Abstract
Ghrelin is a hormone produced predominantly by the stomach that targets a number of specific areas in the central nervous system to promote a positive energy balance by increasing food intake and energy storage. In that respect, similarities exist with the effects of consuming a high-fat diet (HFD), which also increases caloric intake and the amount of stored calories. We determined whether the effects of ghrelin on feeding and adiposity are influenced by the exposure to an HFD. Chronic intracerebroventricular ghrelin (2.5 nmol/d) increased feeding in lean rats fed a low-fat control diet (CD) [192±5 g (ghrelin+CD) vs. 152±5 g (control i.c.v. saline+CD), P<0.001], but the combination of ghrelin plus HFD did not result in significantly greater hyperphagia [150±7 g (ghrelin+HFD) vs. 136±4 g (saline+HFD)]. Despite failing to increase food intake in rats fed the HFD, ghrelin nonetheless increased adiposity [fat mass increase of 14±2 g (ghrelin+HFD) vs. 1±1 g (saline+HFD), P<0.001] up-regulating the gene expression of lipogenic enzymes in white adipose tissue. Our findings demonstrate that factors associated with high-fat feeding functionally interact with pathways regulating the effect of ghrelin on food intake. We conclude that ghrelin's central effects on nutrient intake and nutrient partitioning can be separated and suggest an opportunity to identify respective independent neuronal pathways.—Perez-Tilve, D., Heppner, K., Kirchner, H., Lockie, S. H., Woods, S. C., Smiley, D. L., Tschöp, M., and Pfluger, P. Ghrelin-induced adiposity is independent of orexigenic effects.
Keywords: brain, metabolism, melanocortin
Ghrelin is a 28-aa peptide synthesized in the stomach and esterified with an 8-carbon acyl side chain by the action of the enzyme ghrelin octanoyl acyl transferase (GOAT). In addition to being an endogenous ligand of the growth hormone secretagogue receptor (GHSR), ghrelin has an important role in the control of energy metabolism (6). It is the only known circulating factor that potently increases food intake (6), and it affects nutrient partitioning by reducing fat oxidation and stimulating lipid synthesis, predisposing to increased adiposity (6). Ghrelin stimulates food intake by targeting neural pathways in the central nervous system, including the hypothalamus and the hindbrain (1). In the hypothalamic arcuate nucleus, ghrelin activates its receptor on neurons that cosecrete agouti-related peptide (AGRP) and neuropeptide Y (NPY). Both NPY and AGRP, which is an inverse agonist for the melanocortin 4 receptor, stimulate food intake, and both are involved in ghrelin-induced hyperphagia, since mice lacking both NPY and AGRP do not increase their food intake when administered ghrelin (2). Consistent with this, ghrelin stimulation of NPY/AGRP neurons decreases membrane excitability of nearby proopiomelanocortin (POMC) neurons that secrete the melanocortin receptor agonist, melanocyte-stimulating hormone α (αMSH; ref. 3), which normally suppresses food intake; and ghrelin does not stimulate food intake in mice lacking melanocortin receptors (2).
There is evidence that the anabolic actions of ghrelin involve more than hyperphagia. Whereas chronic peripheral (4, 5) or central (6, 7) administration of ghrelin increases both food intake and fat mass, direct administration of ghrelin into the CNS causes activation of lipogenic genes in white adipose tissue (WAT), promoting the de novo synthesis of triglycerides, and it increases insulin-stimulated glucose uptake in adipocytes (6). Likewise, peripheral (8) or central (9) administration of ghrelin in rats triggers lipogenic gene programs in the liver and increases hepatic lipid content, and mice lacking the ghrelin receptor are protected from ghrelin-induced hepatic steatosis (4).
Although its anabolic role in the regulation of energy balance is well documented, functionally relevant interactions between macronutrient availability and specific components of ghrelin's multifaceted impact on energy balance have not been thoroughly explored. This is important, because dysregulation of energy homeostasis in metabolic disease has been associated with altered endogenous ghrelin. Obese humans and rodents have reduced plasma ghrelin levels (10, 11), and mice with diet-induced obesity (DIO) are relatively insensitive to the orexigenic effect of ghrelin (10, 12, 13). DIO mice have reduced secretion of arcuate neuropeptides (16), as well as fewer projections from the arcuate nucleus to the paraventricular nucleus (PVN) in rats (14), and the activation of arcuate NPY/AGRP neurons by ghrelin (13) is blunted in DIO mice.
The mechanism for the reduced ghrelin sensitivity in DIO animals is unclear. It could be due to toxic effects of dietary fatty acids, to increased flux of newly ingested calories, or to secondary factors associated with the development of obesity. Indeed, mice that develop obesity without exposure to a high-fat diet (HFD), such as leptin receptor-deficient mice (db/db), also have a reduced feeding response to ghrelin (12). The aim of this study was to determine whether ghrelin has actions on body weight that are independent of food intake, and/or whether ghrelin stimulation of food intake is independent of body weight. We used an experimental paradigm in which lean rats were fed a low-fat control diet (CD) or else a calorically dense HFD while receiving ghrelin chronically. We found that ghrelin influences distinct components of energy balance via targeting different neuronal pathways. Specifically, stimulation of food intake by exogenous ghrelin is mediated through neuronal pathways that are also targeted by signals generated by consuming an HFD and not by signals related to obesity per se. In contrast, ghrelin acts to increase fat mass by mechanisms unrelated to consuming an HFD. The persistence of ghrelin-induced adipogenesis despite a lack of ghrelin-induced hyperphagia suggests novel therapeutic opportunities by selectively targeting specific aspects of ghrelin's actions.
MATERIALS AND METHODS
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati. All experimental procedures were carried out in accordance with the U.S. National Institutes of Health guidelines for the care and use of laboratory animals.
Animals
Male Long-Evans (275–300 g) and Wistar (260–290 g) rats were obtained from Harlan (Indianapolis, IN, USA) and maintained in a temperature- and humidity-controlled vivarium with a 12-h light-dark cycle. They were individually housed in tub cages and had free access to food and tap water at all times unless otherwise specified.
Peptides
Ghrelin was synthetized using in situ neutralization for Boc chemistry, purified by preparative chromatography, and characterized by HPLC and mass spectral analysis, as described previously (5). SHU9119 was purchased from Bachem (Torrance, CA, USA).
Diets
The rats were fed pelleted low-fat chow CD (3.5 kcal/g; Teklad; Harlan) or a pelleted HFD (4.54 kcal/g; 38% of calories derived from butter and 2% from soybean oil; D03082706; Research Diets, Brunswick, NJ, USA).
Acute intracerebroventricular (i.c.v.) ghrelin
Male Long-Evans rats were implanted with a stainless-steel cannula into the third ventricle of the brain. The surgical procedure and the test of the placement of the cannula have been previously described (15). The acute i.c.v. injection (1 μl) of ghrelin (10 μg) or vehicle (saline) was performed with a Hamilton syringe (Hamilton, Reno, NV, USA), 2 h after the onset of light. Food intake was monitored for 2 h. To assess the effect of acute exposure to HFD on the orexigenic activity of ghrelin, the CD was replaced by HFD immediately after the administration of ghrelin or saline in some animals. To assess the effect of a short-term exposure to HFD on the orexigenic activity of ghrelin, the CD was replaced by HFD at the onset of the dark, 14 h prior to the injections. In both instances, the HFD remained on the cages until 2 h after the i.c.v. injection.
Chronic i.c.v. infusion of ghrelin or SHU9119
Male Wistar rats were implanted with a stainless-steel cannula in the lateral ventricle; the cannula was connected to an osmotic minipump (1002; Alzet Durect, Cupertino, CA, USA) subcutaneously placed in the interscapular space, as described previously (16). The rats received ghrelin at 2.5 or 25 nmol/d, SHU9119 at 2.5 nmol/d, or vehicle (saline) for 7 d. Prior to the surgery, the rats were fed the CD. After the surgery, the diet of half the rats was changed to HFD during the 7-d infusion period. Food intake and body weight were monitored daily. Body composition was determined by nuclear magnetic resonance (echoMRI, Houston, TX, USA) immediately before surgery and at the end of the infusion period. After d 7 of infusion, the rats were euthanized (2–4 h after the onset of light), and plasma, brain, liver, and epididymal WAT were collected and stored at −80°C.
Plasma determinations
Blood glucose levels were determined using glucose strips (FreeStyle; Abbot, Alameda, CA, USA). Plasma lipid levels were determined using commercial enzymatic kits (Thermo Scientific, Rockford, IL, USA) following the manufacturer's instructions. Adiponectin, insulin, and leptin levels were determined using a multiplex immunoassay (Milipore, Billerica, MA, USA), following the manufacturer's instructions. Thyroxine (T4) and triiodothyronine (T3) levels were determined by RIA (DSL, Webster, TX, USA, and MP Biomedicals, Solon, OH, USA, respectively).
Gene expression analysis
RNA from WAT, liver, and hypothalamus was extracted using a commercially available kit (RNeasy Lipid Tissue Mini Kit; Qiagen Valencia, CA, USA) following the manufacturer's instructions. After DNase I treatment (Invitrogen, Carlsbad, CA, USA), cDNA was synthesized using SuperScript III (Invitrogen) following the manufacturer's instructions. Gene expression was determined by qPCR using SYBR Green qPCR Mix (Bio-Rad Laboratories, Hercules, CA, USA) using the standard curve method, as described previously (17). The levels of expression of the target genes were normalized against the results obtained for the housekeeping gene (HPRT). The primers for qPCR were designed using mRNA National Center for Biotechnology Information (NCBI) reference sequences using the online software Primer3plus (18).
Statistical analysis
Data are presented as means ± se. Student's t test for independent samples was used for comparisons between 2 groups. Two-way ANOVA followed by Bonferroni's post hoc test was used for comparisons between multiple, independent groups.
RESULTS
Effect of short-term exposure to HFD on the orexigenic effect of acute ghrelin
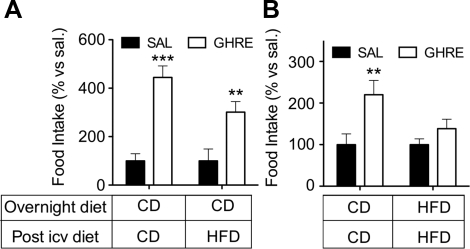
We hypothesized that the orexigenic effect of i.c.v. ghrelin would be blunted by acute, simultaneous exposure to an HFD. We injected ghrelin (10 μg) or vehicle 2 h after the onset of light in male Long-Evans rats normally fed CD. Immediately after the injection, half of the rats were given access to HFD and half remained on CD. Ghrelin significantly increased feeding, independent of the diet that the rats were fed (Fig. 1A); i.e., having simultaneous access to the HFD had no effect in rats that had been fed the CD until then. A different outcome occurred when rats had been allowed to consume HFD during the dark prior to receiving the i.c.v. injection. As expected, ghrelin-treated rats fed with CD had a significant increase in food intake relative to controls (Fig. 1B). In contrast, ghrelin failed to significantly increase food intake in rats that had had access to HFD during the previous dark phase, relative to the control rats (Fig. 1B). Taken together, these data suggest that some aspect of the HFD interferes with ghrelin signaling.
Figure 1.
Short-term exposure to HFD abrogates the orexigenic effect of ghrelin. Male Long-Evans rats were injected i.c.v. (1 μl, third ventricle) with saline or ghrelin (10 μg) 2 h after the onset of the light phase, and food intake was recorded after 2 h. A) Rats had been fed low-fat, chow diet (CD) at all times prior to the injection. Immediately after the i.c.v. injection, rats received CD or an HFD. B) A subset of rats was fed an HFD during the dark phase prior to i.c.v. injection and was fed the same HFD after the treatment. The rest of the rats were fed CD for the entire duration of the experiment. Acute access to an HFD does not prevent the orexigenic response induced by ghrelin (A). In contrast, the short-term (overnight) exposure to HFD prevents the hyperphagia induced by ghrelin when compared to the corresponding saline-infused control group (B). Data are expressed as mean ± se percentage of change vs. corresponding control group (n=8). Ghre, ghrelin; sal, saline. **P<0.01, ***P<0.001 vs. corresponding saline; 2-way ANOVA with Bonferroni post hoc test.
Effect of chronic exposure to HFD on the orexigenic and adipogenic effects induced by chronic administration of ghrelin
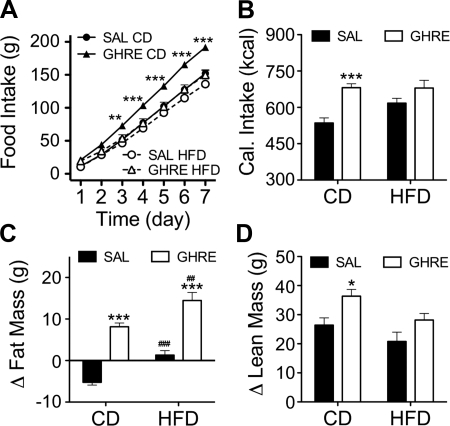
We found that even a minimal, several-hour exposure to HFD significantly attenuated the orexigenic action of ghrelin, perhaps accounting for reports that longer-term exposure to HFD has the same effect. We next asked whether another CNS-mediated effect of ghrelin, its ability to increase adiposity, is also affected in rats fed an HFD. Therefore, we infused i.c.v. ghrelin at a rate of 2.5 nmol/d, or saline, via minipumps for 7 d. All of the rats had been fed CD and had no previous exposure to HFD, but immediately after the i.c.v. cannulation, the diet of half the rats was changed to the HFD. Ghrelin induced a significant increase in food intake in the rats fed CD, but failed to increase feeding in rats fed the HFD relative to controls (Fig. 2A). Notably, calories consumed during the week of treatment did not differ between vehicle-infused control rats fed HFD, ghrelin-infused rats fed HFD, and ghrelin-infused rats fed CD (Fig. 2B). Interestingly, the amount of calories eaten by ghrelin-treated rats was statistically similar to the value observed in the control rats that had free access to HFD (Fig. 2B). As expected, the increased caloric intake induced by ghrelin in CD-fed rats was accompanied by an increase in fat mass (Fig. 2C). Surprisingly, and despite the absence of hyperphagia, central infusion of ghrelin also promoted adiposity in rats, consuming the HFD relative to vehicle-infused rats consuming the same amount of the HFD (Fig. 2C). In fact, and despite comparable caloric intake, ghrelin-treated rats fed the HFD gained significantly more fat than ghrelin-treated CD-fed rats (Fig. 2C).
Figure 2.
High-fat feeding prevents the hyperphagia induced by chronic central infusion of ghrelin but does not prevent the ghrelin-induced adiposity. Male Wistar rats fed CD were infused i.c.v. (lateral ventricle) for 1 wk with saline or ghrelin (2.5 nmol/d). Immediately after initiation of infusion, diet of half of the rats was switched to HFD. A) Ghrelin induced a significant increase in food intake in the rats fed CD, but this orexigenic effect was prevented by feeding the rats HFD, independently of the dose of ghrelin administered. B) Consequently, the cumulative caloric intake over 1 wk increased with the i.c.v. injection of ghrelin in CD- but not in HFD-fed rats, when compared with the corresponding saline-infused control group. C) Despite the similar caloric intake from HFD, i.c.v. ghrelin still induced a significant increase in fat mass in HFD-fed rats compared to saline-infused control rats. D) HF feeding suppressed the increase in lean mass induced by central infusion of ghrelin. Data are expressed as means ± se (n=8–10). Ghre, ghrelin; sal, saline. *P < 0.05, **P < 0.01, ***P < 0.001 vs. corresponding saline; 2-way ANOVA with Bonferroni post hoc test. ##P < 0.01, ###P < 0.001 vs. corresponding CD; t test.
Overall, baseline insulin levels were not significantly different among groups (although a post hoc t test revealed a significant increase (P<0.05) of insulin in ghrelin-infused rats fed the CD relative to their saline controls). As expected, because of the increased fat mass, leptin levels were significantly increased in CD-fed ghrelin-infused rats compared to controls (Table 1). Consuming the HFD increased plasma leptin (P<0.01), but leptin was not significantly elevated in ghrelin-infused HFD rats in comparison with HFD-infused controls, despite the higher fat mass (Table 1). Similarly, HFD, but not central infusion of ghrelin, increased the levels of circulating thyroxine (P<0.01) and triiodothyronine (P<0.001) (Table 1).
Table 1.
Effect of chronic infusion of ghrelin (2.5 nmol/d, 7 d) on plasma parameters
| Parameter | Saline |
Ghrelin |
P value |
||||
|---|---|---|---|---|---|---|---|
| Value | n | Value | n | Treatment | Diet | Ghrelin vs. saline | |
| Glucose (mg/dl) | |||||||
| CD | 102.0 ± 1.7 | 9 | 105.6 ± 4.1 | 9 | |||
| HFD | 106.1 ± 2.8 | 9 | 107.8 ± 2.5 | 9 | |||
| Triglycerides (mg/dl) | <0.05 | ||||||
| CD | 94.6 ± 9.5 | 9 | 98.2 ± 8.7 | 9 | |||
| HFD | 237.4 ± 28.7** | 9 | 198.9 ± 15.9** | 9 | |||
| Insulin (ng/ml) | |||||||
| CD | 3.0 ± 0.2 | 8 | 4.5 ± 0.4 | 8 | |||
| HFD | 3.7 ± 0.9 | 8 | 3.9 ± 0.5 | 8 | |||
| Leptin (ng/ml) | <0.01 | <0.01 | |||||
| CD | 3.5 ± 0.6 | 8 | 5.8 ± 0.3 | 8 | <0.01 | ||
| HFD | 5.5 ± 0.5 | 8 | 6.7 ± 0.6 | 8 | |||
| Adiponectin (ng/ml) | |||||||
| CD | 75.0 ± 30.2 | 8 | 61.2 ± 5.0 | 8 | |||
| HFD | 39.0 ± 2.8 | 8 | 174.9 ± 77.3 | 8 | |||
| Thyroxine (ng/ml) | <0.01 | ||||||
| CD | 38.2 ± 3.3 | 8 | 40.7 ± 2.1 | 8 | |||
| HFD | 47.6 ± 2.7 | 8 | 51.2 ± 2.6 | 8 | |||
| Triiodothyronine (ng/ml) | <0.001 | ||||||
| CD | 0.7 ± 0.0 | 8 | 0.7 ± 0.0 | 8 | |||
| HFD | 1.2 ± 0.1 | 8 | 1.2 ± 0.1 | 8 | |||
Values are means ± se.
P < 0.01 vs. CD.
Central infusion of ghrelin increased the lean mass in rats fed CD but not in rats fed the HFD (Fig. 2D). These data suggest that signals triggered by HFD exposure directly interact with mechanisms activated by ghrelin to increase feeding and lean mass. However, ghrelin apparently induces adiposity by different mechanisms from those involved in the promotion of feeding and lean mass gain, since the HF-associated signals are unable to block the mechanisms, whereby ghrelin induces adiposity.
To determine whether the absence of orexigenic activity of ghrelin in rats fed the HFD was due to using an insufficient dose of ghrelin, we repeated the experiment infusing a 10-fold higher dose of ghrelin (25 nmol/d). Consistent with the data obtained with the lower dose, rats fed the HFD did not have ghrelin-induced hyperphagia or gain of lean mass. Increased adiposity was observed, however, with this higher ghrelin dose in both CD- and HFD-fed rats, and was larger in ghrelin-infused HFD rats compared to ghrelin-infused CD rats, despite similar caloric intake (Table 2). These data suggest that the inhibition of ghrelin-induced hyperphagia and gain of lean mass observed with the lower dose of ghrelin was not due to insufficient pharmacological activation of central ghrelin signaling.
Table 2.
Effect of chronic infusion of ghrelin (25 nmol/d, 7 d) on food and caloric intake, fat mass, and lean mass
| Parameter | Saline |
Ghrelin |
P value |
||||
|---|---|---|---|---|---|---|---|
| Value | n | Value | n | Treatment | Diet | Ghrelin vs. saline | |
| Food intake (g) | <0.001 | <0.001 | |||||
| CD | 155.1 ± 3.7 | 9 | 193.6 ± 4.0 | 8 | <0.001 | ||
| HFD | 146.7 ± 5.3 | 9 | 158.5 ± 5.0*** | 9 | |||
| Caloric intake (kcal) | <0.001 | <0.001 | |||||
| CD | 542.7 ± 12.8 | 9 | 677.4 ± 14.0 | 8 | <0.001 | ||
| HFD | 666.1 ± 24.1*** | 9 | 719.4 ± 22.9 | 9 | |||
| Δ Fat mass (g) | <0.001 | <0.001 | |||||
| CD | 1.9 ± 1.1 | 9 | 11.2 ± 1.3 | 8 | <0.001 | ||
| HFD | 9.3 ± 1.5*** | 9 | 16.6 ± 1.8* | 9 | <0.01 | ||
| Δ Lean mass (g) | <0.05 | <0.01 | |||||
| CD | 31.2 ± 2.2 | 9 | 44.0 ± 2.7 | 8 | <0.05 | ||
| HFD | 27.9 ± 4.2 | 9 | 27.5 ± 2.2*** | 9 | |||
Values are means ± se.
P < 0.05,
P < 0.001 vs. CD.
Effect of HFD on hyperphagia and increased adiposity elicited by blockade of the CNS melanocortin system
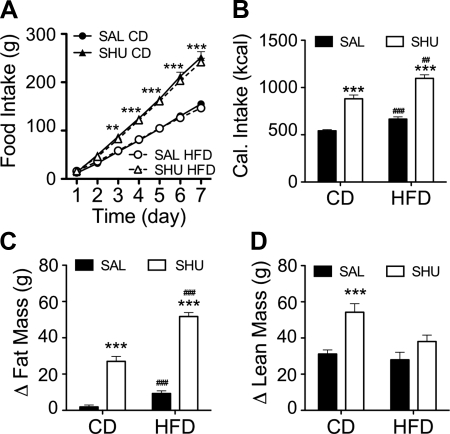
Some of the effects of ghrelin to increase food intake and adipose mass have been attributed to the increased secretion of AgRP, an inverse agonist of the melanocortin (MC) receptors, MC3 and MC4; i.e., increased ghrelin activity is thought to stimulate the secretion of AgRP, leading to reduced melanocortinergic activity in the hypothalamus. We asked whether the inhibitory effect of an HFD on ghrelin-induced hyperphagia occurs downstream of the actions of ghrelin on the melanocortin system. We implanted osmotic minipumps containing the MC3/4 receptor antagonist SHU9119 (SHU), or saline, in male Wistar rats fed CD. Immediately after the surgery, half of the rats were changed to HFD for the 7-d infusion period. In contrast with what we had observed with ghrelin, central chronic infusion of SHU (2.5 nmol/d) elicited a robust hyperphagia that was not altered by the HFD (Fig. 3A); i.e., chronic i.c.v. SHU led to a significant increase in caloric intake independent of diet (Fig. 3B). We also observed a parallel gain in adiposity (Fig. 3C). Central infusion of SHU also significantly increased lean mass in rats fed CD, but not in HFD-exposed rats (Fig. 3D). The lack of gain of lean mass in rats consuming the HFD is similar to what we had observed in ghrelin-infused rats, but in the case of the SHU-infused rats, it cannot be attributed to a decrease in caloric intake induced by the HFD. Therefore, consumption of an HFD does not suppress the orexigenic activity of ghrelin by targeting mechanisms downstream to the MC receptors. The inhibition of gain of lean mass but not of adiposity that occurs on the HFD when rats are given ghrelin suggests that the induction of adiposity is a primary action, whereas the gain of lean mass may be a secondary effect of central ghrelin and melanocortin signaling.
Figure 3.
High-fat feeding does not prevent the hyperphagia induced by chronic central infusion of the melanocortin receptor blocker SHU9119. Male Wistar rats fed CD were infused i.c.v. (lateral ventricle) for 1 wk with saline or SHU9119 (2.5 nmol/d). Immediately after initiation of infusion, diet of half of the rats was switched to HFD. A–C) Intracerebroventricular infusion of SHU for 1 wk induced a significant increase in food intake, irrespectively of the diet (A), which led to a significant increase in caloric intake (B), and fat mass content (C) in comparison with the saline-infused control rats. D) HF feeding suppressed the increase in lean mass induced by central infusion of SHU. Data are expressed as means ± se (n=8–10). Ghre, ghrelin; sal, saline. **P < 0.01, ***P < 0.001 vs. corresponding saline; 2-way ANOVA with Bonferroni post hoc test. ##P < 0.01, ###P < 0.001 vs. corresponding CD; t test.
Effect of consuming an HFD on ghrelin-induced induction of lipogenic genes in WAT
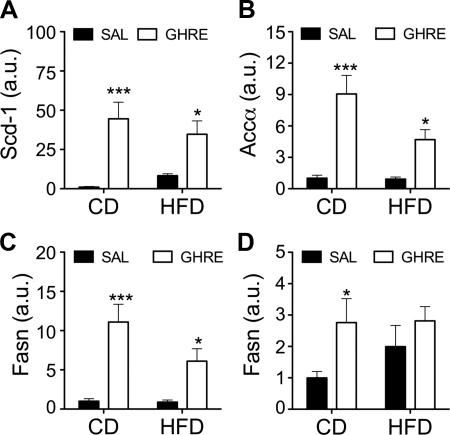
Central ghrelin promotes adiposity by directly increasing de novo lipogenesis in WAT. Specifically, ghrelin activity in the CNS leads to up-regulation of the expression of genes of enzymes involved in the synthesis of triglycerides. This up-regulation is not secondary to the hyperphagia induced by ghrelin, since it cannot be prevented with forced isocaloric pair-feeding. Therefore, we hypothesized that ghrelin would increase adiposity in rats fed an HFD, despite the isocaloric intake by facilitating de novo lipogenesis in WAT through the up-regulation of gene expression of lipogenic enzymes. To test this, we extracted mRNA from the epididymal fat depot of Wistar rats treated for 1 wk with ghrelin (2.5 nmol/d i.c.v.) that were fed CD or HFD, as depicted in Fig. 2. Ghrelin administration significantly up-regulated the expression of genes for lipogenic enzymes, including stearoyl-CoA desaturase-1 (Scd-1), acetyl-CoA carboxylase (Acca), and fatty acid synthase (Fasn) (Fig. 4A–C), in both CD- and HFD-fed rats, in comparison to their corresponding control groups. These data suggest that the signals induced by consuming an HFD that are responsible for the suppression of ghrelin-induced hyperphagia do not also target the central mechanisms responsible for ghrelin-induced regulation of WAT lipogenesis. We also assessed lipogenic genes in another key lipogenic organ, the liver. Intracerebroventricular ghrelin induced a significant increase in the expression of Fasn in the liver of CD-fed rats (Fig. 4D). However, this was suppressed in rats fed the HFD. This suggests that ghrelin either does not directly regulate hepatic triglyceride synthesis, or that it exerts tissue-specific regulation of lipogenesis through a different central mechanism, one that is vulnerable to or cotriggered by yet-to-be-defined signals derived from consuming an HFD.
Figure 4.
Chronic central infusion of ghrelin up-regulates the gene expression of lipogenic enzymes in WAT in CD- and HFD-fed rats. A–C) Chronic infusion of ghrelin significantly increased the gene expression of the lipogenic enzymes Scd-1 (A), Acca (B), and Fasn (C) in epididymal WAT. D). In contrast, gene expression of Fasn in liver was only significantly increased in ghrelin-infused CD-fed rats. Data are expressed as means ± se (n=7). a.u., arbitrary units; ghre, ghrelin; sal, saline. *P < 0.05, ***P < 0.001 vs. corresponding saline; 2-way ANOVA with Bonferroni post hoc test.
Effect of ghrelin and consuming an HFD on the regulation of the expression of neuropeptides and receptors in the mediobasal hypothalamus
Ghrelin has been suggested to target the hypothalamic arcuate nucleus to exert its effects on feeding. To gain insight into the central circuits that could be targeted by signals derived from consuming an HFD that inhibit ghrelin-induced hyperphagia, we analyzed the expression of several neuropeptides and receptors involved in the control of food intake that are expressed in the arcuate nucleus. Chronic i.c.v. infusion of ghrelin induced a modest, but significant, reduction in hypothalamic ghrelin receptor expression (P<0.05; see Table 3). However, neither chronic ghrelin treatment nor consuming an HFD for 1 wk significantly altered the expression of genes for the leptin receptor, AgRP, NPY, CART, or POMC. Thus, the ability of the HFD to attenuate the orexigenic activity of ghrelin is likely independent of changes in the gene expression of neuropeptides expressed in the arcuate nucleus that are involved in the control of feeding.
Table 3.
Effect of chronic infusion of ghrelin (2.5 nmol/d, 7 d) in receptor and neuropeptide expression in the mediobasal hypothalamus
| Peptide or receptor | Saline |
Ghrelin |
P value, treatment | ||
|---|---|---|---|---|---|
| Value | n | Value | n | ||
| GHSR | <0.05 | ||||
| CD | 1.00 ± 0.07 | 7 | 0.85 ± 0.04 | 7 | |
| HFD | 1.01 ± 0.11 | 7 | 0.76 ± 0.09 | 7 | |
| LEPR | |||||
| CD | 1.00 ± 0.05 | 7 | 1.17 ± 0.10 | 7 | |
| HFD | 1.23 ± 0.13 | 7 | 1.09 ± 0.15 | 7 | |
| AGRP | |||||
| CD | 1.00 ± 0.10 | 7 | 1.02 ± 0.13 | 7 | |
| HFD | 1.30 ± 0.18 | 7 | 1.06 ± 0.22 | 7 | |
| NPY | |||||
| CD | 1.00 ± 0.11 | 7 | 1.28 ± 0.20 | 7 | |
| HFD | 1.50 ± 0.28 | 7 | 1.34 ± 0.16 | 7 | |
| POMC | |||||
| CD | 1.00 ± 0.23 | 7 | 0.91 ± 0.36 | 7 | |
| HFD | 1.12 ± 0.20 | 7 | 0.98 ± 0.29 | 7 | |
| CART | |||||
| CD | 1.00 ± 0.20 | 7 | 0.72 ± 0.08 | 7 | |
| HFD | 0.96 ± 0.12 | 7 | 1.06 ± 0.13 | 7 | |
Values are means ± se.
DISCUSSION
The intimate correlation between overfeeding and the development of obesity has been a limiting factor in the exact dissection of pathogenic mechanisms leading to metabolic disease. Here, we show that changing from consumption of a control low-fat diet to a calorically dense HFD blunts ghrelin-induced hyperphagia without affecting ghrelin's ability to increase adiposity. Our data demonstrate that whereas central ghrelin simultaneously regulates feeding and peripheral WAT lipid metabolism, it obviously does so through separable mechanisms.
The effect of consuming an HFD to neutralize ghrelin-induced hyperphagia cannot be attributed to a change in the taste perception for the new diet. This is supported by our data that rats with no prior exposure to the HFD had significantly increased acute food intake when provided with HFD immediately after the i.c.v. injection of ghrelin, in comparison with the controls. In support of this finding, previous reports indicate that ghrelin not only increases food intake, but actually increases the preference for foods with higher fat content (19). However, the exposure to the HFD for only 1 night in lean rats previously fed CD was sufficient to neutralize the hyperphagia induced by the acute central injection of ghrelin. Further, consuming the HFD was able to suppress the hyperphagia induced by the chronic central administration of ghrelin, as well as to an acute ghrelin administration, and this phenomenon was apparent when very high doses of the peptide were infused. Collectively, these data suggest that factors associated with the nutrient composition of the HFD, rather than its specific organoleptic properties, are responsible for the inhibition of the orexigenic action of ghrelin by HFD. Furthermore, the suppression of the ghrelin-induced hyperphagia after a short, several-hour exposure to HF feeding suggests that factors related to acute exposure to the diet per se, and not secondary metabolic alterations due to chronic HFD consumption, such as obesity, are responsible for the loss of hyperphagia. The suppression of the orexigenic action of ghrelin by more chronic consumption of an HFD has been previously reported. Acute i.c.v. ghrelin failed to increase feeding in mice with HFD-induced obesity (13). Moreover, consumption of an HFD prevented the slight increase in food intake induced by a chronic peripheral infusion of ghrelin in mice fed regular chow (20).
When food intake was evaluated by the caloric content of the diet, we found that despite the suppression of the hyperphagia, ghrelin, nonetheless, increased the caloric intake in rats fed the HFD to the same level as occurred in CD rats given ghrelin. Interestingly, the caloric intake of the ghrelin-treated rats fed the HFD was similar to that of control rats fed HFD. This result suggests that ghrelin regulates feeding by raising the caloric intake to a certain threshold level, which is the same that can be reached by consuming an HFD. Thus, the neural mechanisms regulating feeding that are targeted by ghrelin seem to respond to negative feedback once a specific amount of calories has been ingested. This mechanism could act to prevent excessive feeding in response to afferent signals, such as ghrelin.
The central MC system is a critical component in the stimulation of feeding by ghrelin. Ghrelin activates arcuate neurons that coexpress NPY and the MC4R inverse agonist, AgRP (1, 21), and ghrelin loses the ability to increase food intake in animal models lacking melanocortin receptors (2). On the basis of the central role of MC receptors mediating the hyperphagia induced by ghrelin, we asked whether the suppression of feeding induced by HFD occurred at the level of the MC receptors. In contrast to what we had previously observed with the infusion of ghrelin, the increase in food intake induced by the central administration of the MC receptor antagonist SHU9119 was unaffected by exposure to the HFD. Thus, the feeding response induced by the blockade of the melanocortin receptors is not limited to a specific upper level of caloric intake, as occurs with ghrelin, but rather may be limited by other factors. Furthermore, this result suggests that consuming an HFD suppresses the orexigenic action of ghrelin-targeting sites that are either upstream to the regulation of the melanocortin receptor activity, or suppresses the melanocortin system independent ghrelin-induced hyperphagia. In addition to arcuate NYP/AGRP neurons, the ghrelin receptor is expressed in several other nuclei in the CNS that are involved in the control of feeding (22). In fact, administration of ghrelin into extrahypothalamic regions, such as the hindbrain (23) or the ventral tegmental area (VTA; ref. 24), stimulates feeding. Therefore, in addition to any effect on arcuate neurons (13), consuming the HFD might interfere with the hyperphagia induced by the action of ghrelin in extrahypothalamic areas.
In addition to increasing food intake, central infusion of ghrelin or SHU promoted a significant increase in fat mass, as previously reported (6, 16). The increase in adiposity induced by the central action of ghrelin and SHU involves the activation of lipogenic gene programs in WAT (6, 16). Interestingly, despite suppressing the orexigenic effect of ghrelin, consuming an HFD did not inhibit the effect of central ghrelin to increase adiposity. Even with comparable caloric intake as occurred in saline-treated CD and HFD rats, ghrelin treatment in rats fed the HFD still caused a robust increase in fat mass compared to what occurred in the saline-treated groups. The increase of fat mass was associated with an increase in the expression of genes for lipogenic enzymes in WAT. The persistence of the control over the gene expression of lipogenic enzymes despite the suppression of feeding induced by HFD suggests that ghrelin increases adiposity and controls food intake through simultaneously activated, but ultimately independent, mechanisms.
In addition to the activation of lipogenesis in WAT, ghrelin also stimulates lipid synthesis in the liver. Peripheral administration of ghrelin increases the triglyceride content and the gene expression of lipogenic enzymes in the liver without increasing food intake (8). The CNS also influences hepatic lipid metabolism since it can be triggered by central administration of ghrelin (9). The present data are consistent with this and suggest that ghrelin can increase hepatic lipogenesis, as indicated by the higher expression of fatty acid synthase in the liver of CD-fed rats. In contrast to what occurred in WAT, feeding the rats an HFD prevented the increase of lipogenic enzymes in the liver. Altogether, these data suggest that the control of lipid metabolism in different peripheral tissues by a central action of ghrelin might be mediated by different neuronal circuits. Thus, ghrelin regulation of lipogenesis in WAT appears to be mediated by pathways that are unaffected by exposure to an HFD. This is in contrast to ghrelin's stimulation of lipogenesis in the liver, which appears to be controlled by central pathways susceptible to suppression by consuming an HFD. Alternatively, ghrelin-induced lipogenesis in the liver could be secondary to the increased caloric intake.
Ghrelin was initially discovered during the search for the endogenous ligand of the growth hormone secretagogue receptor (GHSR; ref. 25). Thus, in addition to its role in stimulating food intake and adiposity, ghrelin may be important in the secretion of growth hormone (GH). As a result of its stimulatory effect on the somatotropic axis, treatment with ghrelin or other GHSR agonists induces a significant increase in lean mass both in rodents (26) and humans (27–29). Consistent with this, we found that the central infusion of ghrelin significantly increased lean mass in CD-fed rats. In contrast to the gain of fat mass, the increase in lean mass induced by central ghrelin is suppressed by consumption of an HFD. This is perhaps consistent with the reported lack of a stimulatory effect of i.c.v. ghrelin on GH secretion in DIO mice (12, 13). Previous reports have suggested that the ability of ghrelin to regulate adiposity is independent of its effects on growth hormone secretion (9, 30).
Negative energy balance reduces the activity of the hypothalamic-pituitary-thyroid (HPT) axis in humans, resulting in a decrease of thyroid hormone levels that can be partially restored with the administration of leptin (31). In our experiments, the positive energy balance induced by 1 wk of HFD increased circulating levels of thyroxin (T4) and triiodothyronine (T3) (Table 2). Interestingly, the positive energy balance induced by ghrelin did not affect thyroid hormone levels, despite the increase in both caloric intake and plasma leptin levels. This lack of HPT stimulation may contribute to maximizing ghrelin's effect on adiposity.
Blockade or genetic deletion of melanocortin receptor signaling, specifically from MC4R, not only results in the development of obesity, but induces an increase in lean mass, both in rodents and humans (32, 33). It is unknown whether the melanocortin and ghrelin receptors regulate lean mass through separate or similar mechanisms. We found that similar to treatment with ghrelin, HFD consumption suppresses lean mass gain, but not the increased adiposity induced by SHU. The prevention of lean mass gain cannot be solely explained by the lack of sufficient caloric intake since it was observed with ghrelin treatment. In fact, the suppression of gain of lean mass occurs despite the increase in caloric intake induced by SHU in rats fed the HFD. Altogether, our results suggest that consuming an HFD interacts with the mechanisms, whereby ghrelin and melanocortin receptors promote the increase in lean mass and that these are independent of the mechanisms by which ghrelin and the melanocortin system control adiposity.
In summary, our data imply that the processes promoting positive energy balance in response to ghrelin are initiated through different neural pathways that can be differentially influenced by consuming an HFD. Unknown HFD-derived signals attenuate the stimulatory effect of ghrelin on feeding and on gain of lean mass but do not interfere with its anabolic effect on adiposity. On the basis of our data, ghrelin could contribute to the persistence of an overweight phenotype despite controlled caloric intake, if changes in feeding habits, such as reducing the amount of fat contained in the meals are not properly implemented. Furthermore, our results suggest that appropriate diet control might significantly increase the effectiveness of ghrelin-based therapies designed to promote the increase in lean mass. The signals triggered by HFD feeding and the specific neural pathways, whereby those signals interfere with ghrelin action in the CNS still require further investigation.
Acknowledgments
This work was supported by the U.S. National Institutes of Health (DK077975).
REFERENCES
- 1. Cowley M. A., Smith R. G., Diano S., Tschop M., Pronchuk N., Grove K. L., Strasburger C. J., Bidlingmaier M., Esterman M., Heiman M. L., Garcia-Segura L. M., Nillni E. A., Mendez P., Low M. J., Sotonyi P., Friedman J. M., Liu H., Pinto S., Colmers W. F., Cone R. D., Horvath T. L. (2003) The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37, 649–661 [DOI] [PubMed] [Google Scholar]
- 2. Chen H. Y., Trumbauer M. E., Chen A. S., Weingarth D. T., Adams J. R., Frazier E. G., Shen Z., Marsh D. J., Feighner S. D., Guan X. M., Ye Z., Nargund R. P., Smith R. G., Van der Ploeg L. H., Howard A. D., MacNeil D. J., Qian S. (2004) Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 145, 2607–2612 [DOI] [PubMed] [Google Scholar]
- 3. Tong Q., Ye C. P., Jones J. E., Elmquist J. K., Lowell B. B. (2008) Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 11, 998–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davies J. S., Kotokorpi P., Eccles S. R., Barnes S. K., Tokarczuk P. F., Allen S. K., Whitworth H. S., Guschina I. A., Evans B. A., Mode A., Zigman J. M., Wells T. (2009) Ghrelin induces abdominal obesity via GHS-R-dependent lipid retention. Mol. Endocrinol. 23, 914–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tschop M., Smiley D. L., Heiman M. L. (2000) Ghrelin induces adiposity in rodents. Nature 407, 908–913 [DOI] [PubMed] [Google Scholar]
- 6. Theander-Carrillo C., Wiedmer P., Cettour-Rose P., Nogueiras R., Perez-Tilve D., Pfluger P., Castaneda T. R., Muzzin P., Schurmann A., Szanto I., Tschop M. H., Rohner-Jeanrenaud F. (2006) Ghrelin action in the brain controls adipocyte metabolism. J. Clin. Invest. 116, 1983–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wren A. M., Small C. J., Abbott C. R., Dhillo W. S., Seal L. J., Cohen M. A., Batterham R. L., Taheri S., Stanley S. A., Ghatei M. A., Bloom S. R. (2001) Ghrelin causes hyperphagia and obesity in rats. Diabetes 50, 2540–2547 [DOI] [PubMed] [Google Scholar]
- 8. Barazzoni R., Bosutti A., Stebel M., Cattin M. R., Roder E., Visintin L., Cattin L., Biolo G., Zanetti M., Guarnieri G. (2005) Ghrelin regulates mitochondrial-lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 288, E228–E235 [DOI] [PubMed] [Google Scholar]
- 9. Sangiao-Alvarellos S., Vazquez M. J., Varela L., Nogueiras R., Saha A. K., Cordido F., Lopez M., Dieguez C. (2009) Central ghrelin regulates peripheral lipid metabolism in a growth hormone-independent fashion. Endocrinology 150, 4562–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perreault M., Istrate N., Wang L., Nichols A. J., Tozzo E., Stricker-Krongrad A. (2004) Resistance to the orexigenic effect of ghrelin in dietary-induced obesity in mice: reversal upon weight loss. Int. J. Obes. Relat. Metab. Disord. 28, 879–885 [DOI] [PubMed] [Google Scholar]
- 11. Tschop M., Weyer C., Tataranni P. A., Devanarayan V., Ravussin E., Heiman M. L. (2001) Circulating ghrelin levels are decreased in human obesity. Diabetes 50, 707–709 [DOI] [PubMed] [Google Scholar]
- 12. Iwakura H., Akamizu T., Ariyasu H., Irako T., Hosoda K., Nakao K., Kangawa K. (2007) Effects of ghrelin administration on decreased growth hormone status in obese animals. Am. J. Physiol. Endocrinol. Metab. 293, E819–E825 [DOI] [PubMed] [Google Scholar]
- 13. Briggs D. I., Enriori P. J., Lemus M. B., Cowley M. A., Andrews Z. B. (2010) Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology 151, 4745–4755 [DOI] [PubMed] [Google Scholar]
- 14. Bouret S. G., Gorski J. N., Patterson C. M., Chen S., Levin B. E., Simerly R. B. (2008) Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab. 7, 179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clegg D. J., Riedy C. A., Smith K. A., Benoit S. C., Woods S. C. (2003) Differential sensitivity to central leptin and insulin in male and female rats. Diabetes 52, 682–687 [DOI] [PubMed] [Google Scholar]
- 16. Nogueiras R., Wiedmer P., Perez-Tilve D., Veyrat-Durebex C., Keogh J. M., Sutton G. M., Pfluger P. T., Castaneda T. R., Neschen S., Hofmann S. M., Howles P. N., Morgan D. A., Benoit S. C., Szanto I., Schrott B., Schurmann A., Joost H. G., Hammond C., Hui D. Y., Woods S. C., Rahmouni K., Butler A. A., Farooqi I. S., O'Rahilly S., Rohner-Jeanrenaud F., Tschop M. H. (2007) The central melanocortin system directly controls peripheral lipid metabolism. J. Clin. Invest. 117, 3475–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Untergasser A., Nijveen H., Rao X., Bisseling T., Geurts R., Leunissen J. A. (2007) Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 35, W71–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shimbara T., Mondal M. S., Kawagoe T., Toshinai K., Koda S., Yamaguchi H., Date Y., Nakazato M. (2004) Central administration of ghrelin preferentially enhances fat ingestion. Neurosci. Lett. 369, 75–79 [DOI] [PubMed] [Google Scholar]
- 20. Gardiner J. V., Campbell D., Patterson M., Kent A., Ghatei M. A., Bloom S. R., Bewick G. A. (2010) The hyperphagic effect of ghrelin is inhibited in mice by a diet high in fat. Gastroenterology 138, 2468–2476, 2476.e1 [DOI] [PubMed] [Google Scholar]
- 21. Van den Top M., Lee K., Whyment A. D., Blanks A. M., Spanswick D. (2004) Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat. Neurosci. 7, 493–494 [DOI] [PubMed] [Google Scholar]
- 22. Zigman J. M., Jones J. E., Lee C. E., Saper C. B., Elmquist J. K. (2006) Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 494, 528–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Faulconbridge L. F., Grill H. J., Kaplan J. M. (2005) Distinct forebrain and caudal brainstem contributions to the neuropeptide Y mediation of ghrelin hyperphagia. Diabetes 54, 1985–1993 [DOI] [PubMed] [Google Scholar]
- 24. Egecioglu E., Jerlhag E., Salome N., Skibicka K. P., Haage D., Bohlooly Y. M., Andersson D., Bjursell M., Perrissoud D., Engel J. A., Dickson S. L. (2010) Ghrelin increases intake of rewarding food in rodents. Addict. Biol. 15, 304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 [DOI] [PubMed] [Google Scholar]
- 26. Strassburg S., Anker S. D., Castaneda T. R., Burget L., Perez-Tilve D., Pfluger P. T., Nogueiras R., Halem H., Dong J. Z., Culler M. D., Datta R., Tschop M. H. (2008) Long-term effects of ghrelin and ghrelin receptor agonists on energy balance in rats. Am. J. Physiol. Endocrinol. Metab. 295, E78–E84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nass R., Pezzoli S. S., Oliveri M. C., Patrie J. T., Harrell F. E., Jr., Clasey J. L., Heymsfield S. B., Bach M. A., Vance M. L., Thorner M. O. (2008) Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann. Intern. Med. 149, 601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Svensson J., Lonn L., Jansson J. O., Murphy G., Wyss D., Krupa D., Cerchio K., Polvino W., Gertz B., Boseaus I., Sjostrom L., Bengtsson B. A. (1998) Two-month treatment of obese subjects with the oral growth hormone (GH) secretagogue MK-677 increases GH secretion, fat-free mass, and energy expenditure. J. Clin. Endocrinol. Metab. 83, 362–369 [DOI] [PubMed] [Google Scholar]
- 29. White H. K., Petrie C. D., Landschulz W., MacLean D., Taylor A., Lyles K., Wei J. Y., Hoffman A. R., Salvatori R., Ettinger M. P., Morey M. C., Blackman M. R., Merriam G. R. (2009) Effects of an oral growth hormone secretagogue in older adults. J. Clin. Endocrinol. Metab. 94, 1198–1206 [DOI] [PubMed] [Google Scholar]
- 30. Lall S., Tung L. Y., Ohlsson C., Jansson J. O., Dickson S. L. (2001) Growth hormone (GH)-independent stimulation of adiposity by GH secretagogues. Biochem. Biophys. Res. Commun. 280, 132–138 [DOI] [PubMed] [Google Scholar]
- 31. Rosenbaum M., Goldsmith R., Bloomfield D., Magnano A., Weimer L., Heymsfield S., Gallagher D., Mayer L., Murphy E., Leibel R. L. (2005) Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J. Clin. Invest. 115, 3579–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huszar D., Lynch C. A., Fairchild-Huntress V., Dunmore J. H., Fang Q., Berkemeier L. R., Gu W., Kesterson R. A., Boston B. A., Cone R. D., Smith F. J., Campfield L. A., Burn P., Lee F. (1997) Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 [DOI] [PubMed] [Google Scholar]
- 33. Farooqi I. S., Keogh J. M., Yeo G. S., Lank E. J., Cheetham T., O'Rahilly S. (2003) Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 348, 1085–1095 [DOI] [PubMed] [Google Scholar]