Abstract
Although it has long been hypothesized that attachment figures provide individuals with a sense of safety and security, the neural mechanisms underlying attachment-induced safety have not been explored. Here, we investigated whether an attachment figure acts as a safety signal by exploring whether viewing an attachment figure during a threatening experience (physical pain) led to increased activity in a neural region associated with safety signaling, the ventromedial prefrontal cortex (VMPFC), and corresponding reductions in pain. Female participants in long-term romantic relationships were scanned as they received painful stimuli while viewing pictures of their partner and control images (stranger, object). Consistent with the idea that the attachment figure may signal safety, results revealed that viewing partner pictures while receiving painful stimulation led to reductions in self-reported pain ratings, reductions in pain-related neural activity (dorsal anterior cingulate cortex, anterior insula), and increased activity in the VMPFC. Moreover, greater VMPFC activity in response to partner pictures was associated with longer relationship lengths and greater perceived partner support, further highlighting a role for the VMPFC in responding to the safety value of the partner. Last, greater VMPFC activity while viewing partner pictures was associated with reduced pain ratings and reduced pain-related neural activity. An implication of these findings is that, in the same way that stimuli that historically have threatened survival (e.g., snakes, spiders) are considered to be prepared fear stimuli, attachment figures, who have historically benefited survival, may serve as prepared safety stimuli, reducing threat- or distress-related responding in their presence.
Keywords: functional MRI, neuroimaging, close relationship, distress
“…for a person to know that an attachment figure is available and responsive gives him a strong and pervasive feeling of security…”
—John Bowlby, 1988 (1)
One of the central tenets of attachment theory is that the attachment bond—first formed between caregiver and child—provides a sense of safety and security for the child, who is not yet capable of providing or fending for him/herself (2). As suggested by Bowlby (1) above, knowing that an attachment figure is present may serve as a kind of safety signal, letting the individual know that he/she is safe and will be taken care of. Indeed, the presence versus absence of an attachment figure is known to produce quite distinct behavioral profiles in the same child—with courage and exploration seen in the caregiver's presence and timidity and fear observed in the caregiver's absence (2). Although the purpose of this attachment bond is most obvious during childhood, attachment bonds, such as those between adult romantic relationship partners, persist throughout the life cycle and may be beneficial during times of threat or danger (3).
Although it makes sense that an attachment figure provides a “pervasive feeling of security,” how attachment-induced safety manifests behaviorally or neurally is not well understood. In the present study, we explored the psychological and neural underpinnings of attachment-induced safety by examining whether viewing an attachment figure during a threatening experience—receiving physical pain—led to increased activity in a neural region associated with safety signaling and a corresponding reduction in the threatening or distressing experience of physical pain. Indeed, accumulating correlational and experimental evidence has demonstrated the pain-attenuating effects of an attachment figure or other supportive individual. For instance, coronary-bypass patients whose spouses visited them more frequently in the hospital took less pain medication and recovered more quickly than patients whose spouses visited them less frequently (4). Women with supportive individuals present during childbirth were less likely to use pain-relief medication than women who did not have these individuals present (5). Moreover, recent experimental studies suggest that being with or being primed with one's attachment plays a causal role in pain reduction (6–8). In addition, holding the hand of an attachment figure (spouse) has been shown to reduce threat-related neural activity associated with the anticipation of painful experience (9). However, to date, few studies have examined the mechanisms that produce these pain-attenuating effects (cf. ref. 8), and no studies have examined whether these effects are caused by the safety-inducing properties of the supportive individuals themselves.
Although no work has explicitly examined the mechanisms whereby attachment figures may signal safety, previous research has examined the neural mechanisms underlying perceived safety in the context of fear learning. Both animal and human research has shown that the ventromedial prefrontal cortex (VMPFC) is more active in response to cues (tones, shapes) that, through learning, signal safety (e.g., no shock) compared with cues that signal negative outcomes (e.g., shock) (10–16). The VMPFC is also more active during fear extinction, a form of learned safety in which a cue that previously predicted a negative outcome now predicts safety (11, 12, 14, 17). In addition, the VMPFC is thought to have inhibitory control over certain limbic regions, such as the amygdala, leading to reductions in conditioned fear responses in the presence of safety cues or during fear extinction (10, 15). Thus, in rats, stimulating the VMPFC while presenting a fear-inducing cue diminishes fear responding, suggesting a role for this region in inhibiting the fear response (18). Similarly, in humans, increased activity in the VMPFC (including the subgenual anterior cingulate cortex; subACC) has been observed in response to safety signals (relative to fear-predictive stimuli) (11–14), and greater activity in the VMPFC/subACC during fear extinction or learned safety has been associated with reduced fear responding (reductions in skin conductance) (14). Thus, in the context of fear learning, VMPFC activity appears to track stimuli that signal safety, and this activity is thought to modulate other neural regions that are involved in responding to the threat at hand.
Building on these results, research on stressful experience also points to a role for the VMPFC in signaling safety and reducing threat responding. In studies of psychological stress, the VMPFC is typically more active during the control condition (which presumably is safer) than in the stress condition. For example, the VMPFC was more active during a control condition in which participants completed math problems without social evaluation than when they received negative evaluative feedback while completing these problems (19). Moreover, consistent with an inhibitory role for the VMPFC in threat-related responding, greater VMPFC activity during stress has been shown to be associated with reductions in heart rate and self-reported anxiety (20–22) and reduced activity in the dorsal anterior cingulate cortex (dACC) (22), a region positively associated with cardiovascular responses to stress (21–23).
Finally, in the context of physical pain, it has been shown that conditions that cue greater relative safety activate the VMPFC and that this activity relates to reduced pain ratings. In one study, expectations about the intensity of an upcoming painful stimulus were varied so that participants expected a low-pain stimulus on some trials and a high-pain stimulus on others; regardless of the expectation, participants received a medium-level pain stimulus. Results demonstrated that expecting a low-pain compared to a high-pain stimulus led to greater activity in the VMPFC (24), potentially reflecting the greater relative safety value of the low- vs. high-pain cue. In addition, greater VMPFC activity during the “safer” low-pain vs. high-pain cues was associated with lower pain ratings in response to the subsequent medium-level pain stimulus.
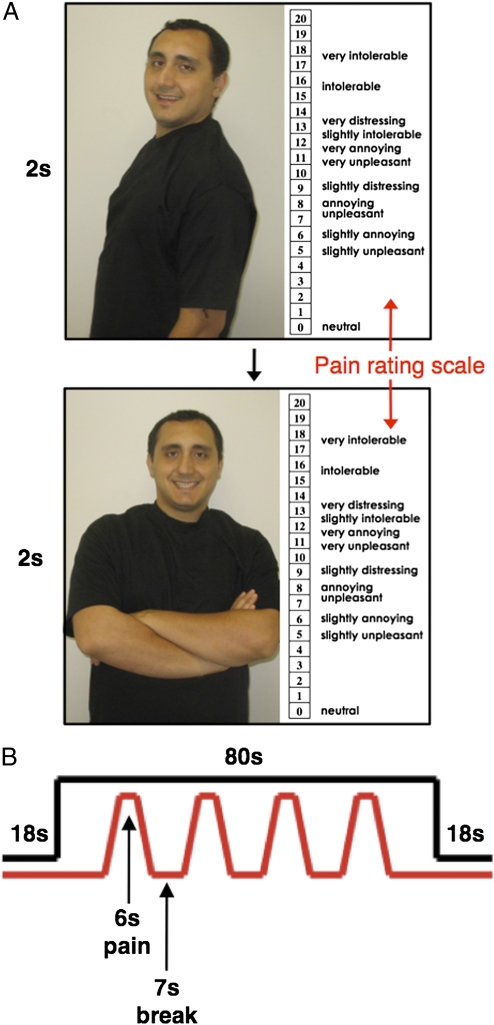
In sum, the VMPFC has been shown to be more active in response to conditions that signify relative safety and to correlate negatively with fear, stress, and pain responses. Building on this information, the present study explored whether viewing pictures of romantic attachment figures while receiving pain activated similar neural regions, leading to reductions in the distressing experience of physical pain. To examine this possibility, female participants in committed romantic relationships completed a functional MRI (fMRI) scan. During the scan, each female participant received painful heat stimuli (at two different temperatures: moderate and high) while viewing pictures of her relationship partner or control images (a stranger or an object). There were four different pictures in each condition. After each heat stimulus, participants were asked to rate its unpleasantness (Fig. 1).
Fig. 1.
(A) In each block, participants viewed four different photographs for 2 s per image. (Shown are two examples of partner images with the pain-rating scale displayed on the right side.) (B) Photographs were shown continuously throughout the 80-s block while participants received four 6-s heat stimulations (depicted in red) separated by 7 s.
To the extent that the relationship partner provides the participant with a safety cue during this negative event, we hypothesized that (i) viewing pictures of the relationship partner (vs. control images) would reduce the threatening experience of physical pain, resulting in lower ratings of pain unpleasantness; (ii) viewing pictures of the relationship partner (vs. control images) would reduce neural activity in regions previously associated with the unpleasantness of physical pain (dACC and anterior insula (25–27); (iii) viewing pictures of the partner would lead to greater activity in the VMPFC, a region associated with safety signaling; and (iv) greater VMPFC activity would be associated with reduced pain ratings and reduced activity in pain-related neural regions.
It is important to note that, in addition to contributing to safety-signaling processes, the VMPFC is also part of a larger set of neural regions known to be involved in reward processing more generally (28–31). Although we do not view safety-signaling processes as being incompatible with reward-related processes, because cues of safety may themselves be experienced as rewarding (16, 32), it is important to explore whether the current task activates neural regions that are more common to studies of safety signaling or more common to studies exploring the rewarding experience of viewing partner pictures. Specifically, research on safety-signaling and fear-extinction processes typically have yielded VMPFC activity without other reward-related regions such as the ventral striatum (11–14) and have shown that the ventral striatum is not needed for safety-signaling processes (33) On the other hand, previous work on viewing romantic partners has led to increased activity in dopamine-rich reward-related regions, such as the caudate and ventral tegmental area, but not to activity in the VMPFC (34–38). Thus, exploring the pattern of neural activity associated with viewing attachment figures during pain would help determine if this experience is more closely aligned with safety-signaling processes or more closely aligned with the potentially rewarding experience of viewing partner pictures.
Results
Hypothesis 1: Viewing Partner Pictures Will Reduce Pain Ratings.
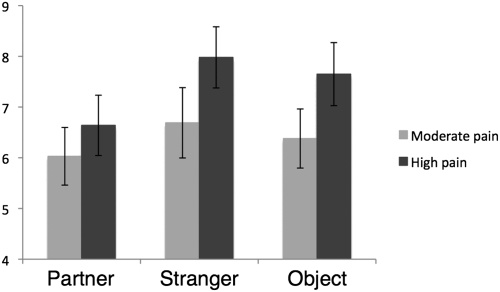
We first examined whether viewing partner vs. control pictures reduced pain ratings in response to the heat stimuli. A repeated-measures ANOVA (with condition and level of painful stimulation as the independent variables) revealed a main effect of condition [F(2,15) = 6.11, P < 0.05], such that pain ratings while viewing pictures of the partner pictures were significantly lower than pain ratings while viewing pictures of a stranger [t(16) = 3.77, P < 0.05] or pictures of an object [t(16) = 2.19, P < 0.05], replicating our prior work (6) (Fig. 2). Pain ratings during the stranger and object conditions were not significantly different from one another (P = 0.15). There also was a main effect of level of painful stimulation, such that participants reported significantly higher pain ratings in response to the high-pain trials than in the moderate-pain trials [F(1,16) 24.88, P < 0.005]. Interestingly, there also was a marginally significant interaction [F(2,15) = 3.46, P = 0.06], such that the difference in pain ratings between the partner and control conditions was larger during the high-pain trials [partner vs. stranger: t(16) = 3.58, P < 0.005; partner vs. object: t(16) = 3.34, P < 0.005] than during the moderate-pain trials [partner vs. stranger: t(16) = 2.26, P < 0.05; partner vs. object: t(16) = 0.92, ns]. Thus, as predicted, viewing partner pictures had pain-attenuating effects, and these effects were more pronounced during the high-pain trials.
Fig. 2.
Average pain unpleasantness ratings for each of the study conditions separated by moderate- and high-pain trials. Error bars represent SEMs.
Hypothesis 2: Viewing Partner Pictures Will Reduce Pain-Related Neural Activity.
Next, we examined whether viewing partner vs. control pictures during pain reduced activity in pain-related neural regions. Specifically, we focused on anatomical regions of interest (ROIs) in the dACC and bilateral anterior insula. As confirmation that these regions were associated with the experience of physical pain in the present study, a parametric modulation analysis revealed that moment-to-moment changes in self-reported pain across the scanning session correlated positively with activity in the dACC ROI [t(16) = 2.36, P < 0.05] and bilateral anterior insula ROI [bilateral: t(16) = 2.50, P < 0.05; left: t(16) = 2.03, P < 0.05; right: t(16) = 2.74, P < 0.05].
We then explored whether these pain-related regions showed differential activity as a function of condition. Based on the results from the self-reported pain analyses, we focused specifically on high-pain trials, because in these trials the pain-modulation effects were most pronounced. In addition, because there were no differences in pain ratings between the two control conditions (stranger, object), we collapsed these two control conditions into one. As expected, participants showed significantly less activity in the dACC ROI [t(16) = 1.80, P < 0.05] and bilateral anterior insula ROI [bilateral: t(16) = 1.80, P < 0.05; left: t(16) = 1.64, P = 0.06, right: t(16) = 1.75, P = 0.05] while viewing partner vs. control pictures during the high-pain trials. Although in the right direction, these effects were not significant during the moderate-pain trials (P > 0.15). Thus, consistent with the self-report data, there was reduced activity in pain-related neural regions while viewing partner vs. control pictures during pain, and this effect was most pronounced in the high-pain trials.
Hypothesis 3: Viewing Partner Pictures Will Activate the VMPFC.
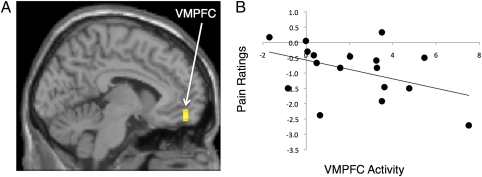
Next, we examined whether viewing partner pictures activated the VMPFC, a region implicated in safety signaling. To do so, we focused specifically on neural activity while viewing partner vs. stranger pictures during pain to rule out activity that might differentiate responses to persons vs. objects and to examine the specificity of VMPFC involvement in viewing the partner while controlling for the effects of simply viewing another person. Participants showed greater activity in the VMPFC [Brodmann's area (BA) 11: −6,51,−15; t = 3.81; k (number of voxels) = 10] (Fig. 3A) in response to viewing partner vs. stranger pictures during pain. They also showed greater activity in the premotor cortex [BA 6: 36,12,33, t = 5.30; k = 28]. There were no other regions that were more active during partner vs. stranger viewing. In addition, even at a lower threshold (P < 0.05, 10 voxels), there was no activity in regions previously associated with viewing partner pictures without pain, such as the caudate or ventral tegmental area (there was also no ventral striatum activity). The only two regions that were more active during stranger vs. partner viewing were in the caudate (−15,21,12; t = 3.93; k = 11) and premotor cortex (BA 6: −45,12,9; t = 4.02; k = 11).
Fig. 3.
(A) VMPFC activation while viewing partner pictures vs. stranger pictures during pain. (B) Scatterplot showing the correlation between VMPFC activity (−6,51,15) and pain ratings (during partner vs. stranger trials).
Interestingly, consistent with the hypothesis that VMPFC activity signals attachment-related safety, participants in longer-term relationships (categorized by the number of years together: 1 = 1 y or less; 2 = 1–2 y; up to 8 = more than 7 y) showed greater activity in this same VMPFC cluster (r = 0.43, P < 0.05). Moreover, participants who rated their partner as being a more significant source of support showed marginally more activity in this VMPFC cluster as well (r = 0.35, P = 0.08).* (Relationship length and perceived partner support were not significantly correlated: r = 0.13, ns). Thus, given the role of the VMPFC in safety signaling and its correlation with relationship length and partner support in the current study, it is possible that the VMPFC activation observed in this task is related to the value of the partner as a safety signal.
Hypothesis 4: VMPFC Activation Will Be Associated with Reduced Pain Ratings and Reduced Pain-Related Neural Activity.
We first examined whether neural activity in the regions observed during partner vs. stranger viewing correlated negatively with pain ratings (during partner vs. stranger trials). The VMPFC cluster identified above (−6,51,−15) was found to correlate negatively with pain ratings (r = −0.43, P < 0.05) (Fig. 3B). The premotor cortex did not correlate significantly with pain ratings (r = −0.24, P > 0.17). Thus, to the extent that the VMPFC was more active while viewing partner pictures, self-reported pain distress was diminished.
We then explored whether VMPFC activity correlated negatively with pain-related neural activity using a psychophysiological interaction analysis. This analysis allowed us to investigate the neural regions in which the time course of neural activity was more negatively correlated with VMPFC activity during the partner-viewing condition vs. the stranger-viewing condition. These analyses were constrained to focus on correlated neural activity in the dACC and anterior insula ROIs. Although VMPFC activity did not correlate negatively with activity in the full dACC or anterior insula ROIs, results revealed a cluster within the dACC ROI (6,24,18, t = 4.49, P < 0.005, k = 12) that correlated negatively with VMPFC activity. Hence, VMPFC activity was more strongly negatively correlated with this region of the dACC during the partner-viewing trials than during the stranger-viewing trials. Moreover, activation in this dACC cluster correlated positively with pain ratings during partner vs. stranger trials (r = 0.35, P = 0.08).† No regions of the anterior insula correlated negatively with VMPFC activity. Although the results are not conclusive, it is possible that the VMPFC contributes to reduced activity in regions that are involved in the distressing experience of physical pain, such as the dACC.
Discussion
The attachment bond provides individuals with a sense of safety and security during times of threat. However, the neural mechanisms underlying attachment-induced safety have not been explored previously. The present investigation examined the hypothesis that an attachment figure may act as a safety signal during a threatening experience, relying on neural regions known to be involved in signaling safety and reducing distress or threat. Consistent with this analysis, we found that viewing an attachment figure (romantic partner) during an experience of physical pain led to greater activity in the VMPFC, a region implicated in safety signaling and fear extinction (10–16), and attenuated reports of pain and pain-related neural activity.
Specifically, we found that viewing partner pictures while receiving pain led to lower pain ratings than viewing control pictures while receiving pain, particularly during the high-pain trials. These findings replicate our previous work on the pain-attenuating effects of viewing relationship partners (6). Moreover, building on these previous findings, viewing partner vs. control pictures while receiving high levels of pain led to significantly less activity in the dACC and bilateral anterior insula, regions associated with the unpleasantness of physical pain in the current study and others (25–27). Although it could be argued that these partner-related reductions in pain were caused by greater distraction by the partner images, a previous behavioral study using this same paradigm ruled out this possibility by showing no differences in reaction times to a probe stimulus in any of the conditions (6).
We also found that viewing one's partner relative to a stranger's picture led to greater activity in the VMPFC. Moreover, greater VMPFC activity in response to viewing partner pictures was associated with longer relationship lengths, consistent with the idea that longer-term relationship partners, who presumably have shown greater evidence of commitment and availability over time, may signal greater safety. In addition, greater VMPFC activity in response to viewing partner pictures was associated with the participant's perception that the partner was a greater source of support.
Finally, greater activity in the VMPFC in response to viewing partner pictures was associated with reduced pain ratings as well as reduced activity in the dACC, a region associated with the unpleasantness of physical pain. Although not conclusive, these findings are consistent with the hypothesis that being primed with the partner (through pictures) during pain may activate safety-related neural regions, such as the VMPFC, which then may relate to reductions in pain-related or distress-related experience as well as reduced activity in the neural regions that process these experiences.
As noted earlier, previous work has highlighted a role for the VMPFC not only in signaling safety but in reward processing more generally (28–31). Although safety-signaling processes may overlap to some extent with reward-related processes, because cues of safety may be intrinsically rewarding or reinforcing (16, 32), several points are inconsistent with the conclusion that the processes observed in the current study are simply the result of the rewarding experience associated with viewing partner pictures. Earlier studies have shown that viewing romantic partners (without receiving pain) activates the caudate and ventral tegmental area but not the VMPFC (34–38). In fact, in the current study, viewing attachment figures during pain led to reduced, rather than increased, activity in the caudate—a pattern inconsistent with the notion that the effects reported here are simply the result of the rewarding experience of viewing partner pictures. Indeed, a previous study demonstrated similar reductions in caudate activity during partner vs. stranger handholding while anticipating pain (9). Thus, the pattern of neural activity observed in the current study is more consistent with the pattern of neural activity observed in previous studies of safety signaling rather than previous studies of simply viewing partner pictures. However, further work will be needed to flesh out fully the role that reward-related processes play in safety signaling more generally as well as in the effects reported here more specifically.
Although not explicitly tested here, one possibility suggested by the present study is that, in much the same way that stimuli that historically have threatened survival (e.g., snakes, spiders) are considered to be prepared fear stimuli [stimuli that are more readily fear conditioned and more difficult to extinguish (39, 40)] attachment figures may be prepared safety stimuli, that is, stimuli that historically have benefited survival and thus may inhibit conditioned fear responding. Although prepared safety stimuli have not yet been investigated, their defining features should parallel those of prepared fear stimuli. Thus, just as prepared fear stimuli augment conditioned fear responding, to the extent that attachment figures serve as prepared safety stimuli, reductions in fear- or threat-related responding should be observed when attachment figures are present (or primed) in the face of threat or danger.
Of course, unlike prepared fear stimuli, in which the specific feared stimulus does not need to be learned and is likely to be universal (e.g., most individuals should show fear of snakes), in the case of attachment figures as prepared safety stimuli, the specific attachment figure does need to be learned and most certainly will not be universal (e.g., not everyone will show safety responses to the same person). Therefore, when referring to attachment figures as “prepared” safety stimuli, the idea is not that a specific person is a prepared stimulus but rather that the prepared stimulus is a schema or placeholder in the attachment behavioral system that then becomes occupied by a specific attachment figure who serves as a secure base or source of safety (2). How a certain individual comes to occupy that prepared slot is still not clear; however, recent work shows that it may occur, in part, through being comforted following distressing experiences. For instance, pairing a distressing stimulus with a smiling face has been shown to lead to greater thoughts of attachment security than pairing a neutral stimulus with a smiling face (41). Thus, a specific attachment figure may come to occupy this prepared slot in the attachment system by providing safety during times of threat.
Interestingly, μ-opioids, which are known to be released in response to positive, close social contact (42), have been shown to play a role in both fear acquisition and fear extinction or learned safety. Thus, blocking endogenous μ-opioid neurotransmission enhances the acquisition of conditioned fear (43) and impairs the acquisition of extinction or learned safety (44–46). The implication of these findings is that μ-opioids are involved in reducing conditioned fear responses and enhancing fear extinction or learned safety. This finding is interesting, in that attachment figures are likely to increase μ-opioid levels and thus may serve as prepared safety stimuli—reducing conditioned fear responses and enhancing learned safety—through μ-opioid–related processes. Moreover, the involvement of μ-opioids in safety-related processes also fits with the findings from the current study showing pain reductions during partner picture presentations, because μ-opioids are known to ameliorate pain responses (47). Future work will be needed to determine the precise role of μ-opioids in the value of attachment figures as safety signals.
In summary, this study yielded evidence consistent with the notion that an attachment figure may signal safety during threatening experiences. Paralleling previous work on safety signals, viewing attachment figures in the context of physical pain activated the VMPFC, a region associated with signaling safety, and greater activity in this region was associated with reduced pain ratings and reduced activity in pain-related neural regions. These attachment-induced safety-signaling processes represent an understudied mechanism in the link between attachment figures, feelings of safety, and distress reduction.
Methods
Participants.
Twenty-one female participants (mean age: 23.4 y, SD = 3.8) completed the study procedures (see SI Methods for inclusion criteria). The average relationship length reported was 3.52 y (range: 9 mo to 13 y); three of the participants were married to their partners. Four participants who were no longer with their partners by the end of the study were not included in the present analyses. Thus, the final sample included 17 participants. All experimental procedures were approved by the University of California, Los Angeles Institutional Review Board.
Procedure.
The study consisted of two sessions. For session 1, each participant arrived at the laboratory with her partner. During this session, each participant's pain threshold was determined using a double random staircase algorithm (48) (see SI Methods for details). The pain threshold was defined as “the point at which the stimulation feels aversive, but tolerable, and requires some effort to deal with” or a “10” (‘moderate discomfort’) on a 0–20 scale (49). During this time, a set of four digital photographs was taken of the participant's partner from four different standardized angles. For session 2, participants came to the scanning facility without their partners. During the scanning session, each participant received two levels of heat stimulations consisting of her threshold temperature (“moderate” pain) and her threshold temperature plus 1 °C (“high” pain); thresholds were rechecked before scanning. Participants were paid $20 for session 1 and an additional $30 for session 2. Partners were paid $10 for session 1.
fMRI Task Design.
During the scanning session, participants received heat stimulations while undergoing three different conditions, in which they viewed a set of four pictures each of (i) their partner, (ii) a male stranger (roughly matched to the partner's age, height, and weight), or (iii) an object (a chair). Participants completed three functional runs with counterbalanced orders of these conditions. Each run contained one 80-s block of each condition type (partner, stranger, object). During each block, participants viewed different pictures continuously (each appearing for 2 s) and received four heat stimulations (two moderate and two high) to their left forearm. An example is shown in Fig. 1. Participants made pain ratings after each stimulus by moving a trackball device to the corresponding number on the pain-rating scale (additional details are given in SI Methods).
fMRI Data Analysis.
Scanning parameters are included in SI Methods. Neuroimaging data were preprocessed and analyzed using Statistical Parametric Mapping (SPM5; Wellcome Department of Cognitive Neurology, Institute of Neurology, London). Preprocessing included image realignment to correct for head motion, normalization into a standard stereotactic space defined by the Montreal Neurological Institute (MNI) and the International Consortium for Brain Mapping, and spatial smoothing using an 8-mm Gaussian kernel, full width at half maximum, to increase signal-to-noise ratio.
The task was modeled as an event-related design. Periods when the participant viewed the various pictures (partner, stranger, object) while receiving pain and while not receiving pain were modeled as separate events, for a total of six different event types. Linear contrasts comparing the event types were computed for each participant. These individual contrast images then were used in random-effects group-level analyses.
Analytic Strategy.
ROI analyses.
To examine whether viewing partner vs. control pictures led to reduced activity in pain-related neural regions, structural ROI analyses of the dACC and bilateral anterior insula were performed (SI Methods). First, to verify that these ROIs were involved in pain-related responding, we conducted a parametric modulation analysis. Thus, we used a parametric modulator consisting of each participant's pain unpleasantness rating in response to each pain trial. Mean parameter estimates from the parametric modulation analysis were extracted across all voxels in each ROI using the Marsbar toolbox (http://marsbar.sourceforge.net) to see if there was a significant association between self-reported pain and neural activity (P < 0.05).
We then examined the effect of condition on neural activity in these ROIs. The Marsbar toolbox was used to extract mean parameter estimates (modeling the amplitude of the blood oxygen level-dependent response when viewing pictures during pain vs. viewing pictures without pain) averaged across all voxels in each ROI. Standard statistical software (SPSS 14.0) then was used to conduct paired-samples t tests to assess differences in neural activity in these ROIS as a function of condition (partner vs. control conditions, P < 0.05). Based on convention, all neuroimaging analyses were one-tailed.
Whole-brain analyses.
To examine whether viewing pictures of one's partner during pain activated neural regions involved in safety signaling, we conducted whole-brain main effect analyses (P < 0.005, 10 voxels) (50). All coordinates are reported in Montreal Neurological Institute (MNI) coordinate space.
Next, we examined whether activity in these regions correlated with reduced pain ratings. There was one outlier on pain ratings during partner vs. stranger trials (>2.5 SDs below the mean). This individual's data were winsorized by moving the data point to 2.5 SDs from the group mean without that subject included in the estimate of the mean. Parameter estimates then were extracted from each significant cluster during partner vs. stranger trials, and correlational analyses were conducted in SPSS to see if cluster-level neural activity correlated with pain ratings (P < 0.05).
Finally, to explore whether activity in the VMPFC was associated with reduced activity in pain-related regions, we conducted psychophysiological interaction analyses. In these analyses, an interaction between neural activity (deconvolved from the hemodynamic response) in a seed region (VMPFC) and task condition (partner vs. stranger trials) was generated for each participant (51). Whole-brain parameter estimates then were regressed onto this interaction to search for activity or regions within the dACC and anterior insula ROIs that were differentially associated with the seed region in different task conditions.
Supplementary Material
Acknowledgments
We thank the University of California, Los Angeles Brain Mapping Center for their assistance with this project.
Footnotes
The authors declare no conflict of interest.
*This relationship might have been stronger if there were more variability in the measure of partner support; however, because participants were selected based on high ratings of partner support, this scale has a restricted range (7–10 on a 10-point scale).
†One participant was discovered through visual inspection to be a multivariate outlier in this analysis. Further analysis of Mahalanobis and Cook's distances confirmed that this participant's data had a disproportionate influence on the regression line for this analysis, and therefore this participant was excluded. Because this participant was not an outlier on any single scale, she was included in the other analyses.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108239108/-/DCSupplemental.
References
- 1.Bowlby J. A Secure Base: Parent-Child Attachment and Healthy Human Development. USA: Basic Books; 1988. p. 27. [Google Scholar]
- 2.Bowlby J. Attachment. USA: Basic Books; 1969. [Google Scholar]
- 3.Hazan C, Shaver P. Romantic love conceptualized as an attachment process. J Pers Soc Psychol. 1987;52:511–524. doi: 10.1037//0022-3514.52.3.511. [DOI] [PubMed] [Google Scholar]
- 4.Kulik JA, Mahler HIM. Social support and recovery from surgery. Health Psychol. 1989;8:221–238. doi: 10.1037//0278-6133.8.2.221. [DOI] [PubMed] [Google Scholar]
- 5.Kennell J, Klaus M, McGrath S, Robertson S, Hinkley C. Continuous emotional support during labor in a US hospital. A randomized controlled trial. JAMA. 1991;265:2197–2201. [PubMed] [Google Scholar]
- 6.Master SL, et al. A picture's worth: Partner photographs reduce experimentally induced pain. Psychol Sci. 2009;20:1316–1318. doi: 10.1111/j.1467-9280.2009.02444.x. [DOI] [PubMed] [Google Scholar]
- 7.Montoya P, Larbig W, Braun C, Preissl H, Birbaumer N. Influence of social support and emotional context on pain processing and magnetic brain responses in fibromyalgia. Arthritis Rheum. 2004;50:4035–4044. doi: 10.1002/art.20660. [DOI] [PubMed] [Google Scholar]
- 8.Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing pictures of a romantic partner reduces experimental pain: Involvement of neural reward systems. PLoS ONE. 2010;5:e13309. doi: 10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coan JA, Schaefer HS, Davidson RJ. Lending a hand: Social regulation of the neural response to threat. Psychol Sci. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- 10.Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Kalisch R, et al. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milad MR, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Mobbs D, et al. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Natl Acad Sci USA. 2010;107:20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 15.Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: Reversal of fear in the human brain. J Neurosci. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- 18.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 19.Pruessner JC, et al. Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 20.Wager TD, et al. Brain mediators of cardiovascular responses to social threat: Part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: A functional neuroimaging investigation in humans. J Physiol. 2000;523:259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wager TD, et al. Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Critchley HD, et al. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 24.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 27.Rainville P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12:195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 28.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haber S, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology Reviews. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 31.O'Doherty JP. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Rogan MT, Leon KS, Perez DL, Kandel ER. Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron. 2005;46:309–320. doi: 10.1016/j.neuron.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Josselyn SA, Falls WA, Gewirtz JC, Pistell P, Davis M. The nucleus accumbens is not critically involved in mediating the effects of a safety signal on behavior. Neuropsychopharmacology. 2005;30:17–26. doi: 10.1038/sj.npp.1300530. [DOI] [PubMed] [Google Scholar]
- 34.Acevedo BP, Aron A, Fisher HE, Brown LL. Neural correlates of long-term intense romantic love. Soc Cogn Affect Neurosci. 2011 doi: 10.1093/scan/nsq092. 10.1093/scan/nsq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aron A, et al. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- 36.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Ortigue S, Bianchi-Demicheli F, Patel N, Frum C, Lewis JW. Neuroimaging of love: fMRI meta-analysis evidence toward new perspectives in sexual medicine. J Sex Med. 2010;7:3541–3552. doi: 10.1111/j.1743-6109.2010.01999.x. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, et al. Reward and motivation systems: A brain mapping study of early-stage intense romantic love in Chinese participants. Hum Brain Mapp. 2011;32:249–257. doi: 10.1002/hbm.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- 40.Seligman MEP. Phobias and preparedness. Behav Ther. 1971;2:307–320. [Google Scholar]
- 41.Beckes L, Simpson JA, Erickson A. Of snakes and succor: Learning secure attachment associations with novel faces via negative stimulus pairings. Psychol Sci. 2010;21:721–728. doi: 10.1177/0956797610368061. [DOI] [PubMed] [Google Scholar]
- 42.Panksepp J. Affective Neuroscience. New York: Oxford Univ Press; 1998. [Google Scholar]
- 43.Eippert F, Bingel U, Schoell E, Yacubian J, Büchel C. Blockade of endogenous opioid neurotransmission enhances acquisition of conditioned fear in humans. J Neurosci. 2008;28:5465–5472. doi: 10.1523/JNEUROSCI.5336-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNally GP, Pigg M, Weidemann G. Opioid receptors in the midbrain periaqueductal gray regulate extinction of Pavlovian fear conditioning. J Neurosci. 2004;24:6912–6919. doi: 10.1523/JNEUROSCI.1828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNally GP, Westbrook RF. Opioid receptors regulate the extinction of Pavlovian fear conditioning. Behav Neurosci. 2003;117:1292–1301. doi: 10.1037/0735-7044.117.6.1292. [DOI] [PubMed] [Google Scholar]
- 46.Kim JH, Richardson R. The effect of the μ-opioid receptor antagonist naloxone on extinction of conditioned fear in the developing rat. Learn Mem. 2009;16:161–166. doi: 10.1101/lm.1282309. [DOI] [PubMed] [Google Scholar]
- 47.Price DD, Von der Gruen A, Miller J, Rafii A, Price C. A psychophysical analysis of morphine analgesia. Pain. 1985;22:261–269. doi: 10.1016/0304-3959(85)90026-0. [DOI] [PubMed] [Google Scholar]
- 48.Gracely RH, Lota L, Walter DJ, Dubner R. A multiple random staircase method of psychophysical pain assessment. Pain. 1988;32:55–63. doi: 10.1016/0304-3959(88)90023-1. [DOI] [PubMed] [Google Scholar]
- 49.Gracely RH, McGrath F, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5:5–18. doi: 10.1016/0304-3959(78)90020-9. [DOI] [PubMed] [Google Scholar]
- 50.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.