4Z,6E)-trideca-2,4,6-trienoates (27 and 28) by using alkyne elementometalation–Pd-catalyzed Negishi coupling protocol.
(b) Carbonyl olefination–Pd-catalyzed Negishi coupling protocol.
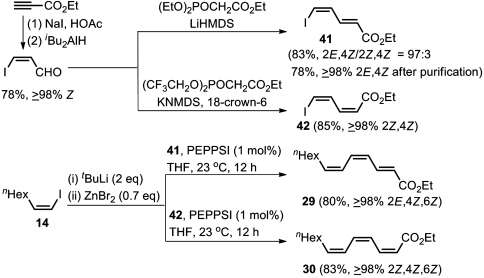
Although the syntheses of 27 and 28 summarized in Scheme 8 relying heavily on Pd-catalyzed Negishi coupling do proceed in good yields and in high selectivity, they do require altogether five steps in the longest linear sequences. We then noticed the availability of ≥98% stereoisomerically pure (Z)-3-iodoacrolein (65) in two steps from commercially available ethyl propiolate. Both HWE and SG reactions of (Z)-3-iodoacrolein proceeded in excellent yields. Although the stereoselectivity of 97% after the HWE reaction was slightly lower than the threshold level of ≥98% we set at the outset of this investigation, simple chromatographic purification improves it to ≥98%. We were further pleased to find that the Negishi coupling of the ethyl esters of (2E,4Z)- and (2Z,4Z)-5-iodo-2,4-pentadienoic acid (41 and 42) with (Z)-1-octenylzinc bromide generated in situ by treatment of (Z)-1-iodo-1-octene with tBuLi (2 equivalents) and ZnBr2 (0.7 M equivalent) in the presence of 1 mol % of PEPPSI proceeded in 80–85% yields to give without any isomeric purification the final two isomers, i.e., (2E,4Z,6Z)-29 and (2Z,4Z,6Z)-30, in ≥98% stereoselectivity and in good yields. Thus, a complete set of all eight possible stereoisomers of a 2,4,6-trienoic acid ester have been synthesized as isomerically pure compounds (Scheme 9).
Scheme 9.
Highly selective synthesis of ethyl (4Z,6Z)-trideca-2,4,6-trienoates (29 and 30) by using carbonyl olefination–Pd-catalyzed Negishi coupling protocol.
In this study, we deliberately chose the ethyl esters of di- and trienoic acids so that we may readily compare our results and methods with conventionally often-used methods, such as HWE, SG, and Heck olefination. As is well-known, however, the Pd-catalyzed alkenylation routes to conjugated di- and trienes, represented by Negishi and Suzuki couplings, do not require the ester functional groups, which are either required or very desirable in the above-mentioned olefination reactions. Although not demonstrated in this study, we are inclined to believe and predict that, in many other cases of the syntheses of conjugated di- and trienes, highly satisfactory results may be obtained through the use of the Pd-catalyzed Negishi and/or Suzuki alkenylation. Further studies along these lines are currently ongoing in our laboratories.
Conclusions
-
As a preliminary step, critical analysis and evaluation of selected Pd-catalyzed alkenylation and carbonyl olefination reactions were made with regard to the syntheses of all four stereoisomers of ethyl undeca-2,4-dienoate (7–10). Negishi alkenylation is uniformly applicable and highly satisfactory for preparing all four isomers 7–10 in > 80% yields and ≥98% selectivity (Scheme 1). By using CsF or
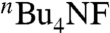 as a base, Suzuki alkenylation permits the synthesis of ethyl (2Z, 4E)-undeca-2,4-dienoate (8) in ≥98% selectivity, and that the other three stereoisomers are also preparable in ≥98% selectivity.
as a base, Suzuki alkenylation permits the synthesis of ethyl (2Z, 4E)-undeca-2,4-dienoate (8) in ≥98% selectivity, and that the other three stereoisomers are also preparable in ≥98% selectivity.The other protocols are significantly more limited in scope and selectivity.
A systematic investigation of HWE and SG olefinations for the synthesis of 2,4-dienoic esters has confirmed the following:- (2E,4E)-Dienoic esters are readily preparable in good yields. However, their steroisomeric purity is generally 85–90%, and a very high (≥98%) level of stereoselectivity is observable only with α,β-unsaturated aldehydes including aryl aldehydes and certain (2E)-alkenals as well as with some sterically hindered alkyl aldehydes.
- SG olefination appears to be satisfactory for preparing various (2Z,4E)-di- and -oligoenoic esters in high yields and ≥98% selectivity.
- SG olefination has thus far remained unsatisfactory for the preparation of (2Z,4Z)-dienoic esters.
One of the most notable advances made in this study is the synthesis of all eight stereoisomers of ethyl trideca-2,4,6-trienoate (23–30) in good yields and ≥98% selectivity by using the Negishi or Suzuki version of Pd-catalyzed alkenylation. It is anticipated and hoped that the study on triene syntheses reported herein will form a reliable and useful foundation for its applications and extensions in selective syntheses of a variety of conjugated trienes and higher oligoenes as well.
For various reasons, it is very desirable to explore and develop Pd-catalyzed alkenylation–carbonyl olefination synergy. In view of the superior features associated with SG olefination in the synthesis of (2Z,4E)-dienoic esters (vide supra), its applicability to the synthesis of conjugated trienoic esters has been probed. Both (2Z,4E,6E)- and (2Z,4E,6Z)-tri-deca-2,4,6-trienoic ethyl esters (24 and 26) were prepared in high yields and ≥98% stereoselectivity by Pd-catalyzed alkenylation–SG olefination tandem processes.
Materials and Methods
General.
All experiments were conducted in flame-dried glassware under argon atmosphere. THF and diethyl ether were dried and distilled from sodium/benzophenone under nitrogen. ZnBr2 was flame-dried under vacuum prior to use. Details for the synthesis of all four stereoisomers (7–10) of ethyl undeca-2,4-dienoate and all eight stereoisomers (23–30) of ethyl trideca-2,4,6-trienoate, as well as their spectroscopic data, are available in SI Appendix.
Representative Procedure for Hydrozirconation–Pd-Catalyzed Negishi Coupling Protocol.
To a solution of ZrCp2Cl2 (0.438 g, 1.5 mmol) in THF (4.5 mL) was added dropwise a solution of 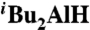 (1.5 mL, 1.0 M solution in hexane, 1.5 mmol) at 0 °C in dark under argon atmosphere, and the resulting suspension was stirred at 0 °C for 30 min followed by the addition of a solution of 1-octyne (0.19 mL, 1.3 mmol) in THF (2 mL). The reaction mixture was warmed to 23 °C and stirred at 23 °C for 30 min. To a solution of ethyl (E)-3-bromoacrylate (0.179 g, 1.0 mmol) and PEPPSI (7 mg, 0.01 mmol) in THF (3 mL) was added the above reaction mixture, and the resulting reaction mixture was stirred at 23 °C for 12 h. The reaction mixture was quenched with water and extracted with ether (2 × 20 mL). The combined organic layers were dried over anhydrous MgSO4, filtered, and concentrated to give crude product with ≥98% isomeric purity determined by 1H NMR. Flash chromatography (silica gel, 5% ethyl acetate in hexanes) afforded 0.188 g of (2E, 4E)-ethyl undeca-2, 4-dienoate (7) in 90% yield with ≥98% 2E, 4E isomeric purity determined by 1H NMR.
(1.5 mL, 1.0 M solution in hexane, 1.5 mmol) at 0 °C in dark under argon atmosphere, and the resulting suspension was stirred at 0 °C for 30 min followed by the addition of a solution of 1-octyne (0.19 mL, 1.3 mmol) in THF (2 mL). The reaction mixture was warmed to 23 °C and stirred at 23 °C for 30 min. To a solution of ethyl (E)-3-bromoacrylate (0.179 g, 1.0 mmol) and PEPPSI (7 mg, 0.01 mmol) in THF (3 mL) was added the above reaction mixture, and the resulting reaction mixture was stirred at 23 °C for 12 h. The reaction mixture was quenched with water and extracted with ether (2 × 20 mL). The combined organic layers were dried over anhydrous MgSO4, filtered, and concentrated to give crude product with ≥98% isomeric purity determined by 1H NMR. Flash chromatography (silica gel, 5% ethyl acetate in hexanes) afforded 0.188 g of (2E, 4E)-ethyl undeca-2, 4-dienoate (7) in 90% yield with ≥98% 2E, 4E isomeric purity determined by 1H NMR.
Supplementary Material
Acknowledgments.
We thank the National Institutes of Health (GM 36792) and Purdue University for support of this research. We also thank Sigma-Aldrich, Albemarle, and Boulder Scientific for their support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105155108/-/DCSupplemental.
References
- 1.Negishi E, Lew G, Yoshida T. Stereoselective synthesis of conjugated trans-enynes readily convertible into conjugated cis, trans-dienes and its application to the synthesis of the pheromone bombykol. J Chem Soc Chem Commun. 1973:874–875. [Google Scholar]
- 2.Eiter K. Insect sexual attractants. Fortschr Chem Org Naturst. 1970;28:204–255. [PubMed] [Google Scholar]
- 3.Negishi E, Abramovitch A. A highly efficient chemo-, regio-, and stereoselective synthesis of (7E,9E)-dodecadien-1-yl acetate, a sex pheromone of Lobesia botrana, via a functionalized organoborate. Tetrahedron Lett. 1977;18:411–414. [Google Scholar]
- 4.Labovitz JN, Henrick CA, Corbin VL. Synthesis of (7E,9E)-7,9-dodecadien-1-yl acetate, a sex pheromone of Lobesia botrana. Tetrahedron Lett. 1975;16:4209–4212. [Google Scholar]
- 5.Pitsinos E, Athinaios N, Xu Z, Wang G, Negishi E. Total synthesis of (+)-scyphostatin featuring an enantioselective and highly efficient route to the side-chain via Zr-catalyzed asymmetric carboalumination of alkenes (ZACA) Chem Commun. 2010;46:2200–2202. doi: 10.1039/b920261g. and pertinent references cited therein. [DOI] [PubMed] [Google Scholar]
- 6.Xu C, Negishi E. A highly efficient and selective synthesis of lissoclinolide featuring hydrogen transfer hydrozirconation, trans-selective Pd-catalyzed cross coupling of alkenylzirconiums with 1,1-dibromoalkenes and Ag-catalyzed lactonization providing (Z)-γ-alkylidenebutenolides. Tetrahedron Lett. 1999;40:431–434. [Google Scholar]
- 7.Davidson BS, Ireland CM. Lissoclinolide, the first non-nitrogenous metabolite from a lissoclinum tunicate. J Nat Prod. 1990;53:1036–1038. doi: 10.1021/np50070a049. [DOI] [PubMed] [Google Scholar]
- 8.Wang G, Huang Z, Negishi E. Efficient and selective syntheses of (all-E) and (6E,10Z)-2′-O-methylmyxalamides D via Pd-catalyzed alkenylation-carbonyl olefination synergy. Org Lett. 2008;10:3223–3226. doi: 10.1021/ol801115s. and pertinent references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolaou KC, Daines RA, Ogawa Y, Chakraborty TK. Total synthesis of amphotericin B. 3. The final stages. J Am Chem Soc. 1988;110:4696–4705. and pertinent references cited therein. [Google Scholar]
- 10.Cereghetti DM, Carreira EM. Amphotericin B: 50 Years of Chemistry and Biochemistry. Synthesis. 2006:914–942. [Google Scholar]
- 11.Hu Q. West Lafayette, IN: Purdue University; 2007. Study towards selective synthesis of highly conjugated oligoene and oligoyne compounds via palladium-catalyzed cross-coupling; p. 143. PhD dissertation. [Google Scholar]
- 12.Negishi E, Wang G. In: Science of Synthesis. Rawal V, Kozmin S, editors. Vol 46. Stuttgart: Thieme; 2009. pp. 239–352. [Google Scholar]
- 13.Negishi E, Wang G. In: Science of Synthesis. Jacobsen EN, de Meijere A, editors. Vol 47. Stuttgart: Thieme; 2010. pp. 909–1016. [Google Scholar]
- 14.Maryanoff BE, Reitz AB. The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chem Rev. 1989;89:863–927. [Google Scholar]
- 15.Horner L, Hoffmann H, Wippel HG, Klahre G. Phosphororganische verbindungen, XX. Phosphinoxyde als olefinierungsreagenzien. Chem Ber. 1959;92:2499–2505. [Google Scholar]
- 16.Wadsworth WS, Jr, Emmons WD. The utility of phosphonate carbanions in olefin synthesis. J Am Chem Soc. 1961;83:1733–1738. [Google Scholar]
- 17.Still WC, Gennari C. Direct synthesis of Z-unsaturated esters. A useful modification of the Horner-Emmons olefination. Tetrahedron Lett. 1983;24:4405–4408. [Google Scholar]
- 18.Ando K. Practical synthesis of Z-unsaturated esters by using a new Horner-Emmons reagent, ethyl diphenylphosphonoacetate. Tetrahedron Lett. 1995;36:4105–4108. [Google Scholar]
- 19.Peterson DJ. Carbonyl olefination reaction using silyl-substituted organometallic compounds. J Org Chem. 1968;33:780–784. [Google Scholar]
- 20.Julia M, Paris JM. Syntheses a l’aide de sulfones v(+)-methode de synthese generale de doubles liaisons. Tetrahedron Lett. 1973;14:4833–4836. [Google Scholar]
- 21.Brown HC, Zweifel G. Hydroboration. XI. The hydroboration of acetylenes—A convenient conversion of internal acetylenes into cis-olefins and of terminal acetylenes into aldehydes. J Am Chem Soc. 1961;83:3834–3840. [Google Scholar]
- 22.Zweifel G, Arzoumanian H, Whitney CC. A convenient stereoselective synthesis of substituted alkenes via hydroboration-iodination of alkynes. J Am Chem Soc. 1967;89:3652–3653. [Google Scholar]
- 23.Zweifel G, Polston NL. Selective hydroboration of conjugated diynes with dialkylboranes. A convenient route to conjugated cis-enynes, α,β-acetylenic ketones, and cis,cis-dienes. J Am Chem Soc. 1970;92:4068–4071. [Google Scholar]
- 24.Zweifel G, Miller RL. Novel stereoselective synthesis of 1,3-dienes from alkynes via the addition of cuprous chloride to vinylalanes. J Am Chem Soc. 1970;92:6678–6679. [Google Scholar]
- 25.Zweifel G, Fisher RP, Snow JT, Whitney CC. Stereoselective introduction of olefinic side chains onto cyclic systems via the hydroboration-iodination of alkynes. J Am Chem Soc. 1971;93:6309–6311. [Google Scholar]
- 26.Negishi E, Yoshida T. A highly stereoselective and general synthesis of conjugated trans,trans-dienes and trans-alkyl ketones via hydroboration. J Chem Soc Chem Commun. 1973:606–607. [Google Scholar]
- 27.Baba S, Negishi E. A novel stereospecific alkenyl-alkenyl cross-coupling by a palladium- or nickel-catalyzed reaction of alkenylalanes with alkenyl halides. J Am Chem Soc. 1976;98:6729–6731. [Google Scholar]
- 28.Okukado N, Van Horn DE, Klima WL, Negishi E. A highly stereo-, regio-, and chemoselective synthesis of conjugated dienes by the palladium-catalyzed reaction of (E)-1-alkenylzirconium derivatives with alkenyl halides. Tetrahedron Lett. 1978;19:1027–1030. [Google Scholar]
- 29.Negishi E, Okukado N, King AO, Van Horn DE, Spiegel BI. Selective carbon-carbon bond formation via transition metal catalysts. 9. Double metal catalysis in the cross-coupling reaction and its application to the stereo- and regioselective synthesis of trisubstituted olefins. J Am Chem Soc. 1978;100:2254–2256. [Google Scholar]
- 30.Negishi E. Palladium- or nickel-catalyzed cross coupling. A new selective method for carbon-carbon bond formation. Acc Chem Res. 1982;15:340–348. [Google Scholar]
- 31.Negishi E. Transition metal-catalyzed organometallic reactions that have revolutionized organic synthesis. Bull Chem Soc Jpn. 2007;80:233–257. [Google Scholar]
- 32.Miyaura N, Suzuki A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem Rev. 1995;95:2457–2483. [Google Scholar]
- 33.Miyaura N, Yamada A, Suzuki A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979;20:3437–3440. [Google Scholar]
- 34.Miyaura N, Suzuki A. Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst. J Chem Soc Chem Commun. 1979:866–867. [Google Scholar]
- 35.Huo S, Negishi E. In: The Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E, editor. New York: Wiley; 2002. pp. 335–408. [Google Scholar]
- 36.Negishi E, Wang G, Rao H, Xu Z. Alkyne elementometalation-Pd-catalyzed cross-coupling. Towards synthesis of any alkenes in high yields, efficiently, selectively, economically, and safely- “green” way. J Org Chem. 2010;75:3151–3182. doi: 10.1021/jo1003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heck RF, Nolley JP., Jr Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J Org Chem. 1972;37:2320–2322. [Google Scholar]
- 38.Mizoroki T, Mori K, Ozaki A. Arylation of olefin with aryl iodide catalyzed by palladium. Bull Chem Soc Jpn. 1971;44:581. [Google Scholar]
- 39.Oestreich M, editor. The Mizoroki-Heck Reaction. Chichester, UK: Wiley; 2009. p. 608. [Google Scholar]
-
40.Zeng F, Negishi E. A highly efficient, selective, and general method for the synthesis of conjugated (all-E)-oligoenes of the
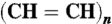 type via iterative hydrozirconation-palladium-catalyzed cross-coupling. Org Lett. 2002;4:703–706. doi: 10.1021/ol0102794. [DOI] [PubMed] [Google Scholar]
type via iterative hydrozirconation-palladium-catalyzed cross-coupling. Org Lett. 2002;4:703–706. doi: 10.1021/ol0102794. [DOI] [PubMed] [Google Scholar] - 41.Yanagi T, Oh-e T, Miyaura N, Suzuki A. Stereoselective synthesis of conjugated 2,4-alkadienoates via the palladium-catalyzed cross-coupling of 1-alkenylboronates with 3-bromo-2-alkenoates. Bull Chem Soc Jpn. 1989;62:3892–3895. [Google Scholar]
- 42.Molander GA, Felix LA. Stereoselective Suzuki-Miyaura cross-coupling reactions of potassium alkenyltrifluoroborates with alkenyl bromides. J Org Chem. 2005;70:3950–3956. doi: 10.1021/jo050286w. [DOI] [PubMed] [Google Scholar]
- 43.Molander GA, Ellis N. Organotrifluoroborates: Protected boronic acids that expand the versatility of the Suzuki coupling reaction. Acc Chem Res. 2007;40:275–286. doi: 10.1021/ar050199q. [DOI] [PubMed] [Google Scholar]
- 44.Khrimian A. The geometric isomers of methyl-2,4,6-decatrienoate, including pheromones of at least two species of stink bugs. Tetrahedron. 2005;61:3651–3657. [Google Scholar]
- 45.Franci X, et al. A comparison of the Still–Gennari and Ando HWE-methodologies with α,β-unsaturated aldehydes: Unexpected results with stannyl substituted systems. Tetrahedron Lett. 2003;44:7735–7740. [Google Scholar]
- 46.Smith AB, III, Dong S, Brenneman JB, Fox RJ. Total synthesis of (+)-sorangicin A. J Am Chem Soc. 2009;131:12109–12111. doi: 10.1021/ja906115a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weir JR, Patel BA, Heck RF. Palladium-catalyzed triethylammonium formate reductions. 4. Reduction of acetylenes to cis-monoenes and hydrogenolysis of tertiary allylic amines. J Org Chem. 1980;45:4926–4931. [Google Scholar]
- 48.Ma S, Lu X. A novel stereospecific hydrohalogenation reaction of propiolates and propiolic acid. J Chem Soc Chem Commun. 1990;22:1643–1644. [Google Scholar]
- 49.Huang Z, Negishi E. A convenient and genuine equivalent to HZrCp2Cl generated in situ from ZrCp2Cl2-DIBAL-H. Org Lett. 2006;8:3675–3678. doi: 10.1021/ol061202o. [DOI] [PubMed] [Google Scholar]
- 50.Kantchev EAB, O′Brien CJ, Organ MG. Palladium complexes of N-heterocyclic carbenes as catalysts for cross-coupling reactions—A synthetic chemist’s perspective. Angew Chem Int Ed Eng. 2007;46:2768–2813. doi: 10.1002/anie.200601663. [DOI] [PubMed] [Google Scholar]
- 51.Alexakis A, Cahier G, Normant JF. Z-1-Iodohexene. Org Synth. 1984;62:1–8. [Google Scholar]
- 52.Wang C, Tobrman T, Xu Z, Negishi E. Highly regio- and stereoselective synthesis of (Z)-trisubstituted alkenes via propyne bromoboration and tandem Pd-catalyzed cross-coupling. Org Lett. 2009;11:4092–4095. doi: 10.1021/ol901566e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, Xu Z, Tobrman T, Negishi E. Arylethyne bromoboration-Negishi coupling route to E- orZ-aryl-substituted trisubstituted alkenes of ≥98% isomeric purity. New horizon in the highly selective synthesis of trisubstituted alkenes. Adv Synth Catal. 2010;352:627–631. doi: 10.1002/adsc.200900766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Negishi E, Tobrman T, Rao H, Xu S, Lee C-T. Highly (≥98%) selective trisubstituted alkene synthesis of wide applicability via fluoride-promoted Pd-catalyzed cross-coupling of alkenylboranes. Isr J Chem. 2011;50:696–701. doi: 10.1002/ijch.201000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Larhed M, Hallberg A. In: The Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E, editor. New York: Wiley; 2002. pp. 1133–1178. [Google Scholar]
- 56.Lu X, Huang X, Ma S. A convenient stereoselective synthesis of conjugated (2Z)-en-4-ynoic and (2Z,4Z)- and (2Z,4E)-dienoic acid derivatives from propiolic acid derivatives. Tetrahedron Lett. 1992;33:2535–2538. [Google Scholar]
- 57.Flock S, Skattebøl L. Syntheses of three metabolites of icosapentaenoic and docosahexaenoic acids. J Chem Soc Perkin 1. 2000:3071–3076. [Google Scholar]
- 58.Byrne B, Lafleur Lawter LM, Wengenroth KJ. The stereoselective synthesis of ethyl 2(E),4(Z)-decadienoate. J Org Chem. 1986;51:2607–2609. [Google Scholar]
- 59.Nicolaou KC, Chakraborty TK, Daines RA, Simpkins NS. Retrosynthetic and synthetic chemistry on amphotericin B. Synthesis of C(1)-C(20) and C(21)-C(38) fragments and construction of the 38-membered macrocycle. J Chem Soc Chem Commun. 1986;5:413–416. [Google Scholar]
- 60.Nicolaou KC, Daines RA, Chakraborty TK, Ogawa Y. Total synthesis of amphoteronolide B and amphotericin B. 2. Total synthesis of amphoteronolide B. J Am Chem Soc. 1988;110:4685–4696. [Google Scholar]
- 61.Boschelli D, Takemasa T, Nishitani Y, Masamune S. Synthesis of amphotericin B. 2. Fragment C-D of the aglycone. Tetrahedron Lett. 1985;26:5239–5242. [Google Scholar]
- 62.Wang G, Huang Z, Negishi E. Highly stereoselective and efficient synthesis of ω-heterofunctional di- and trienoic esters for Horner-Wadsworth-Emmons reaction via alkyne hydrozirconation and Pd-catalyzed alkenylation. Tetrahedron Lett. 2009;50:3220–3223. [Google Scholar]
- 63.Takacs JM, Jaber MR, Clement F, Walters C. A useful procedure for the preparation of (E,E)-2,4-dienoates: Lithium hydroxide-promoted dienylation by 4-phosphonocrotonate. J Org Chem. 1998;63:6757–6760. [Google Scholar]
- 64.Molander GA, Ellis NM. Highly stereoselective synthesis of cis-alkenyl pinacolboronates and potassium cis-alkenyltrifluoroborates via a hydroboration/protodeboronation approach. J Org Chem. 2008;73:6841–6844. doi: 10.1021/jo801191v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marek I, Meyer C, Normant JF. A simple and convenient method for the preparation of (Z)-β-iodoacrolein and of (Z)- or (E)-γ-iodo allylic alcohols: (Z)- and (E)-1-iodohept-1-en-3-ol. Org Synth. 1997;74:194–204. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.