Abstract
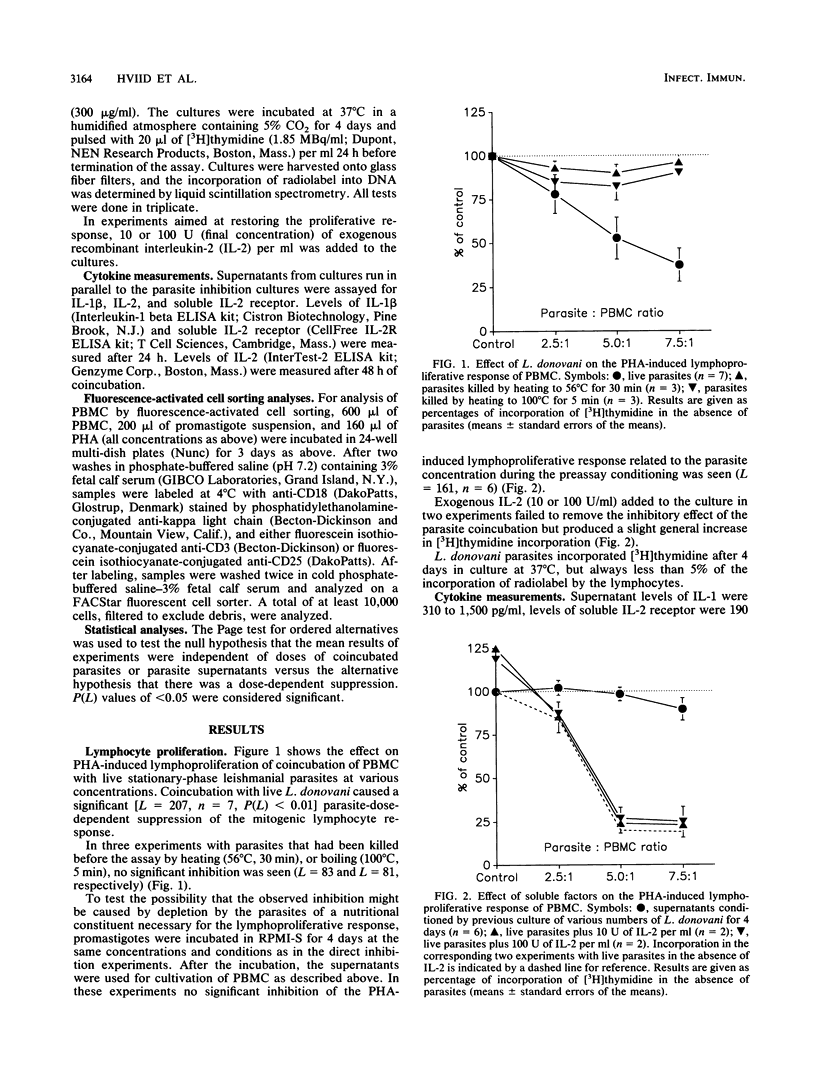
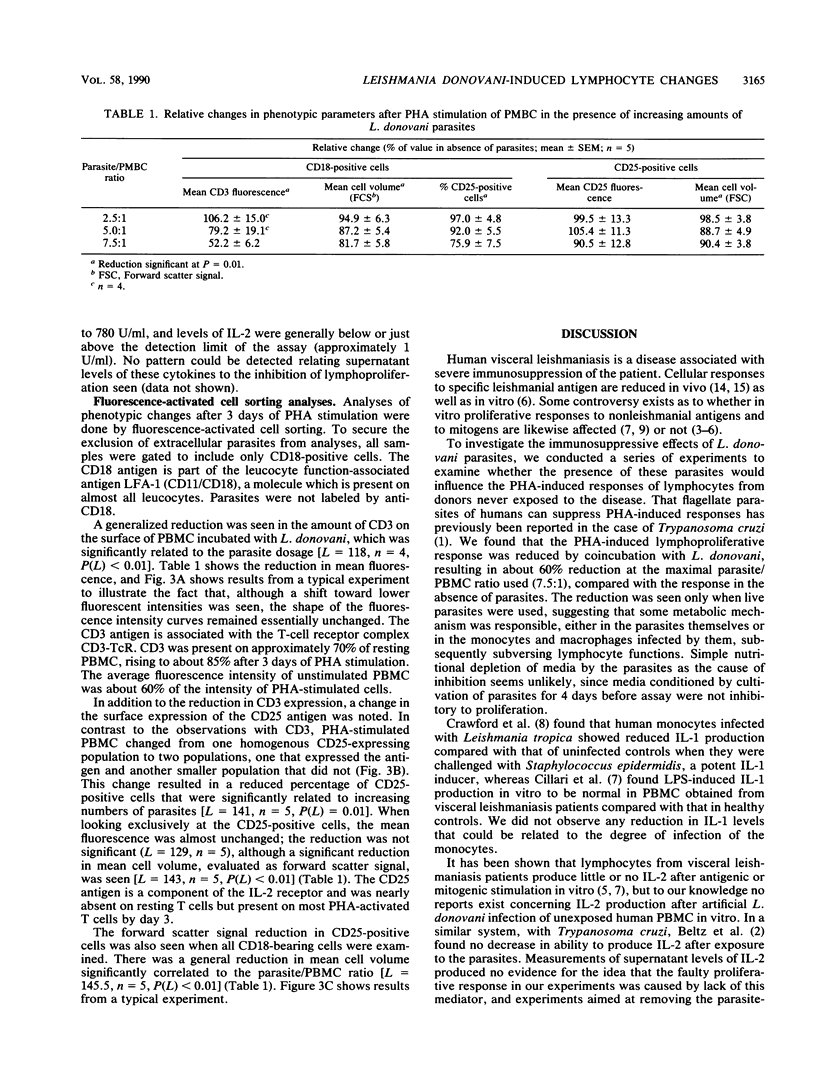
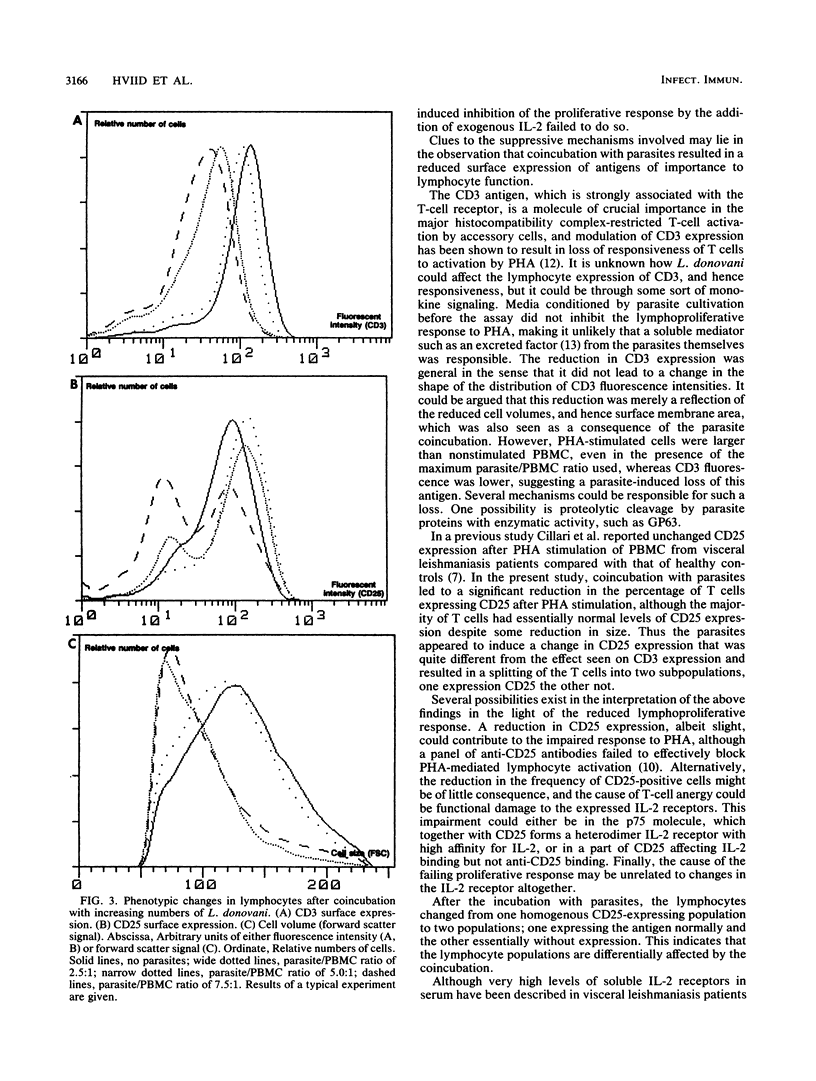
In this paper we describe functional and phenotypic changes in T cells after in vitro coincubation of peripheral blood mononuclear cells (PBMC) and Leishmania donovani parasites at different parasite/peripheral blood mononuclear cell ratios. The phytohemagglutinin (PHA)-induced lymphoproliferative response was reduced by the coincubation, and at the maximal parasite/peripheral blood mononuclear cell ratio used (7.5:1), the average response was less than 40% of the response in the absence of parasites. The cause of the reduction in lymphoproliferation is not clear, but it requires live parasites. Interleukin-1 production was unaffected, the levels of soluble interleukin-2 receptor in supernatants were not changed by the coincubation, and the addition of exogenous interleukin-2 failed to revert the suppressive effect of the parasites. In addition to the reduction in lymphocyte proliferation, phenotypic lymphocyte changes were observed. Cell surface expression of the CD3 antigen, which is part of the CD3-T-cell receptor complex, was significantly reduced with increasing parasite/peripheral blood mononuclear cell ratios; the reduction was general in the sense that the parasites caused a shift in the fluorescent intensities of anti-CD3 labeled cells toward lower values, without affecting the distribution pattern. In contrast, the parasites altered the CD25 (interleukin-2 receptor) expression on PHA-stimulated cells from a homogenous CD25-positive population to two populations, one small and without CD25 expression and the other, larger population with only a slight reduction in size and CD25 expression. In addition to the changes in expression of surface antigens, a general reduction in the size of PHA-stimulated lymphocytes after coincubation with the parasites was observed. The data presented thus suggest that the inhibition of the proliferative response to PHA by live L. donovani in vitro is associated with early processes in lymphocyte activation. Further studies on the inhibitory phenomena described may be of potential significance in the investigation of the suppressive mechanisms in human visceral leishmaniasis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beltz L. A., Kierszenbaum F. Suppression of human lymphocyte responses by Trypanosoma cruzi. Immunology. 1987 Feb;60(2):309–315. [PMC free article] [PubMed] [Google Scholar]
- Beltz L. A., Sztein M. B., Kierszenbaum F. Novel mechanism for Trypanosoma cruzi-induced suppression of human lymphocytes. Inhibition of IL-2 receptor expression. J Immunol. 1988 Jul 1;141(1):289–294. [PubMed] [Google Scholar]
- Carvalho E. M., Bacellar O. A., Reed S., Barral A., Rocha H. Visceral leishmaniasis: a disease associated with inability of lymphocytes to activate macrophages to kill leishmania. Braz J Med Biol Res. 1988;21(1):85–92. [PubMed] [Google Scholar]
- Carvalho E. M., Bacellar O., Barral A., Badaro R., Johnson W. D., Jr Antigen-specific immunosuppression in visceral leishmaniasis is cell mediated. J Clin Invest. 1989 Mar;83(3):860–864. doi: 10.1172/JCI113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Teixeira R. S., Johnson W. D., Jr Cell-mediated immunity in American visceral leishmaniasis: reversible immunosuppression during acute infection. Infect Immun. 1981 Aug;33(2):498–500. doi: 10.1128/iai.33.2.498-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillari E., Liew F. Y., Lo Campo P., Milano S., Mansueto S., Salerno A. Suppression of IL-2 production by cryopreserved peripheral blood mononuclear cells from patients with active visceral leishmaniasis in Sicily. J Immunol. 1988 Apr 15;140(8):2721–2726. [PubMed] [Google Scholar]
- Crawford G. D., Wyler D. J., Dinarello C. A. Parasite-monocyte interactions in human leishmaniasis: production of interleukin-1 in vitro. J Infect Dis. 1985 Aug;152(2):315–322. doi: 10.1093/infdis/152.2.315. [DOI] [PubMed] [Google Scholar]
- Ho M., Koech D. K., Iha D. W., Bryceson A. D. Immunosuppression in Kenyan visceral leishmaniasis. Clin Exp Immunol. 1983 Feb;51(2):207–214. [PMC free article] [PubMed] [Google Scholar]
- Josimovic-Alasevic O., Feldmeier H., Zwingenberger K., Harms G., Hahn H., Shrisuphanunt M., Diamantstein T. Interleukin 2 receptor in patients with localized and systemic parasitic diseases. Clin Exp Immunol. 1988 May;72(2):249–254. [PMC free article] [PubMed] [Google Scholar]
- Kammer G. M., Kurrasch R., Scillian J. J. Capping of the surface OKT3 binding molecule prevents the T-cell proliferative response to antigens: evidence that this molecule conveys the activation signal. Cell Immunol. 1984 Aug;87(1):284–294. doi: 10.1016/0008-8749(84)90152-7. [DOI] [PubMed] [Google Scholar]
- Londner M. V., Frankenburg S., Slutzky G. M., Greenblatt C. L. Action of leishmanial excreted factor (EF) on human lymphocyte blast transformation. Parasite Immunol. 1983 May;5(3):249–256. doi: 10.1111/j.1365-3024.1983.tb00741.x. [DOI] [PubMed] [Google Scholar]
- MANSON-BAHR P. E. Immunity in kala-azar. Trans R Soc Trop Med Hyg. 1961 Nov;55:550–555. doi: 10.1016/0035-9203(61)90078-5. [DOI] [PubMed] [Google Scholar]
- Rezai H. R., Ardehali S. M., Amirhakimi G., Kharazmi A. Immunological features of kala-azar. Am J Trop Med Hyg. 1978 Nov;27(6):1079–1083. doi: 10.4269/ajtmh.1978.27.1079. [DOI] [PubMed] [Google Scholar]



