Abstract
We describe a novel transgenic system for tissue-specific and inducible control of gene expression in mice. The system employs a tetracycline-responsive CMV promoter that controls transcription of a short-hairpin RNA (shRNA) that remains nonfunctional until an interrupting reporter cassette is excised by Cre recombinase. Insertion of Dicer and Drosha RNase processing sites within the shRNA allows generation of siRNA to knock down a target gene efficiently. Tissue-specific shRNA expression is achieved through the use of appropriate inducer mice with tissue-specific expression of Cre. We applied this system to regulate expression of junctophilins (JPs), genes essential for maintenance of membrane ultrastructure and Ca2+ signaling in muscle. Transgenic mice with skeletal muscle-specific expression of shRNA against JP mRNAs displayed no basal change of JP expression before treatment with doxycycline (Dox), while inducible and reversible knockdown of JPs was achieved by feeding mice with Dox-containing water. Dox-induced knockdown of JPs led to abnormal junctional membrane structure and Ca2+ signaling in adult muscle fibers, consistent with essential roles of JPs in muscle development and function. This transgenic approach can be applied for inducible and reversible gene knockdown or gene overexpression in many different tissues, thus providing a versatile system for elucidating the physiological gene function in viable animal models.— Ko, J. -K., Choi, K. -H., Zhao, X., Komazaki, S., Pan, Z., Weisleder, N., Ma, J. A versatile single-plasmid system for tissue-specific and inducible control of gene expression in transgenic mice.
Keywords: cre, doxycycline, junctophilin, muscle, shRNA
RNA interference (RNAi) has emerged as a powerful molecular tool to explore the physiological function of genes in cellular and animal models (1–4). As with the germline-knockout approach, RNAi-mediated suppression of target genes cannot be used in viable animal models if constitutive knockdown of the gene causes death during development. To overcome lethality associated with knockdown of essential genes, RNAi-based conditional gene knockdown systems have been developed in which short hairpin RNA (shRNA) expression is combined with Cre/loxP recombination (5–7) or with drug- or hormone-controlled induction systems (4, 8–10). While these systems have shown promising results, their broad application for physiological evaluation is constrained by several limitations. Although the Cre-recombination system can target shRNA expression in a tissue-specific manner, irreversibility of gene knockdown may produce toxic effects on cells if the target gene of shRNA is essential for cell survival. Application of the tetracycline- or ecdysone-induction system relies on viral infections to achieve expression of shRNA in target tissues (4, 8–10). However, chronic application of viruses may cause toxicity and immunogenicity that can interfere with gene function and/or complicate interpretation of results (11–13). Moreover, many of the available inducible systems that employ the U6 promoter for control of shRNA expression often display a relatively high level of leaky expression of shRNA before induction and low efficiency of expression in certain cell types (14) and thus are not optimal for use in transgenic models.
Many applications of RNAi in animal model studies require tight control of siRNA expression in an inducible, reversible, and preferably tissue-specific manner. To develop a transgenic system to meet these goals, we took advantage of a Tet-on system regulated by the tetracycline transcriptional silencer (tTS) and reverse tetracycline transcriptional activator (rtTA) expressed as a bicistronic cDNA for inducible modulation of gene expression (15, 16). We followed the approach used by Silva et al. (3) to design shRNA containing the Dicer and Drosha RNase processing sites that would result in efficient production of small-interference RNA (siRNA) (3, 4, 8). The siRNA expression cassette, driven by a Tet-responsive CMV promoter, is not functional until an interrupting GFP expression cassette flanked by loxP sites is excised by Cre-mediated recombination (9, 15). Thus, tissue- or lineage-specific expression of siRNA can be achieved by the use of appropriate inducer mice expressing tissue-specific Cre.
We applied this unique system to investigate the physiological role of junctophilins in skeletal muscle (17–21). Two junctophilin subtypes, JP1 and JP2, are expressed in skeletal muscle, where they are essential for proper maintenance of the junctional membrane structure and excitation-contraction coupling (17–21). Because of lethality associated with germline ablation of either JP1 or JP2, the physiological function of JPs in adult muscles could not be examined using the traditional knockout approaches. The Tet-on expression system for shRNA targeting JP1 and JP2 was stably incorporated into multiple founder mouse lines. Through breeding of founder mice with inducer mice that produce muscle-specific expression of Cre-recombinase, skeletal muscle-specific knockdown of JP1 and JP2 was achieved in a doxycycline (Dox)-inducible and reversible manner. Knockdown of JPs led to abnormal junctional membrane structure and disruption of store-operated Ca2+ entry (SOCE) in skeletal muscle, demonstrating the essential role of JPs in regulation of intracellular Ca2+ homeostasis in adult muscle fibers. Our study indicate that this single-plasmid transgenic system can be a powerful tool to modulate gene expression in a tissue-specific and inducible manner, allowing physiological dissection of gene functions using viable animal models.
MATERIALS AND METHODS
Plasmid construction
Detailed procedures for construction of vectors are described in Supplemental Table S1. PCR primers and synthetic genes used for the subcloning studies were purchased from Integrated DNA Technologies Inc. (Coralville, Iowa, USA). All constructs were confirmed by sequencing analysis.
Cell culture and Western blotting
HEK293 and CHO cells were cultured in DMEM (Gibco Life Technologies, Inc., Gaithersburg, MD, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% antibiotic/antimycotic solution (Gibco Life Technologies). Gene transfections were performed with the GeneJammer reagent (Stratagene, La Jolla, CA, USA) according to the manufacturer's protocol. To induce activity of the Tet-responsive promoter, Dox (Clontech, Palo Alto, CA, USA), a stable analog of tetracycline, was added to the normal growth medium at 1.0 μg /ml unless otherwise noted. For Western blotting, the monoclonal anti-JP1 mouse antibody and polyclonal anti-JP2 rabbit antibodies were as described previously (19). Anti-β-actin and anti-α-tubulin antibodies were purchased from Sigma (St. Louis, MO, USA). Total protein (10 μg) extract was subjected to SDS-PAGE, transferred onto a PVDF membrane, and analyzed by Western blotting.
Luciferase reporter gene assay
For reporter gene assay, HEK293 or CHO cells were cotransfected in 24-well plates by using Gene Jammer (Stratagene) with 0.5 μg of indicated shRNA vectors and 0.1 μg of pDualuc vector expressing firefly luciferase (fLuc) and Renilla luciferase (rLuc) from two separate expression cassettes independently. At 12 h after cotransfection, cells were treated with Dox (1.0 μg/ml) as indicated. Firefly and Renilla luciferase activity were measured by using the Dual Luciferase Assay Kit (Promega, Madison, WI, USA) according to the manufacturer's instruction.
In vitro Cre-recombination assay
To analyze in vitro Cre-recombination, 200 ng of pTLcG-mirJP plasmid was incubated with 1 U of Cre enzyme (New England Biolab, Beverley, MA, USA) at 37°C for 30 min. Followed by heat inactivation of Cre at 70°C for 10 min, the plasmid was purified from the reaction mixture using a gel extraction kit (Qiagen, Valencia, CA, USA) and subjected to PCR analysis. PCR using forward primer 5′-CCGGGACCGATCCAGCCTCCG and reverse primer 5′-GACGACGAGGCTTGCAGGATC produced 2030- and 470-bp products for unrecombined and recombined DNA, respectively. These primers were also used for detection of recombination on the genomic DNA isolated from transgenic mice harboring the Cre recombinase gene.
Generation of the mirJP transgenic mouse
To construct transgenic mice, pTLcG-mirJP vector was linearized by treatment with PvuI restriction enzyme and purified using the conventional phenol extraction method. Injection of DNA into fertilized C57BL/6J mouse eggs and generation and identification of positive mice were performed by Transgenic and Knockout Mouse Core Facility of the University of Medicine and Dentistry of New Jersey–Robert Wood Johnson Medical School–Cancer Institute of New Jersey (Piscataway, NJ, USA). Resulting positive mice were bred with C57BL/6J mice, and 4 founder mice (strain F0-mirJP) carrying pTLcG-mirJP were identified by Southern blotting. To determine the genotype of mice, each 10 μg of tail DNA was digested with EcoRI, and then electrophoresed on 1.2% agarose. Two replicated membranes were prepared and hybridized with 32P-labeled probe targeting GFP or rtTA sequences. Two probes for Southern blotting were synthesized by PCR amplification using pTLcG-mirJP as a template and the following primers: for a probe of GFP, forward 5′-ATCTGCGCAGCACCATGGCC and reverse 5′-TTAGGACTTGTACAGCTCGTC; for a probe for rtTA sequence, forward 5′-AGCTGTACGGGCCAGATATACGCG and reverse 5′-GAAACTACCCACCGTACTCGTCAATT. All 4 founders successfully passed pTLcG-mirJP transgene to their progeny (strain F1-mirJP), and all transgenic mice developed and bred normally. Two F1-mirJP lines carrying a high transgene copy number, as identified by Southern blotting, were mated with homozygous human α-skeletal actin (HSA)-Cre mice [strain Tg(ACTA1-cre)79Jme/J, The Jackson Laboratory, Bar Harbor, MA, USA] to produce offspring (strain F2-mirJP/cre) that simultaneously contain both the pTLcG-mirJP and HSA-Cre transgenes. The HSA-Cre transgenic mice have the Cre recombinase gene driven by the HSA or ACTA1 promoter. Cre activity is restricted to adult striated muscle fibers and embryonic striated muscle cells. The F2-mirJP/cre mice and littermates were analyzed for their genotypes by genomic PCR using the following primers: for GFP, forward 5′-ACGTAAACGGCCACAAGTTC and reverse 5′-GATCTTGAAGTTCACCTTGATGC; for rtTA, forward 5′-CATTCAGATACACGGCCTACAG and reverse 5′-CATGCGCCATCGCCACGTCC; and for Cre, forward 5′-GCGGTCTGGCAGTAAAAACTATC and reverse 5′-CGTATATCCTGGCAGCGATCGC. Each pair of primers produced 440 bp of PCR product for GFP, 544 bp for rtTA, and 235 bp for Cre.
To induce shRNA expression of mirJP/cre transgenic mice, 8-wk-old mice were provided with water containing Dox (2 mg/ml; Clontech) in light-protected bottles for 2 wk. Tail tips (1 cm length) were cut, and muscle proteins were extracted 1 wk after application of Dox to test the response of the mirJP/cre transgenic mice. To examine JP1 expression level, 10 μg of proteins was examined by Western blotting using anti-JP1 antibody. Mice showing decreased expression of JP1 were considered to be Dox responsive and were further fed with Dox-containing water for another week. After 2 wk of total exposure, one part of this group was sacrificed (Dox + group) and the other part was supplied with Dox-free water for 4 wk to inactivate the Tet-on system and recover expression of JP1 and JP2 (Dox +→− group).
Single muscle fiber isolation and SOCE measurement
Flexor digitorum brevis (FDB) muscle fibers were isolated from transgenic adult mice according to our published procedure (22). To measure SOCE in FDB fibers, the quenching of Fura-2 fluorescence by Mn2+ ions was used as described in our previous study (23). FDB fibers were plated onto a ΔTC dish (Bioptechs Inc., Butler, PA, USA) and allowed to attach to the bottom of the dish, then loaded with 10 μM Fura-2 AM (Molecular Probes, Eugene, OR, USA) at room temperature for 1 h. To prevent motion artifact in muscle fibers, 20 μM N-benzyl-p-toluene sulfonamide (BTS, Sigma), a specific myosin II inhibitor, was applied into the bathing solution 15 min prior to sarcoplasmic reticulum (SR) Ca2+ depletion. Muscle fibers were then examined on the inverted microscope (×400, N.A. 1.3) of a PTI spectrofluorometer system (Photon Technology International, Monmouth Junction, NJ, USA). Fura-2 was excited at 360 nm, a wavelength insensitive to changes in [Ca2+]i, while emission at a wavelength of 510 nm was recorded. SR Ca2+ stores of fibers were depleted by the addition of 20 μM thapsigargin (TG; Sigma) in Tyrode (0 Ca2+) solution for 7 min, and then the addition of 0.5 mM Mn2+ in Tyrode (0 Ca2+) solution for 5 min led to quenching of Fura-2 fluorescence at excitation wavelength of 360 nm (F360), indicating activation of TG-induced SOCE in FDB fibers. The fluorescence signal by Mn2+ quenching was normalized by using the formula [F360 − Fmin]/[Fmax − Fmin], where Fmax is the initial intensity of F360 fluorescence before the addition of Mn2+, and Fmin is minimal intensity determined by lysis of the cells with 1% Triton X-100 at the end of the experiment. During the experiments for SOCE measurement, basal value of F360/F390 indicating resting cytosolic Ca2+ concentration ([Ca2+]cyt; ref. 24) was measured before the addition of TG, and the change of F360/F390 ratio (ΔF360/F390), indicating TG-induced Ca2+ release from SR, was measured after the addition of TG.
Contractility of extensor digitorum longus (EDL) muscle
Intact EDL muscle bundles were dissected from the mirJP/cre mice (8 wk old) following 2 wk of feeding with water containing 2 mg/ml Dox, using our previous protocol (21). The EDL muscle bundles were attached to a force transducer, and contractile force was measured following stimulation with different electrical pulse protocols. After equilibration for 30 min, muscles were exposed to a fatiguing stimulation protocol (20 Hz of 50% duty cycle, 1 s interval) for 10 min, followed by stimulation of 20 Hz applied every minute to allow for recovery of contractile force (see Fig. 7E).
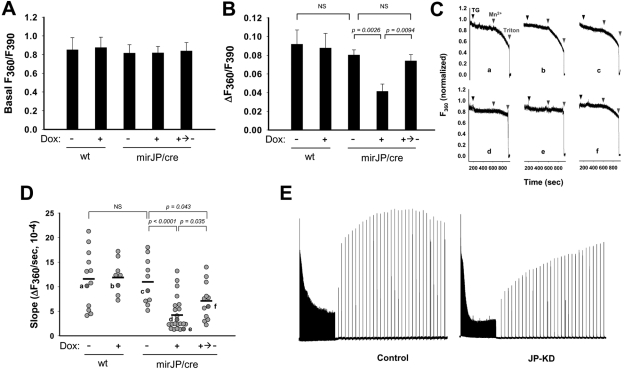
Figure 7.
Knockdown of JPs decreases thapsigargin-induced Ca2+-release from SR and SOCE in transgenic skeletal muscle fibers. To measure Ca2+ release from SR and SOCE, FDB muscle fibers were isolated and loaded with 10 μM Fura-2 AM. SR Ca2+ stores of fibers were depleted by the addition of 20 μM TG (7 min). Addition of 0.5 mM Mn2+ (5 min) leads to quenching of Fura-2 fluorescence at excitation wavelength of 360 nm (F360), indicating activation of TG-induced SOCE in FDB fibers. During the experiments, basal value of F360/F390, indicating resting cytosolic Ca2+ concentration ([Ca2+]cyt), was measured before the addition of TG, and the change of F360/F390 ratio (ΔF360/F390), indicating TG-induced Ca2+ release from SR, was measured after the addition of TG. A) Average data for the basal value of F360/F390. Result shows no significant difference among groups. B) Average data for the TG-induced change of F360/F390. C) Representative traces of F360, measured from FDB fibers of Dox-untreated wt mice (a), Dox-treated wt mice (b), Dox-untreated mirJP/cre mice (c), Dox-treated mirJP/cre mice (d, e), and water-switched mirJP/cre mice after Dox-treatment (f). Fura-2 fluorescence is normalized to a maximum of 1.0 and a minimum of 0 for the value after permeabilization with Triton X-100. D) Statistical analysis of multiple SOCE measurements. Light gray circles indicate rate of Mn2+ quenching of Fura-2 fluorescence from individual fibers; dark gray circles indicate data from F360 traces in C. Horizontal bar indicates mean values in each group. Experiments were performed on the fibers (n=8–22) from ≥3 mice/group. NS, not significant (P>0.05. Note that some fibers with Dox treatment show apparently normal values of SOCE compared with the untreated fibers, which likely reflects the incomplete Cre-recombination system in the muscle background. E) In vitro fatigability and force recovery profile of control (left panel) and JP-KD (right panel) EDL muscle bundles. Traces are representative of 6 muscle bundles in the control group; 4 muscle bundles in the JP-KD group.
Electron microscopy (EM) and histology
For EM studies, EDL muscles were dissected from a right hindlimb of adult mice and prepared for EM following our previous protocols (25). Briefly, skeletal muscles were fixed in 3% paraformaldehyde, 2.5% glutaraldehyde, and 0.1 M cacodylate buffer (pH 7.4) and later postfixed in 1% OsO4 and 0.1 M cacodylate buffer (pH 7.4). Microthin sections were double stained with uranyl acetate and lead citrate. These sections were examined under a transmission electron microscope (JEM-1010; Jeol, Tokyo, Japan). After preparing EDL muscles, gastrocnemious muscle was excised from a left hindlimb, embedded in optimal cutting temperature (OCT) compound (Tissue-Tek, Elkhart, IN, USA), and frozen in liquid nitrogen. Frozen sections were then cut with a cryostat (5 μm thickness) and placed on slides. For observation of GFP fluorescence, nonfixed frozen sections were observed by fluorescence microscopy (Axiovert 200 system; Carl Zeiss International, New York, NY, USA).
RESULTS
Construction of the plasmid system for tissue-specific and inducible expression of shRNA
To achieve inducible control of gene expression, the promoter system must provide minimal leaky expression under basal conditions and be activated efficiently in the induced state. For this purpose, the RNA polymerase III-based U6 promoter was frequently used to control shRNA expression. Published studies using a modified U6 promoter containing a Tet-operator sequence flanking the TATA box (promoter tetU6-1; see Supplemental Fig. S1) showed a certain degree of Dox-dependent induction of shRNA expression in different systems (14, 26). In our cell culture assay, we found this tetU6-1 system to display leaky expression at basal conditions, with low induction of shRNA expression following Dox treatment. Even modified promoters containing up to 7 consecutive TetO2 sequences (7xTetO2) could not produce Dox-inducible control of the U6 promoter (see Supplemental Fig. S1). This leaky expression and low inducibility of the U6 promoter made it unsuitable for our intended studies with transgenic mouse models.
Due to the poor inducibility and leaky expression observed with the U6-promoter-based systems, we focused our effort on developing a minimal CMV promoter (PminiCMV) linked to a Tet-responsive element (TRE) consisting of 7xTetO2 (Fig. 1A). Consistent with previous studies (27, 28), we found that this TRE-PminiCMV construct displayed tight control over transcription of either GFP (Fig. 1B) or luciferase reporter genes in the absence of Dox, with robust expression of the reporter genes in the presence of Dox (<10% leaky expression and up to a 17-fold induction of luciferase activity; Fig. 1C). Such properties could provide inducible control of the transgene; however, it would require modification of the construct to allow processing of the shRNA into efficient siRNA for silencing of the target gene. The shRNA product generated by the RNA polymerase II-CMV promoter often contained flanking sequences at the 5′ and 3′ ends that can interfere with siRNA function, so removal of these extra sequences would be critical for efficient siRNA silencing.
Figure 1.
Tight control of reporter gene expression using the TRE-PminiCMV system. A) pTL is the backbone plasmid that only contains rtTA and tTS cDNAs. TRE/loxP-inducible GFP (pTLiG) and TRE/loxP-inducible luciferase (pTLiL) are plasmids used to test the Dox-inducibility of reporter gene expression. In pTLcG, the GFP reporter gene is constitutively driven by the PSV40 promoter. B) Examination of Dox-inducible GFP expression of pTLiG in HEK293 and CHO cells. HEK293 or CHO cells in 6-well plates were transfected with 1.0 μg of pTLiG or pTLcG plasmid. At 24 h after transfection, the cells were cultured further in a medium with or without Dox (1.0 μg/ml) for 24 h. GFP expression from transfected HEK293 or CHO cells was observed by fluorescence microscopy. C) Examination of Dox-inducible luciferase expression of pTLiL in HEK293 and CHO cells. HEK293 or CHO cells on 24-well plates were transfected with 0.5 μg of pTLiL or pTL plasmid. At 24 h after transfection, the cells were cultured further with or without Dox (1.0 μg/ml) for 24 h. Expression levels of luciferase were measured from HEK293 or CHO cells. Data show tight regulation of gene expression (<12% of leaky expression) in the absence of Dox, as compared with modified U6 promoters containing TRE sequence (see Supplemental Fig. S1) and robust induction in the presence of Dox (8- to 17-fold induction of luciferase reporter genes, depending on cell type).
Elegant studies from Silva et al. (3) demonstrated that a new generation shRNA library making use of the endogenous Dicer and Drosha RNases could be used for systematic analyses of RNAi-induced phenotypes in mammalian cells. Engineered RNA processing sites for Dicer and Drosha were introduced into the shRNA probe, allowing removal of the extra flanking sequences using the natural system for generation of siRNA to produce precise 19- to 21-nt sequences. We adapted this Dicer/Drosha system to produce shRNA probes for gene silencing using the transgenic mouse model. For convenient use in our in vitro and in vivo studies, we incorporated all 3 essential transgene elements into a single plasmid system named pTRE-loxP-constitutive GFP-shRNA-mirJP (pTLcG-mirJP; Fig. 2A). First, for tight control of the TRE-PminiCMV promoter activity, the tTS and rtTA genes were cloned behind the SV40 promoter, for generation of the Tet-on silencer and activator gene products using the bicistronic system (29, 30). Second, a GFP reporter gene was cloned behind the TRE-PminiCMV promoter that functions to interrupt transcription of the downstream shRNA sequence. The GFP reporter gene was flanked by two loxP sequences that can be excised by Cre-mediated recombination, allowing for expression of the shRNA in a tissue-specific manner (6, 7, 31). Third, for efficient knockdown of the target gene, the Dicer and Drosha processing sites were introduced into the shRNA sequence to mimic native miRNA processing and produce the specific siRNA probe (Fig. 2B, inset).
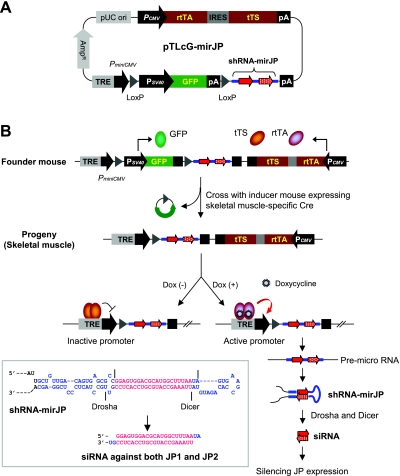
Figure 2.
Single-plasmid-based system for inducible and tissue-specific control of gene silencing in transgenic mice. A) Schematic diagrams of pTLcG-mirJP system. Minimal CMV promoter PminiCMV is linked to a TRE, a specific binding site for Tet-responsible transcription factors tTS and rtTA, which are expressed constitutively by a separate SV40 promoter. An internal ribosomal entry site (IRES) sequence permits translation of the 2 open reading frames for tTS and rtTA from single mRNA. A sequence of shRNA-mirJP targeting both JP1 and JP2 mRNAs is located downstream of an internal GFP expression cassette, which is flanked by 2 unidirectional loxP sites. B) Schematic of the experimental strategy in transgenic mice. First, the transgenic founder mice constitutively express tTS, rtTA, and GFP but will not express shRNA-mirJP in any cell. Transgenic offspring mice carrying both pTLcG-mirJP and muscle-specific Cre recombinase produce a functional Tet-on system for shRNA expression due to excision of GFP expression cassette by Cre-mediated recombination in a muscle-specific manner. Without Dox, the tTS molecules bind TRE and actively silence the transcription of the downstream shRNA-mirJP. In the presence of Dox, the shRNA-mirJP can be expressed actively by rtTA and processed by Drosha and Dicer to produce siRNA against JP1 and JP2 mRNA (inset).
We applied this single-plasmid system to elucidate the physiological function of JP1 and JP2 in adult striated muscle. Both JP1 and JP2 are abundantly expressed in skeletal muscle and are essential for formation of the triad junction and Ca2+ signaling in skeletal muscle (17–20). These two genes share a high degree of sequence homology and play complementary roles in mediating the membrane crosstalk in striated muscles (19). To avoid the possible redundant functions of JP1 and JP2, we designed the shRNA probe targeting both JP1 and JP2. Three different targeting sequences were tested in cell culture studies, and one mirJP probe was found to be highly efficient in knocking down both JP1 and JP2 expression by Western blot (see Supplemental Fig. S2). This mirJP probe will be used for our subsequent in vitro and in vivo studies.
Our strategy for generating the transgenic mice with muscle-specific expression of mirJP is outlined in Fig. 2B. We expect the first-generation transgenic mice to express tTS, rtTA and GFP in all tissues constitutively. The presence of an internal poly(A) sequence within the GFP expression cassette interrupts TRE-PminiCMV promoter activity to prevent transcription of the downstream mirJP sequence. Cross-breeding of the pTLcG-mirJP founder mice with transgenic mice expressing Cre in skeletal muscle generates progeny with an active Tet-controllable expression cassette for mirJP in skeletal muscle by excision of the GFP cassette using the loxP recombination mechanism. Without Dox, tTS binding to the TRE suppresses transcription of the downstream mirJP. In the presence of Dox, rtTA replaces tTS for activation of the PminiCMV promoter to allow transcription of mirJP. Further processing of the mirJP by the endogenous Drosha and Dicer produces the siRNA probe for efficient silencing of JP1 and JP2 in skeletal muscle. Thus, muscle-specific expression of the siRNA can be achieved to provide Dox-inducible and reversible suppression of JP1 and JP2 in viable transgenic mice.
Dox-controlled and reversible knockdown of JP expression through the mirJP mechanism
For testing the functionality of the pTLcG-mirJP system, we first constructed the pTLi-mirJP plasmid mimicking Cre-recombined pTLcG-mirJP by excision of GFP expression cassette (Fig. 3A). HEK293 cells were cotransfected with pTLcG-mirJP or pTLi-mirJP together with expression plasmids for JP1 and JP2, and the changes in exogenous JP1 and JP2 protein levels were determined by Western blots. At 24 h after cotransfection, the cells were treated with 1 μg/ml of Dox and further cultured for 24 h. Western blotting showed that both JP1 and JP2 levels were significantly decreased in cells transfected with pTLi-mirJP following Dox treatment compared with cells grown in the Dox-free medium. The expression of JP1 and JP2 did not change in cells transfected with pTLcG-mirJP irrespective of treatment with Dox, suggesting that the GFP expression cassette efficiently blocked the transcriptional activity of the TRE-PminiCMV promoter (Fig. 3B). This Tet-on system is highly responsive to Dox, since >50% reduction of both JP1 and JP2 protein levels was detected following treatment with 100 ng/ml of Dox (Fig. 3C). The knockdown of JP was also time dependent, as significant reduction of JP1 was observed at 12 h after treatment with Dox. In addition, the Tet-on state was efficiently turned off by removal of Dox from the culture medium, with JP1 protein levels returning to normal at ∼48 h after removal of Dox from the culture medium (Fig. 3D).
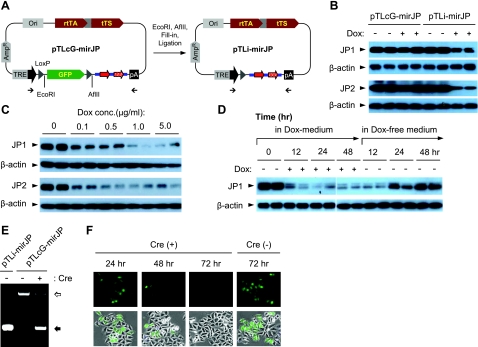
Figure 3.
In vitro assay with Dox-induced knockdown of JP1 and JP2 expression using pTLcG-mirJP system. A) For testing the Dox-induced silencing of JP1 and JP2 expression, the GFP expression cassette was excised from the pTLcG-mirJP plasmid to generate the pTLi-mirJP construct (which mimics Cre-recombination of the pTLcG-mirJP system). B) HEK293 cells in 6-well plates were cotransfected with 1.0 μg of pTLcG-mirJP or pTLi-mirJP together with 0.2 μg of either pCMS-EGFP-mJP1 or pCMS-RFP-mJP2 (41). Dox (1.0 μg/ml) was applied 24 h after transfection. Total cell lysates were prepared at 24 h after addition of Dox, and 10 μg of proteins was analyzed by Western blotting against mouse JP1 or JP2. C) HEK293 cells were cotransfected with 1.0 μg of pTLi-mirJP and 0.2 μg of pCMS-EGFP-mJP1 or pCMS-RFP-mJP2. At 24 h after transfection, cells were treated with indicated concentrations of Dox. Cells were cultured further for 24 h and assayed by Western blotting. D) HEK293 cells cotransfected with 1.0 μg of pTLi-mirJP and 0.2 μg of pCMS-EGFP-mJP1 were treated with Dox (1.0 μg/ml) at 24 h after transfection. After 48 h incubation in the presence of Dox, cells were washed and incubated further in fresh medium as indicated. Expression of JP1 was detected by Western blotting. E) To analyze in vitro Cre recombination, 200 ng of pTLcG-mirJP was incubated with Cre enzyme (1 U) at 37°C for 30 min, followed by heat inactivation of Cre at 70°C for 10 min. Plasmids were purified from the reaction mixture, and the recombined plasmids were analyzed by PCR, using 2 primers flanking the GFP expression cassette. Open arrow indicates unrecombined product (2030 bp) containing the GFP expression cassette; solid arrow indicates recombined product (470 bp). F) HEK293 cells were transfected with 0.2 μg of pTLcG-mirJP with or without 1.0 μg of pTurbo-Cre. From 24 h after transfection, cells were observed by fluorescence microscopy to detect GFP expression.
We next tested the Cre-mediated excision of the GFP expression cassette from the pTLcG-mirJP plasmid. For this assay, pTLcG-mirJP plasmid DNA was incubated with the Cre enzyme for 30 min. PCR reaction using primers flanking the GFP expression cassette was used to measure the efficiency of Cre-mediated recombination, using pTLi-mirJP as a control template. While PCR amplification of pTLcG-mirJP generated 2.03 kb of product in the absence of Cre enzyme, a product of 470 bp was generated with the same plasmid following treatment with the Cre enzyme. This 470-bp band is identical to that generated with pTLi-mirJP as the template (Fig. 3E). We also tested the Cre-loxP recombination in cell culture. As shown in Fig. 3F, HEK293 cells cotransfected with pTLcG-mirJP and pTurbo-Cre, an eukaryotic expression plasmid for nucleus-targeting Cre, showed time-dependent decrease of GFP fluorescence, indicative of the clearance of GFP expression by Cre-mediated recombination, whereas the GFP fluorescence in cells transfected with pTLcG-mirJP remain unchanged at 72 h after transfection. These results demonstrate that the two loxP sites on the pTLcG-mirJP plasmid are functional for the Cre-mediated recombination.
In parallel studies, we also examined the Dox-inducible gene silencing by targeting fLuc for quantitative analysis of the efficacy of this regulatory system. For this purpose, the mirJP sequence in the pTLcG-mirJP and pTLi-mirJP plasmid was replaced with mirLuc, an efficient RNAi sequence for silencing of the fLuc mRNA (3), to generate the pTLcG-mirLuc and pTLi-mirLuc constructs, respectively (Fig. 4A). CHO or HEK293 cells were cotransfected with pTLcG-mirLuc or pTLi-mirLuc together with pDualuc, a mammalian expression vector containing fLuc and rLuc driven by independent promoters. After cotransfection, the level of fLuc was normalized to that of untargeted rLuc. In cells transfected with pTLi-mirLuc, the fLuc activity was decreased significantly in a Dox-dependent manner with >70% reduction in HEK293 cells and >80% reduction in CHO cells, following addition of 1 μg/ml of Dox (Fig. 4B). Cells transfected with pTLcG-mirLuc did not show any change of fLuc activity following treatment with Dox. Consistent with our studies with pTLcG-mirJP, cells transfected with pTLi-mirLuc also showed strong dose- and time-dependent reduction of fLuc activity with Dox treatment (Fig. 4C), with reversible recovery of fLuc activity following removal of Dox from the culture medium (Fig. 4D).
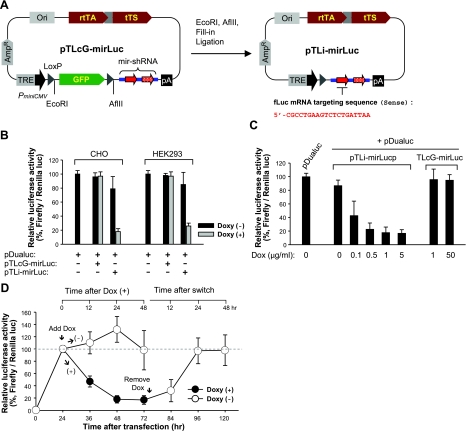
Figure 4.
Quantitative assay of Dox-inducibility and reversibility of luciferase reporter activity using the pTLcG system. A) For a quantitative analysis of the system, shRNA-mirJP sequence in the pTLcG-mirJP and pTLi-mirJP was replaced with shRNA-mirLuc sequence targeting fLuc mRNA to construct pTLcG-mirLuc and pTLi-mirLuc. B) CHO or HEK293 cells in 24-well plates were cotransfected with 0.5 μg of pTLcG-mirLuc or pTLi-mirLuc and 0.1 μg of pDualuc. At 24 h after transfection, cells were treated with or without Dox (1.0 μg/ml) and incubated further for 24 h. Dual luciferase assays were carried out in triplicate; results represent ratio of Renilla to firefly luciferase activity (mean±se). C) HEK293 cells in 24-well plates were cotransfected with 0.5 μg of pTLcG-mirLuc or pTLi-mirLuc and 0.1 μg of pDualuc. At 24 h after transfection, cells were treated with indicated concentrations of Dox. Cells were cultured further for 24 h and analyzed by dual luciferase assay. D) HEK293 cells cotransfected with pTLi-mirLuc and pDualuc were treated with Dox (1.0 μg/ml) at 24 h after transfection. After 48 h incubation in the presence of Dox, cells were washed and further incubated in Dox-free medium. At indicated time points, cells were prepared and analyzed by dual luciferase assay.
Together, these results indicate that the TRE-PminiCMV and Cre-loxP systems in the pTLcG plasmid operate properly as designed. Cre-loxP-mediated recombination allows activation of TRE-PminiCMV promoter activity, facilitating Dox-dependent transcriptional control over expression of mirJP. The Tet-on system is controlled tightly in this configuration, as minimal leaky expression of mirJP was observed in the absence of Dox.
Muscle-specific and Dox-dependent knockdown of JP1 and JP2 in transgenic mice
For application of the pTLcG-mirJP system in the mouse model, blastocytes were injected with linearized pTLcG-mirJP plasmid, and 4 founder lines were generated (F0-mirJP). All 4 lines stably transmitted the transgene to their progeny (F1-mirJP). To achieve skeletal muscle-specific expression of shRNA-mirJP, F1-mirJP mice were crossed with HSA-Cre transgenic mice that express Cre specifically in skeletal muscle. Transgenic mice carrying both pTLcG-mirJP and HSA-Cre (F2-mirJP/cre) and littermates including wild-type (F2-wt), pTLcG-mirJP alone (F2-mirJP), and HAS-Cre alone (F2-cre) were produced without defect in their development and growth.
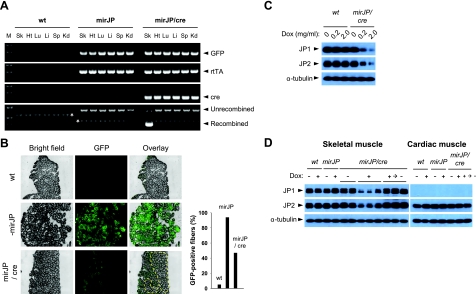
Cre-mediated recombination in various tissues was assayed by genomic PCR. As shown in Fig. 5A, a PCR product of 470 bp was detected only in skeletal muscle but not in other tissues isolated from the mirJP/cre mice. This product was not detected in tissues from wt and mirJP mice, indicating that the Cre-loxP recombination of the pTLcG-mirJP transgene occurred specifically in skeletal muscle in the mirJP/cre mice. Gastrocnemious muscles isolated from the mirJP mice showed even GFP fluorescence throughout the muscle sections, whereas muscles derived from the mirJP/cre mice showed significantly reduced GFP signal (Fig. 5B). The distribution of GFP fluorescence on the cross-sections from mirJP/cre muscle was distributed unevenly, likely reflecting the heterogeneity of Cre expression among individual muscle fibers. Some tissues, such as heart and kidney, showed weak GFP fluorescence, and thus it is difficult to distinguish among tissues isolated from various mouse groups because of their high background of autofluorescence (data not shown).
Figure 5.
Muscle-specific knockdown of JP1 and JP2 expression using the mirJP/Cre transgenic mice. pTLcG-mirJP transgenic (mirJP) mice were mated with HSA-Cre79 transgenic (cre) mice to generate offspring carrying both transgenes (mirJP/Cre). Eight-week-old F2-mirJP/cre mice were examined to detect Cre-mediated DNA recombination (A, B) and Dox-dependent JP knockdown (C, D). A) Genomic PCR was performed in various tissues of wild-type (wt), pTLcG-mirJP (mirJP), and pTLcG-mirJP/HSA-cre (mirJP/cre) transgenic mice. Cre-mediated recombination was determined by detection of two different sizes of PCR products, 2030 bp for unrecombined and 470 bp for recombined products. M, 100-bp DNA ladder marker; Sk, skeletal muscle; Ht, heart; Lu, lung; Li, liver; Sp, spleen; Kd, kidney. Asterisks indicate nonspecific PCR products. B) Gastrocnemious muscles prepared from the mice were cross-sectioned (5 μm thickness) and examined under fluorescence microscopy to visualize the expression pattern of GFP. Area enclosed by dashed line corresponds to fibers showing negative or very weak GFP fluorescence on the section. GFP-positive fibers were counted from the same sections (right panel). C) Examination of Dox-dependent reduction of the JP expression in skeletal muscle. Eight-week-old wt and mirJP/cre mice were supplied with water containing 0.2 or 2.0 mg/ml of Dox for 1 wk. Gastrocnemious muscles were isolated, and expression levels of JP1 and JP2 were examined by Western blotting. D) Reversible control of Dox-inducible knockdown of JPs in skeletal muscle. Eight-week-old wt and transgenic mice were provided with water either with or without Dox (2 mg/ml) for 2 wk. One cohort of Dox-treated mirJP/cre mice was sacrificed (Dox + group), and the other cohort was supplied with Dox-free water for 4 wk to inactivate Tet-on system for shRNA-mirJP expression and recover expression level of JP1 and JP2 (Dox +→− group). Gastrocnemious and cardiac muscles were prepared and examined by Western blotting for JP-1 and JP-2. Each lane shows Western blotting for the muscle isolated from different mice.
Dox-induced knockdown of JP1 and JP2 was tested after 2 wk feeding of the mirJP/cre mice with water containing 0.2 or 2.0 mg/ml of Dox. As shown in Fig. 5C, dose-dependent reduction of JP1 and JP2 in the mirJP/cre skeletal muscle was observed. Compared with wt mice, the expression of both JPs in mirJP/cre muscle was reduced by >50% with 0.2 mg/ml Dox and >70% by 2.0 mg/ml Dox (n=7). The effect of Dox on the knockdown of JPs can be controlled reversibly in the mirJP/cre mice. Providing the Dox-treated mirJP/cre mice with Dox-free water for 4 wk resulted in complete restoration of JP1 and JP2 expression levels to those observed in wt or mirJP mice (Fig. 5D). These results indicate that the TRE-PminiCMV system for mirJP expression can be switched efficiently on and off in adult muscle in the viable transgenic mirJP/cre mice. While endogenous JP2 is expressed abundantly in cardiac muscle, its expression in cardiac muscle was not altered by the Dox treatment with the mirJP/cre mice (Fig. 5D), further demonstrating that knockdown of JP expression is specific for skeletal muscle in these transgenic mice.
Reversible changes of junctional membrane structure and defects of SOCE in transgenic skeletal muscle following knockdown of JP1 and JP2
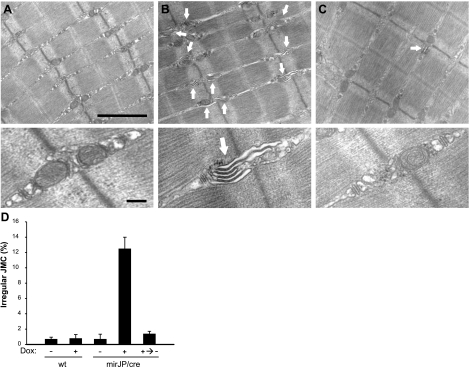
Previously, abnormal triad junction ultrastructure was reported in neonatal skeletal muscle isolated from the JP1-knockout mice (17, 20), and from the adult skeletal muscle fibers infected with virus packaged with shRNA against JP mRNAs (25). To test the effect of transient knockdown of JPs on junctional membrane structures in the skeletal muscle, EM analysis was performed on EDL muscles isolated from the mirJP/cre mice. While muscle fibers derived from the untreated mirJP/cre mice showed normal triad junctions (Fig. 6A), 2 wk treatment of the mirJP/cre mice with Dox led to significant remodeling of the triad junctions (Fig. 6B). Of the 7 mice tested, 6 showed significant irregularities in membrane structures, with poorly developed junction contact between the transverse-tubule and sarcoplasmic reticulum membranes, as reflected by the frequent appearance of a multiple-layer membrane network (Fig. 6B, arrows). The one muscle that did not show significant defects in junctional membrane structure was later tested to have less reduction of JP expression (∼30% of knockdown, not shown). These irregular junctional structures were rarely observed in EDL muscles isolated from untreated mirJP/cre mice (Fig. 6A), or in Dox-treated or untreated wt mice (data not shown). The Dox-induced changes in junctional membrane structure of the mirJP/cre mice could be reversed by switching the mice back to a Dox-free water supply. As shown in Fig. 6C, apparently normal junctional membrane networks were observed with the mirJP/cre muscle 4 wk after switching back to the Dox-free water. Data from multiple experiments are summarized in Fig. 6D, which clearly show that reversible changes in junctional membrane structure could be observed with Dox-induced knockdown of JP expression in skeletal muscle. We also performed parallel analysis with the cardiac muscle derived from the mirJP/cre mice and found no significant differences in membrane structure with or without treatment with Dox (data not shown), further confirming the skeletal muscle-specific nature of the Cre-loxP system in our transgenic model.
Figure 6.
Inducible knockdown of JPs leads to abnormal triad junction ultrastructure in transgenic skeletal muscles. EM analysis was performed on EDL muscles isolated from 3–7 mice/experimental group. A–C) Representative electron micrographs of muscle tissue from Dox-untreated mirJP/cre mice (A), Dox-treated mirJP/cre mice (B), and water-switched mirJP/cre mice after Dox-treatment (C). Arrows indicate irregular triad junctions. Top panels: lower-magnification EM images of longitudinal EDL section. Bottom panels: representative high-magnification images of triad junctions. D) Statistical analysis of changes in junctional membrane structures. At least 400 junctional membrane structures from each EDL muscle fiber were examined. Data are presented as means ± se. Scale bars = 1 μm (top panels); 0.2 μm (bottom panels).
Disruption of the junctional membrane structure associated with knockdown of JP1 and JP2 produced significant effect on Ca2+ signaling in skeletal muscle. Isolated FDB muscle fibers derived from the Dox-treated mirJP/cre mice were loaded with Fura-2 Ca2+ indicator for imaging analysis. As shown in Fig. 7A, the resting cytosolic Ca2+ levels were not different in the 5 tested groups, suggesting that Dox-mediated knockdown of JP1 and JP2 did not alter the overall basal Ca2+ transport process across the sarcolemmal membrane at the resting state. Interestingly, treatment with TG revealed significant reduction of the total Ca2+ releasable pool from the SR in FDB fibers isolated from Dox-treated mirJP/cre mice (Fig. 7B) compared with the wt controls and untreated mirJP/cre muscle. The reduced SR Ca2+ store in the Dox-treated mirJP/cre muscle could be recovered 4 wk after removal of Dox from the mouse water supply.
Previously, we showed that acute knockdown of JP expression by viral vector-delivered shRNA also led to reduced SR Ca2+ store, which was linked to compromised function of SOCE (25). We next measured the SOCE activity in the mirJP/cre FDB fibers using an Mn2+-quenching of Fura-2 fluorescence assay (23, 32). The fibers were loaded with Fura-2 and then treated with TG to deplete the SR Ca2+ store, leading to activation of SOCE. As shown in Fig. 7C, SOCE activities were significantly decreased in FDB fibers from the Dox-treated mirJP/cre mice compared with that of Dox-untreated mirJP/cre fibers, whereas Dox-untreated FDB fibers from mirJP/cre mice did not show significant difference in SOCE from wt fibers (Fig. 7C, D). Under our experimental conditions, many of those fibers (13 of 22 tested fibers isolated from 5 mice) displayed undetectable levels of SOCE in response to TG treatment (see Fig. 7Ce for representative trace). Many of the FDB fibers from mirJP/cre mice after recovery in Dox-free water showed significant recovery of SOCE activities.
We showed previously that reduced SOCE contributes to various aspects of muscle function, including increased susceptibility to muscle fatigue (21, 23, 25, 33). Using the EDL muscle bundles isolated from the Dox-treated mirJP/Cre mice, we examined whether knockdown of JP affected fatigability of the skeletal muscle. As shown in Fig. 7E, muscle fibers with knockdown of JP (based on Western blot assay, not shown) displayed increased fatigability, reflected by the reduced contractile force at the end of the 10-min stimulation protocol (JP-KD, right panel), when compared with the control EDL fibers (left panel). The data shown in Fig. 7E are representative of 4 preparations from the JP-KD group and 6 preparations from the control group.
DISCUSSION
In this study, we report a single-plasmid system for inducible and tissue-specific expression of shRNA to knock down gene expression in both cellular and animal models. We generated this integrated expression system by exploiting the native microRNA processing system to allow efficient production of siRNA derived by a Tet-controlled CMV promoter. Tissue-specific expression of shRNA was achieved using the Cre/loxP recombination system. Based on this approach, we established the mirJP/cre transgenic mouse line for inducible and reversible knockdown of JP1 and JP2 expression in adult skeletal muscle fibers, bypassing the perinatal or embryonic lethality associated with germline ablation of these genes. Our data revealed that reversible knockdown of JP1 and JP2 led to uncoupling of the junctional membrane structure that was accompanied by defective operation of SOCE and increased fatigability of adult skeletal muscle.
The mirJP/cre mouse provided a valuable tool to examine the physiological function for JPs in maintenance of junctional membrane complexes and modulation of Ca2+ signaling in muscle fibers and potentially other excitable cells. The compromised SOCE function associated with reduced expression of JP1 and JP2 in the mirJP/cre muscle might result from alterations of the junctional membrane structure, which can affect the signal transduction from the molecular sensor at the SR membrane (STIM1) to activation of the Orai channels at the sarcolemmal membrane (34–36). It is also possible that JPs might interact with the SOCE machinery and directly influence the function of SOCE. A recent study by Li et al. (37) showed that cultured myotubes with knockout of JP1 also showed defective SOCE, with significant reduction of resting cytosolic Ca2+ levels. Biochemical assays showed that the JP1-knockout myotubes showed reduced expression levels of Orai1 and STIM1. In the present study, we could not detect significant changes in the resting cytosolic Ca2+ levels with Dox-induced knockdown of JP1 and JP2 in the mirJP/cre muscle, and biochemical studies showed no significant changes in expression levels of Orai1, STIM1, and other Ca2+ regulatory proteins, such as RyR1, SERCA, and DHPR, in the adult skeletal muscle derived from the Dox-treated mirJP/cre mice (data not shown). The different resting Ca2+ levels in the adult skeletal muscle fiber and the cultured myotubes may reflect difference in the membrane ultrastructure of these two model systems and also different expression levels of other muscle specific genes, as reported previously (38, 39). A recent study by Woo et al. (40) showed that JP2 could interact with TRPC3 to affect Ca2+ homeostasis in cultured myotubes. Thus, further studies to investigate interaction between JPs and SOCE-involved molecules will provide a new insight for Ca2+ homeostasis and signaling in muscle physiology.
An advantage of our mirJP/cre mouse model is that knockdown of the target gene can be achieved in a reversible manner. Thus, removal of Dox from the mouse diet led to recovery of JP1 and JP2 in skeletal muscle, followed by restoration of junctional membrane defects and SOCE function. This feature will be very useful for testing the adaptive function of genes in a physiological setting, such as aging or stress-induced response associated with physiology and pathophysiological conditions. In addition, we demonstrated that the Dox-induced changes in JP1 and JP2 expression in the mirJP/cre mice occurs only in skeletal muscle, but not in the cardiac tissue; thus, tissue-specific function can be examined. It should be mentioned that our plasmid design can be modified for inducible and tissue-specific overexpression of a cDNA transgene by replacing the shRNA sequence with the transgene of interest, providing a useful tool to examine the physiological function of restoring a gene defect associated with human pathophysiology.
Overall, our single-plasmid system can be a versatile tool for the expression of shRNA among the currently developed systems by achieving tissue specific, Dox-controllable, and efficient gene silencing in an animal system, which can overcome the lethality problem associated with germline ablation of target genes.
Supplementary Material
Acknowledgments
This work was supported in part by U.S. National Institutes of Health grants RO1-HL069000 and RO1-AG028614 to J.M., K99/R00-AR054793 to N.W., and AHA 10SDG2630086 to X. Z.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Dykxhoorn D. M., Novina C. D., Sharp P. A. (2003) Killing the messenger: short RNAs that silence gene expression. Nat. Rev. Mol. Cell. Biol. 4, 457–467 [DOI] [PubMed] [Google Scholar]
- 2. Mittal V. (2004) Improving the efficiency of RNA interference in mammals. Nat. Rev. Genet. 5, 355–365 [DOI] [PubMed] [Google Scholar]
- 3. Silva J. M., Li M. Z., Chang K., Ge W., Golding M. C., Rickles R. J., Siolas D., Hu G., Paddison P. J., Schlabach M. R., Sheth N., Bradshaw J., Burchard J., Kulkarni A., Cavet G., Sachidanandam R., McCombie W. R., Cleary M. A., Elledge S. J., Hannon G. J. (2005) Second-generation shRNA libraries covering the mouse and human genomes. Nat. Genet. 37, 1281–1288 [DOI] [PubMed] [Google Scholar]
- 4. Stegmeier F., Hu G., Rickles R. J., Hannon G. J., Elledge S. J. (2005) A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 102, 13212–13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang H. S., Lin C. H., Chen Y. C., Yu W. C. (2004) Using siRNA technique to generate transgenic animals with spatiotemporal and conditional gene knockdown. Am. J. Pathol. 165, 1535–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tiscornia G., Tergaonkar V., Galimi F., Verma I. M. (2004) CRE recombinase-inducible RNA interference mediated by lentiviral vectors. Proc. Natl. Acad. Sci. U. S. A. 101, 7347–7351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ventura A., Meissner A., Dillon C. P., McManus M., Sharp P. A., Van Parijs L., Jaenisch R., Jacks T. (2004) Cre-lox-regulated conditional RNA interference from transgenes. Proc. Natl. Acad. Sci. U. S. A. 101, 10380–10385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dickins R. A., Hemann M. T., Zilfou J. T., Simpson D. R., Ibarra I., Hannon G. J., Lowe S. W. (2005) Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat. Genet. 37, 1289–1295 [DOI] [PubMed] [Google Scholar]
- 9. Gupta S., Schoer R. A., Egan J. E., Hannon G. J., Mittal V. (2004) Inducible, reversible, and stable RNA interference in mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 101, 1927–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Szulc J., Wiznerowicz M., Sauvain M. O., Trono D., Aebischer P. (2006) A versatile tool for conditional gene expression and knockdown. Nat. Methods 3, 109–116 [DOI] [PubMed] [Google Scholar]
- 11. Elbashir S. M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 [DOI] [PubMed] [Google Scholar]
- 12. Hannon G. J., Rossi J. J. (2004) Unlocking the potential of the human genome with RNA interference. Nature 431, 371–378 [DOI] [PubMed] [Google Scholar]
- 13. Silva J., Chang K., Hannon G. J., Rivas F. V. (2004) RNA-interference-based functional genomics in mammalian cells: reverse genetics coming of age. Oncogene 23, 8401–8409 [DOI] [PubMed] [Google Scholar]
- 14. Henriksen J. R., Lokke C., Hammero M., Geerts D., Versteeg R., Flaegstad T., Einvik C. (2007) Comparison of RNAi efficiency mediated by tetracycline-responsive H1 and U6 promoter variants in mammalian cell lines. Nucleic Acids Res. 35, e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewandoski M. (2001) Conditional control of gene expression in the mouse. Nat. Rev. Genet. 2, 743–755 [DOI] [PubMed] [Google Scholar]
- 16. Zhu Z., Zheng T., Lee C. G., Homer R. J., Elias J. A. (2002) Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling. Semin. Cell. Dev. Biol. 13, 121–128 [DOI] [PubMed] [Google Scholar]
- 17. Komazaki S., Ito K., Takeshima H., Nakamura H. (2002) Deficiency of triad formation in developing skeletal muscle cells lacking junctophilin type 1. FEBS Lett. 524, 225–229 [DOI] [PubMed] [Google Scholar]
- 18. Komazaki S., Nishi M., Takeshima H. (2003) Abnormal junctional membrane structures in cardiac myocytes expressing ectopic junctophilin type 1. FEBS Lett. 542, 69–73 [DOI] [PubMed] [Google Scholar]
- 19. Takeshima H., Komazaki S., Nishi M., Iino M., Kangawa K. (2000) Junctophilins: a novel family of junctional membrane complex proteins. Mol. Cell 6, 11–22 [DOI] [PubMed] [Google Scholar]
- 20. Ito K., Komazaki S., Sasamoto K., Yoshida M., Nishi M., Kitamura K., Takeshima H. (2001) Deficiency of triad junction and contraction in mutant skeletal muscle lacking junctophilin type 1. J. Cell Biol. 154, 1059–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pan Z., Yang D., Nagaraj R. Y., Nosek T. A., Nishi M., Takeshima H., Cheng H., Ma J. (2002) Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat. Cell Biol. 4, 379–383 [DOI] [PubMed] [Google Scholar]
- 22. Wang X., Weisleder N., Collet C., Zhou J., Chu Y., Hirata Y., Zhao X., Pan Z., Brotto M., Cheng H., Ma J. (2005) Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat. Cell Biol. 7, 525–530 [DOI] [PubMed] [Google Scholar]
- 23. Zhao X., Weisleder N., Thornton A., Oppong Y., Campbell R., Ma J., Brotto M. (2008) Compromised store-operated Ca2+ entry in aged skeletal muscle. Aging Cell 7, 561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mao H., Wong L. B. (1998) Fluorescence and laser photon counting: measurements of epithelial [Ca2+]i or [Na+]i with ciliary beat frequency. Ann. Biomed. Eng. 26, 666–678 [DOI] [PubMed] [Google Scholar]
- 25. Hirata Y., Brotto M., Weisleder N., Chu Y., Lin P., Zhao X., Thornton A., Komazaki S., Takeshima H., Ma J., Pan Z. (2006) Uncoupling store-operated Ca2+ entry and altered Ca2+ release from sarcoplasmic reticulum through silencing of junctophilin genes. Biophys. J. 90, 4418–4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin X., Yang J., Chen J., Gunasekera A., Fesik S. W., Shen Y. (2004) Development of a tightly regulated U6 promoter for shRNA expression. FEBS Lett. 577, 376–380 [DOI] [PubMed] [Google Scholar]
- 27. Freundlieb S., Schirra-Muller C., Bujard H. (1999) A tetracycline controlled activation/repression system with increased potential for gene transfer into mammalian cells. J. Gene Med. 1, 4–12 [DOI] [PubMed] [Google Scholar]
- 28. Zhu Z., Ma B., Homer R. J., Zheng T., Elias J. A. (2001) Use of the tetracycline-controlled transcriptional silencer (tTS) to eliminate transgene leak in inducible overexpression transgenic mice. J. Biol. Chem. 276, 25222–25229 [DOI] [PubMed] [Google Scholar]
- 29. Jackson R. J., Howell M. T., Kaminski A. (1990) The novel mechanism of initiation of picornavirus RNA translation. Trends Biochem. Sci. 15, 477–483 [DOI] [PubMed] [Google Scholar]
- 30. Jang S. K., Krausslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. (1988) A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J. Virol. 62, 2636–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubinson D. A., Dillon C. P., Kwiatkowski A. V., Sievers C., Yang L., Kopinja J., Rooney D. L., Zhang M., Ihrig M. M., McManus M. T., Gertler F. B., Scott M. L., Van Parijs L. (2003) A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat. Genet. 33, 401–406 [DOI] [PubMed] [Google Scholar]
- 32. Merritt J. E., Jacob R., Hallam T. J. (1989) Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J. Biol. Chem. 264, 1522–1527 [PubMed] [Google Scholar]
- 33. Zhao X., Weisleder N., Han X., Pan Z., Parness J., Brotto M., Ma J. (2006) Azumolene inhibits a component of store-operated calcium entry coupled to the skeletal muscle ryanodine receptor. J. Biol. Chem. 281, 33477–33486 [DOI] [PubMed] [Google Scholar]
- 34. Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S. H., Tanasa B., Hogan P. G., Lewis R. S., Daly M., Rao A. (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185 [DOI] [PubMed] [Google Scholar]
- 35. Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., Velicelebi G., Stauderman K. A. (2005) STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 169, 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vig M., Peinelt C., Beck A., Koomoa D. L., Rabah D., Koblan-Huberson M., Kraft S., Turner H., Fleig A., Penner R., Kinet J. P. (2006) CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li H., Ding X., Lopez J. R., Takeshima H., Ma J., Allen P. D., Eltit J. M. (2010) Impaired Orai1-mediated resting Ca2+ entry reduces the cytosolic [Ca2+] and SR Ca2+ loading in quiescent junctophilin 1 knockout myotubes. J. Biol. Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferrer-Martinez A., Montell E., Montori-Grau M., Garcia-Martinez C., Gomez-Foix A. M., Roberts M. A., Mansourian R., Mace K. (2006) Long-term cultured human myotubes decrease contractile gene expression and regulate apoptosis-related genes. Gene 384, 145–153 [DOI] [PubMed] [Google Scholar]
- 39. Raymond F., Metairon S., Kussmann M., Colomer J., Nascimento A., Mormeneo E., Garcia-Martinez C., Gomez-Foix A. M. Comparative gene expression profiling between human cultured myotubes and skeletal muscle tissue. BMC Genomics 11, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Woo J. S., Hwang J. H., Ko J. K., Weisleder N., Kim do H., Ma J., Lee E. H. (2010) S165F mutation of junctophilin 2 affects Ca2+ signalling in skeletal muscle. Biochem. J. 427, 125–134 [DOI] [PubMed] [Google Scholar]
- 41. Landstrom A. P., Weisleder N., Batalden K. B., Bos J. M., Tester D. J., Ommen S. R., Wehrens X. H., Claycomb W. C., Ko J. K., Hwang M., Pan Z., Ma J., Ackerman M. J. (2007) Mutations in JPH2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. J. Mol. Cell. Cardiol. 42, 1026–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.