Abstract
We developed a tissue-engineered vascular graft composed of biodegradable scaffold seeded with autologous bone marrow-derived mononuclear cells (BMMCs) that is currently in clinical trial and developed analogous mouse models to study mechanisms of neovessel formation. We previously reported that seeded human BMMCs were rapidly lost after implantation into immunodeficient mice as host macrophages invaded the graft. As a consequence, the resulting neovessel was entirely of host cell origin. Here, we investigate the source of neotissue cells in syngeneic BMMC-seeded grafts, implanted into immunocompetent mouse recipients. We again find that seeded BMMCs are lost, declining to 0.02% at 14 d, concomitant with host macrophage invasion. In addition, we demonstrate using sex-mismatched chimeric hosts that bone marrow is not a significant source of endothelial or smooth muscle cells that comprise the neovessel. Furthermore, using composite grafts formed from seeded scaffold anastomosed to sex-mismatched natural vessel segments, we demonstrate that the adjacent vessel wall is the principal source of these endothelial and smooth muscle cells, forming 93% of proximal neotissue. These findings have important implications regarding fundamental mechanisms underlying neotissue formation; in this setting, the tissue-engineered construct functions by mobilizing the body's innate healing capabilities to “regenerate” neotissue from preexisting committed tissue cells.—Hibino, N., Villalona, G., Pietris, N., Duncan, D. R., Schoffner, A., Roh, J. D., Yi, T., Dobrucki, L. W., Mejias, D., Sawh-Martinez, R., Harrington, J. K., Sinusas, A., Krause, D. S., Kyriakides, T., Saltzman, W. M., Pober, J. S., Shin'oka, T., Breuer, C. K. Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel.
Keywords: bone marrow chimera, composite vascular implant, microcomputed tomography angiography
Tissue engineering is a multidisciplinary science that applies engineering and biological principles toward the creation of neotissue from its cellular components that can be used for a variety of reconstructive surgical applications such as the creation of neovessels for use as vascular grafts (1). One commonly used approach to create a neotissue is to seed differentiated cells or their progenitors onto a biodegradable scaffold and then implant the seeded construct in vivo (2). The scaffold provides sites for initial cell attachment and 3-dimensional space for cell growth. It is hypothesized that as the scaffold degrades, the neotissue forms from the seeded cells, ultimately resulting in a purely biological structure without any synthetic components (2) that becomes incorporated into the host and has the ability to repair, remodel, and grow (1).
We employed this paradigm to develop a tissue-engineered vascular graft (TEVG) for use in congenital heart surgery where neovessel growth can obviate the need for repetitive surgical revisions (3). Our TEVG is constructed by seeding autologous bone marrow-derived mononuclear cells (BMMCs) onto a biodegradable tubular matrix (4–6). In juvenile lamb recipients, the neovessels that form are living vascular conduits that histologically resemble native blood vessels and demonstrate the ability to grow, repair, and remodel (6). We then performed the first clinical trial evaluating the use of this TEVG in humans (7–9). Initial results of our pilot study demonstrated an excellent safety profile for the TEVG, again with the additional benefit of growth potential (8). Late-term clinical followup of this cohort of patients demonstrated that all TEVGs were patent without any graft related mortality (9).
To analyze the process of neovessel formation, we miniaturized our construct so that it could be implanted as an inferior vena cava (IVC) interposition graft in a mouse recipient (10). These tubular scaffolds are composed of the same materials and design used in our clinical TEVGs, thus maintaining similar structural, mechanical, and degradation properties (10). We recently reported results using a human BMMC-seeded scaffold implanted into a CB-17 SCID/bg-immunodeficient host mouse, demonstrating that the cells seeded onto the scaffold disappeared concomitant with infiltration of the scaffold by host-derived macrophages (11). The definitive neovessel that developed was composed of cells entirely of mouse origin. However, the loss of the human BMMCs in this model could be caused by a form of xenorejection mediated by the innate immune system. To better understand the origin of the cells that give rise to neovessels in our human patients, we have now performed studies in a mouse model using syngeneic BMMC and immunocompetent hosts.
MATERIALS AND METHODS
Scaffold fabrication
Scaffolds were constructed from a nonwoven polyglycolic acid (PGA) mesh (Concordia Fibers, Coventry, RI, USA) and a copolymer sealant solution of poly-l-lactide and ε-caprolactone [P(CL/LA)] using the dual cylinder chamber molding system as described previously (10).
BMMC isolation and TEVG assembly
Bone marrow was collected from the femurs of syngeneic CB57BL/6 mice (Jackson Laboratories, Bar Harbor, ME, USA). Following purification of the mononuclear cell component using Histopaque-1086 (Sigma, St. Louis, MO, USA) centrifugation, 1 × 106 mononuclear cells were manually pipetted onto the scaffold. The seeded scaffold was incubated in Dulbecco's modified Eagle's medium (Life Technologies, North Andover, MA, USA) overnight before implantation as described previously (10).
TEVG implantation
TEVG implantations were performed using a microsurgical technique. The scaffolds were inserted into the infrarenal IVC of 6- to 8 -wk-old female mice (Jackson Laboratories) as described previously (10, 11). In total, 65 animals were implanted with TEVGs. All animal experiments were done in accordance with institutional guidelines for the use and care of animals, and the Institutional Review Board at Yale University approved the experimental procedures described.
Transgenic animals
Transgenic inbred C57BL/6 mice that express green fluorescent protein (GFP) under the control of ubiquitin-C promoter were obtained (Jackson Laboratories) and bred in our laboratories. All animal procedures were approved by the Institutional Animal Care and Committee at Yale University.
Fate of TEVG-seeded cells
Six- to 8-wk-old (20–25 g) C57BL/6 mice underwent implantation of IVC interposition grafts using a previously approved protocol, seeded with 1 × 106 GFP+ BMMCs. Mice were killed postoperatively at 6 h (n=4), 1 d (n=4), 3 d (n=4), 7 d (n=4), and 14 d (n=4), and the grafts were explanted at discrete time points to assess for fate of seeded cells using quantitative real-time PCR (qRT-PCR).
Cell quantification assay
The cellularity of each GFP+ BMMC-seeded scaffold was determined by measuring DNA content with the PicoGreen detection assay (Molecular Probes, Eugene, OR, USA). At 6 h and 1, 3, and 7 d of incubation, seeded scaffold sections were rinsed 3 times in 1 ml PBS, placed in 200 μl of distilled water, and stored at −80°C. At the time of evaluation, scaffold sections were thawed at 37°C. A black 96-well plate was loaded with 50 μl from each sample. A 30 μl aliquot of the PicoGreen dye was mixed thoroughly with 6 ml of Tris-EDTA buffer (pH 7.5), and 50 μl was added to each sample in the 96-well plate. All samples were performed in triplicate. The plate was incubated in the dark at room temperature for 10 min. Fluorescence was measured at 488 nm excitation and 525 nm emission. The number of cells maintained on each scaffold was determined from a standard curve generated from a known quantity of mouse BMMCs. A negative control of unseeded scaffold sections was used for comparison (7, 12, 13).
Cell viability assay
To determine GFP+ BMMC viability following seeding onto scaffold sections and incubation at 6 h and 1, 3, and 7 d, a CellTiter 96 AQueous nonradioactive cell proliferation assay (Promega BioSciences, San Luis Obispo, CA, USA) was performed. After seeding, all scaffold sections were incubated in 2 ml of RPMI 1640 medium (1% penicillin/streptomycin and 10% fetal bovine serum) for the respective incubation times. Medium was changed every 2 d. Following incubation, the scaffold sections were washed 3 times with 1 ml PBS and the assay reagent, tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS), and an electron coupling reagent, phenazine methosulfate, was added to each scaffold section in a 1:4 ratio with medium and allowed to incubate for 4 h at 37°C. After 4 h, a 100-μl aliquot of each sample was added to a clear 96-well plate, and the absorbance at 490 nm was read. The relative cell viability was determined by the ratio of absorbance from the seeded scaffold sections incubated at different time points.
Bone marrow transplantation
After myeloablation with 900 cGy total body irradiation using a 137Cs source, 6-wk-old female wild-type C57BL/6 mice received a tail vein injection of 5 × 106 unfractionated nucleated bone marrow cells (BMCs) harvested from age-matched, sex-mismatched (male) GFP+ transgenic mice, as described previously (14). We confirmed engraftment of the bone marrow 1 mo after bone marrow transplantation by determining the percentage of GFP+ cells on peripheral blood using FACS. Subsequently, TEVGs (n=57) were implanted as IVC interposition grafts. Specimens for immunostaining (n=27) were explanted at postoperative d 3 (n=6), 7 (n=6), and 14 (n=5), wk 10 (n=5), and mo 6 (n=5), and specimens for qRT-PCR (n=30) were explanted at postoperative d 3 (n=6), 7 (n=6), and 14 (n=6), wk 10 (n=6), and mo 6 (n=6).
Composite graft construction
The vena cava was harvested from a male mouse. Sections of the vena cava (1 mm) were anastomosed to the distal end of a scaffold. The composite scaffold was then seeded and incubated as described above. The composite TEVG was implanted in a female host (n=8). Specimens were harvested at postoperative d 7 (n=2) and 14 (n=2), wk 10 (n=2), and mo 6 (n=2).
Micro-computed tomography angiography (micro-CTA)
In vivo patency and morphology of the TEVGs were evaluated 24 wk after implantation using micro-CTA. Mice were positioned in supine position and placed on animal bed of micro-CT scanner (eXplore CT120; GE Healthcare, Piscataway, NJ, USA). All animals were imaged using standard micro-CT imaging protocol (220 views, 16-ms X-ray exposure time, penetration energy: 70 kV/32 mA). Omnipaque contrast (GE Healthcare) was utilized as a contrast agent. Micro-CT images were reconstructed and visualized with MicroView (GE Healthcare) and Amide (amide.sf.net) software packages to assess graft patency and vessel lumen size.
Histology
Explanted grafts were pressure fixed in 10% formalin overnight and then embedded in paraffin or glycolmethacrylate using previously published methods (10, 11). Sections were stained with hematoxylin and eosin.
Immunohistochemistry
Primary antibodies included rat-anti-mouse Mac-3 (BD Bioscience, San Jose, CA, USA), F4/80 (AbD Serotec, Raleigh, NC, USA), mouse-anti-human calponin (Dako, Carpinteria, CA, USA), rabbit-anti-human von Willebrand Factor (vWF; Dako), rabbit-anti-mouse to c-kit (Abcam), mouse-anti-mouse to Sox-2 (R&D Systems, Cambridge, UK), and rat-anti-mouse Sca-1 (R&D Systems). Antibody binding was detected using appropriate biotinylated secondary antibodies, followed by binding of streptavidin-HRP and color development with 3,3-diaminobenzidine (Vector, Burlingame, CA, USA). Nuclei were then counterstained with hematoxylin. For immunofluorescence detection, a goat-anti-rabbit IgG-Alexa Fluor 568 (Invitrogen, Carlsbad, CA, USA) or a goat-anti-mouse IgG-Alexa Fluor 488 (Invitrogen) was used with subsequent 4′,6-diamidino-2-phenylindole nuclear counterstaining.
qRT-PCR
Explanted tissue grafts were incubated overnight in 180 μl of lysis buffer (Qiagen, Valenica, CA, USA) and proteinase K (12 mAu/reaction) at 56°C. Following tissue digestion, DNA was isolated from samples using DNeasy blood and tissue kit (Qiagen) following the manufacturer's instructions. The following primers and TaqMan probe were designed to amplify a specific 93-bp region of the GFP gene: forward, 5′-ACCACATGAAGCAGCACGACTTCT-3′; reverse, 5′-TGTAGTTGCCGTCGTCCTTGAAGA-3′; probe, 5′-AAGGCTACGTCCAGGAGCGCACCAT-3′. DNA amplification and quantification were performed using iCycler iQ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Each qRT-PCR reaction consisted of the following steps: 2 min UNG incubation at 50°C to remove possible amplicon contamination, followed by 10 min at 95°C to activate the polymerase, and 40 cycles of 15 s denaturing at 95°C and 1 min at 60°C of extension and annealing. Data were collected at the end of each elongation step and analyzed with iCycler iQ Real Time Detection System Software (Bio-Rad).
Fluorescent in situ hybridization (FISH)
FISH was performed on paraffin sections using digoxigenin-labeled mouse Y chromosome probe detected using a rhodamine-conjugated antibody to digoxigenin (Roche Diagnostics, Mannheim, Germany) as described previously (15, 16). Counting of Y+ nuclei was accomplished by systematically examining the FISH-stained tissue, field by field, at ×40, using a Zeiss Axiovert 200M Fluorescence/Live cell Imaging Microscope (Carl Zeiss Imaging Solutions, Thornwood, NY, USA). Digital images were acquired using the Zeiss LSM510 confocal computer system (Carl Zeiss Imaging Solutions). Images were pseudocolored using image processing software (Adobe Photoshop, San Jose, CA, USA). Cell counts were obtained by first counting all of the Y chromosome-positive (YChr+) cells in a defined area on the tissue and then counting the total number of cells in that area using the immunostained photographs.
RESULTS
TEVGs form neovessels that resemble native veins
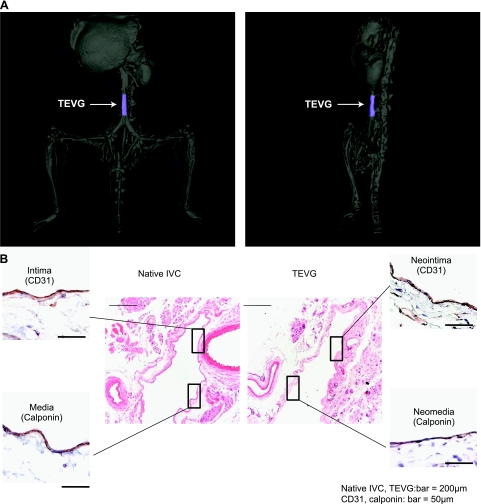
Miniaturized PGA-P(CL/LA) tubular scaffolds were seeded with syngeneic BMMCs and implanted into the IVC of CB57BL/6 mice. Scaffold materials completely degraded by 6 mo, at which point TEVGs displayed tissue architecture similar to a native vein. Three-dimensional computed tomography angiography of TEVGs at 6 mo showed no evidence of stenosis, aneurismal dilation, or thrombosis (Fig. 1A). Furthermore, histological characterization demonstrated a mature laminated structure, consisting of an endothelialized intimal layer and smooth muscle medial layer (Fig. 1B).
Figure 1.
A) Representative CT angiogram of a TEVG IVC interposition graft 6 mo after implantation. TEVG is colored blue. B) Representative photomicrographs of immunohistochemical characterization of the native IVC compared with the TEVG 6 mo after implantation (hematoxylin and eosin, ×100; CD31 and Cal, ×400).
Fate of TEVG-seeded cells
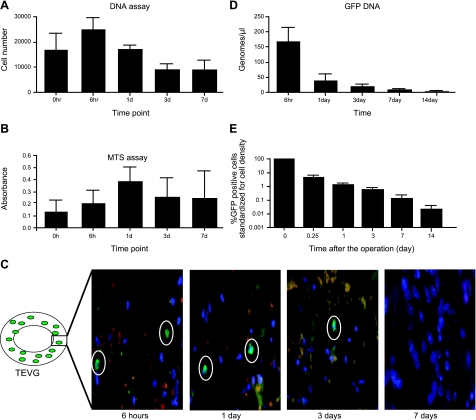
Based on qRT-PCR for the GFP gene, the quantity of cellular DNA within scaffolds was maximal at 6 h after overnight incubation, and the cellularity decreased after 1 d of incubation (Fig. 2A). These findings suggest that the seeded cells decrease in number over time after in vitro culture, probably because they do not attach to the scaffold and, therefore, are washed off with medium changes. The relative cell viability and activity of the seeded cells increased over a 7-d incubation period (Fig. 2B). Despite low attachment of these cells to the TEVG, these cells remain active and viable up to 7 d, suggesting that the cells are not dying with in vitro cultivation. After this 7-d period, we implanted TEVGs (n=30), constructed by seeding the scaffold with GFP-labeled syngeneic BMMCs, into 6- to 8-wk-old C57Bl/6 mice and then harvested the scaffolds at 6 h and 1, 3, 7, and 14 d, quantifying the percentage of GFP DNA at various time points. We found that the seeded syngeneic cells disappeared as the scaffold became populated by host-derived cells identified as F4/80-expressing macrophages (not shown). Immunofluorescence of grafts demonstrated the presence of GFP-labeled cells within the graft at 6 h and 1 and 3 d, but no remaining cells at 7 d postimplantation (Fig. 2C). GFP DNA qRT-PCR corroborated the findings that over the course of 14 d following implantation, the concentration of GFP genomes decreased rapidly, from 173 genomes/μl at 6 h to 0.12 genomes/μl at 14 d (Fig. 2D). We then standardized the GFP data to cell density to confirm that the seeded cells disappeared. The percentage of GFP+ cells per TEVG was found to decrease from 4.37% at 6 h to 0.02% at 14 d (Fig. 2E).
Figure 2.
A) DNA assay of scaffolds from 0 h to 7 d of in vitro incubation showed increased number of cells in first 24 h and loss of cells after 72 h. B) MTS assay of scaffolds from 0 h to 7 d in vitro incubation showed initial increase in metabolic activity followed by steady state. C) In vivo tracking using immunofluorescence (×630) demonstrated GFP+ cells within the graft at 6 h and 1 and 3 d but no cells at 7 d postimplantation. D) These findings were corroborated by qRT-PCR for the GFP gene from TEVGs (n=30) seeded with GFP+ cells, implanted in GFP-negative hosts, and harvested between 6 h and 14 d after implantation. Level of detection is 10 genomes/μl. E) Percentage of GFP-labeled cells per TEVG standardized for cell density decreased from 4.37% at 6 h to 0.02% at 14 d. Data are expressed as means ± sd.
BMCs are not the ultimate source of vascular neotissue
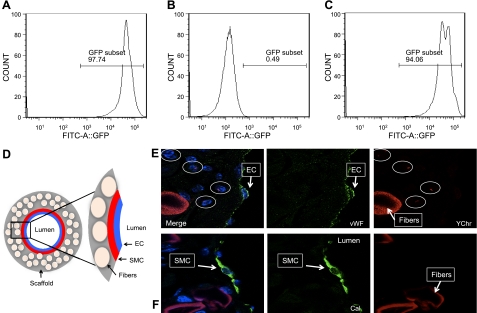
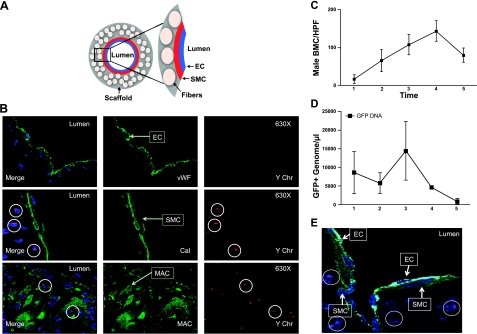
We next designed a series of experiments to determine the source of the cells that populated the scaffold. To assess the role of host BMCs in neovessel formation of our TEVG, chimeras were created by rescuing lethally irradiated C57BL/6 female mice with sex-mismatched male GFP+ total BMCs from transgenic GFP-labeled mice. Donor cell engraftment in the surviving recipients was determined at 5 wk after bone marrow transplantation. FACS analysis of peripheral blood GFP-labeled mice demonstrated 97.74% circulating GFP+ cells in a positive control mouse (Fig. 3A), 0.49% in a negative control mouse (Fig. 3B) and 94.06% reconstitution in transplanted mice (Fig. 3C). Once chimeras were established, we implanted our TEVGs into female mice. All mice were killed at 3, 7, and 14 d, 10 wk, and 6 mo postimplantation, and all grafts were harvested for histological analysis. At 3 and 7 d after implantation, YChr+ cells were found in the graft wall. Most of these male-derived BMCs were macrophages determined by colocalization for FISH Y+ cells and F4/80 antibody using immunofluorescence (not shown). At 14 d, as the number of BMCs increased within the graft wall, endothelial cells and smooth muscle cells began to appear. These vascular cells were positive for vWF and SMA markers, but negative for YChr cells (Fig. 3D, E). At 10 wk after implantation, a confluent neointima (enodthelial cells) and neomedia (smooth muscle cells) were present; both layers were negative for YChr cells (not shown). Finally, at 6 mo postimplantation, the neovessel endothelial layer was composed of vWF+ YChr− cells (Fig. 4A). The smooth muscle layer was composed of calponin+ YChr− cells (Fig. 4B), and the scaffold was mostly degraded, with few inflammatory cells present that were positive for MAC-3 and YChr (Fig. 4B). The numbers of infiltrating BM-derived YChr+ cells in the graft wall increased up to 10 wk, during the period in which graft degradation was occurring, but at 6 mo postimplantation, these cells markedly decreased (Fig. 4C). This acute inflammatory response and later decline were corroborated using qRT-PCR for GFP DNA within the graft over a 6-mo period (Fig. 4D). These experiments demonstrated that the macrophages that initially infiltrated the scaffold were of male origin. At later times, a confluent layer of endothelial cells and smooth muscle cells was present in the neovessel with no evidence of colocalization of either smooth muscle cell markers or endothelial cell markers with the Y chromosome (Fig. 4E).
Figure 3.
A–C) FACS analysis of GFP+ mouse as positive control (A), C57BL/6 mouse for negative control (B), and engraftment 5 wk post-transplantation (C). D) Schematic demonstrating components and orientation of photomicrographs. E, F) Confocal microscopic images of TEVG implanted into female host that had undergone transplantation with male bone marrow harvested 14 d after implantation, demonstrating no colocalization with the Y chromosome (Y-chromosome FISH) and either endothelial cells (EC; vWF, ×630; E) or smooth muscle cells (SMC; calponin, ×630; F).
Figure 4.
A) Schematic demonstrating components and orientation of photomicrographs. B) Confocal microscopic images of TEVG implanted into female host that had undergone transplantation with male bone marrow harvested 6 mo after implantation, demonstrating colocalization of macrophages (MAC) and the Y-chromosome (Y-chromosome FISH) but no colocalization with the Y chromosome (Y-chromosome FISH) and either endothelial cells (vWF) or smooth muscle cells (Calponin; ×400). C) Numbers of infiltrating BM-derived Y chromosome (Y-chromosome FISH) cells (bone marrow cells/high power field) in the graft wall increased up to 10 wk, while graft degradation was occurring, but at 6 mo postimplantation, these cells markedly decreased. D) This was quantified and corroborated using qRT-PCR for GFP DNA within the graft over a 6-mo period. E) Confocal microscopic image of triple staining of a 6-mo neovessel with normal IVC configuration with single-layer neointima (EC; vWF/light blue), neomedia (SMC; calponin/green), and neoadventitia with scattered inflammatory cells (male; Ychr/red).
Neovessel formation arises from ingrowth of vascular cells from the neighboring blood vessel
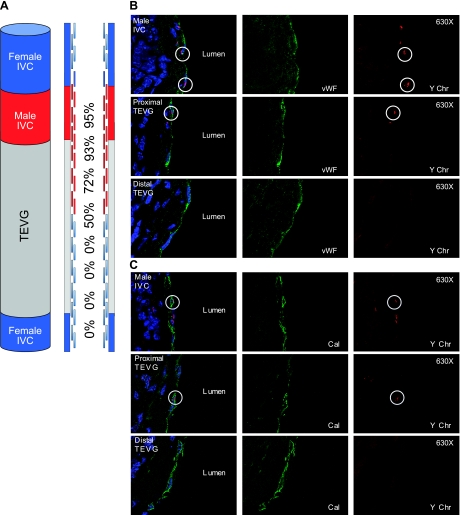
To determine whether the endothelial cells or smooth muscle cells derived from the neighboring blood vessel, we developed a composite vascular graft created by anastomosing a segment of syngeneic male IVC with a TEVG (Fig. 5A). These composite vascular grafts (n=8) were implanted into female hosts and harvested on postoperative d 7 and 14, wk 10, and mo 6, and processed for histological analysis and paraffin embedding. All composite grafts were cut with 5 μm in a longitudinal fashion to include the male (donor) IVC, TEVG, and female (host) IVC (Fig. 5A). The composite graft and neighboring IVC were then evaluated using FISH to identify the Y chromosome. At 7 and 14 d postimplantation, most of the graft wall was populated by MAC 3+/YChr− cells (host origin), and no confluent endothelial or smooth muscle cells layers were noted (not shown). An endothelial (vWF+) and smooth muscle layer (calponin+) was noted initially at 14 d and then more confluent at 10 wk postimplantation (not shown). Finally, at 6 mo postimplantation, the host-derived neovessel was composed of a confluent neointima, a neomedia, and a neoadventitia. Detailed analysis of the neointima and neomedia using confocal microscopy demonstrated that the percentage of YChr+ endothelial and smooth muscle cells was greatest closer to the implanted male IVC segment (Fig. 5B, C). These findings suggest that the resulting neovessel is not derived from seeded cells or bone marrow progenitor cells,but instead through a locally mediated process of ingrowth of neighboring endothelial and smooth muscle cells from the surrounding native vessel. On immunohistochemistry, 6-mo sections were negative for stem cell markers and markers of progenitor cells, including Sca-1, c-kit, and Sox-2.
Figure 5.
A) Schematic demonstrating composite TEVG (n=8) created by anastomosis of a syngeneic male IVC with a TEVG, implanted into a female host and harvested at 6 mo after implantation, and the percentage of cells with Y-chromosome as a function of distance from the male IVC. A) Confocal microscopic images demonstrating colocalization of endothelial cells (vWF) and Y chromosome (Y-chromosome FISH; ×400). C) Photomicrographs demonstrating colocalization of smooth muscle cells (calponin) and Y chromosome (Y-chromosome FISH; ×400).
DISCUSSION
The purpose of this study was to determine the source of the cells that make up the neotissue in our TEVGs. To accomplish this goal, we investigated the origins of the vascular neotissue cells in a syngeneic immunocompetent mouse recipient using bone marrow chimeric hosts and composite vascular implants. Miniaturized PGA-P(CL/LA) tubular scaffolds were seeded with syngeneic BMMCs and implanted into the mouse IVC to mimic the high-flow, low-pressure venous setting seen in the clinical application for congenital heart surgery.
The results of this study validate our previous work demonstrating the rapid disappearance of the seeded cells from the TEVG; however, this time performed in a clinically relevant, immune-competent murine model (11). To trace the fate of the BMMCs in this study, we implanted TEVGs constructed by seeding the scaffolds with GFP-labeled syngeneic BMMCs and then harvested the scaffolds over a 2-wk time course, quantifying the percentage of GFP DNA at various time points. As we had observed in the human into mouse xenogeneic model, we found that the seeded syngeneic cells disappeared as the scaffold became populated by host-derived cells identified as F4/80-expressing macrophages. To demonstrate that this was not simply a dilutional effect due to increased cell density secondary to the infiltrating host inflammatory cells, we standardized the GFP data to cell density and confirmed that the seeded cells disappeared. Also, as with previous studies in other animal models, scaffold materials completely degraded by 6 mo, at which point TEVGs displayed similar vascular architecture to native vein (4–6).
To analyze the role of bone marrow-derived cells in vascular neotissue formation, we implanted TEVGs into female mice that had undergone bone marrow transplantation with syngeneic male bone marrow and then harvested the TEVG at various times over a 6-mo period; we analyzed the specimens using FISH for the Y chromosome and a variety of other cellular markers, including markers for macrophages (F4/80), smooth muscle cells (calponin), and endothelial cells (vWF). These experiments demonstrated that the macrophages that infiltrated the scaffold were of male origin and were therefore derived from the bone marrow. When we looked at later times, there was no evidence of colocalization of either smooth muscle cell markers or endothelial cell markers with the Y chromosome marker, suggesting that the bone marrow was not the source of the definitive vascular neotissue.
To determine if the endothelial cells or smooth muscle cells derived from the neighboring blood vessel, we developed a composite vascular graft created by anastomosing a segment of syngeneic male IVC with a TEVG. These composite vascular grafts were implanted into female hosts and harvested over a 6 mo period. The composite graft and neighboring IVC were then evaluated using FISH to identify the Y chromosome. Both endothelial cells and smooth muscle cells were found to contain the Y chromosome. In addition, the percentage of YChr+ cells was greatest closer to the implanted male IVC segment. These data suggest that definitive neovessel cells arise from ingrowth of cells, mostly likely differentiated endothelial and smooth muscle cells, from the neighboring blood vessel segment. Although we cannot rule out the possibility that there is a rare population of stem cells resident within the adjacent vascular segment, we found negative immunohistochemical staining for markers of both stem cells and progenitor cells, suggesting that it is the differentiated endothelial and smooth muscle cells themselves that migrate in to create the neotissue.
The most interesting new finding of this study is that the smooth muscle cells and endothelial cells that make up the intima and media of the neovessel formed in our model are derived from the neighboring blood vessel wall. Therefore, the cells of the neotissue are not derived from either the seeded cells or from bone marrow derived progenitors as has previously been suggested (4). While this may be a model-specific phenomena, it suggests that neotissue formation can be a form of augmented tissue repair or regeneration. This represents a shift away from the traditional tissue engineering theory in which the seeded cells are viewed as the building blocks of the neotissue and a move toward a new paradigm in which the tissue-engineered construct promotes or augments the body's own reparative mechanisms to induce tissue regeneration. These findings have significant implications for rationally designing improved second-generation tissue engineered grafts based on modulation of the host response to the tissue engineered construct.
Acknowledgments
The authors thank Lin Wang for assisting with Y chromosome FISH analysis. Histological processing and embedding was done by the Yale Core Center for Musculoskeletal Disorders [U.S. National Institutes of Health (NIH) grant AR46032].
Research support was provided through the following grants: NIH K08HL083980, NIH R01HL098228, NIH UL1RR024139, and NIH P30DK072442; a Howard Hughes Medical Institute grant; and a Doris Duke Charitable Foundation Clinical Scientist Development Award. Gunze Ltd. has provided research support for the clinical trial evaluating the use of the use of tissue-engineered vascular grafts. None of the funding for the work done in this study was provided by Gunze Ltd.
REFERENCES
- 1. Vacanti J. P., Langer R. (1999) Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 354(Suppl. 1), S132–S134 [DOI] [PubMed] [Google Scholar]
- 2. Langer R., Vacanti J. P. (1993) Tissue engineering. Science 260, 920–926 [DOI] [PubMed] [Google Scholar]
- 3. Shinoka T., Shum-Tim D., Ma P. X., Tanel R. E., Isogai N., Langer R., Vacanti J. P., Mayer J. E. (1998) Creation of viable pulmonary artery autografts through tissue engineering. J. Thorac. Cardiovasc. Surg. 115, 536–545 [DOI] [PubMed] [Google Scholar]
- 4. Matsumura G., Miyagawa-Tomita S., Shinoka T., Ikada Y., Kurosawa H. (2003) First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation 108, 1729–1734 [DOI] [PubMed] [Google Scholar]
- 5. Hibino N., Shinoka T., Matsumura G., Ikada Y., Kurosawa H. (2005) The tissue-engineered vascular graft using bone marrow without culture. J. Thorac. Cardiovasc. Surg. 129, 1064–1070 [DOI] [PubMed] [Google Scholar]
- 6. Brennan M. P., Dardik A., Hibino N., Roh J. D., Nelson G. N., Papademitris X., Shinoka T., Breuer C. K. (2008) Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann. Surg. 248, 370–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shinoka T., Imai Y., Ikada Y. (2001) Transplantation of a tissue-engineered pulmonary artery. N. Engl. J. Med. 344, 532–533 [DOI] [PubMed] [Google Scholar]
- 8. Shinoka T., Matsumura G., Hibino N., Naito Y., Watanabe M., Konuma T., Sakamoto T., Nagatsu M., Kurosawa H. (2005) Midterm clinical results of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J. Thorac. Cardiovasc. Surg. 129, 1330–1338 [DOI] [PubMed] [Google Scholar]
- 9. Hibino N., McGillicuddy E., Matsumura G., Ichihara Y., Naito Y., Breuer C., Shinoka T. (2010) Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 139, 431–436 [DOI] [PubMed] [Google Scholar]
- 10. Roh J. D., Nelson G. N., Brennan M. P., Mirensky T. L., Yi T., Hazlett T. F., Tellides G., Sinusas A. J., Pober J. S., Saltzman W. M., Kyriakides T. R., Breuer C. K. (2008) Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials 29, 1454–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roh J. D., Sawh-Martinez R., Brennan M. P., Jay S. M., Devine L., Rao D. A., Mirensky T. L., Nalbandian A., Udelsman B., Hibino N., Shinoka T., Saltzman W. M., Snyder E., Kyriakides T. R., Pober J. S., Breuer C. K. (2010) Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc. Natl. Acad. 107, 4669–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roh J. D., Nelson G. N., Udelsman B. V., Brennan M. P., Lockhart B., Fong P. M., Lopez-Soler R. I., Saltzman W. M., Breuer C. K. (2007) Centrifugal seeding increases seeding efficiency and cellular distribution of bone marrow stromal cells in porous biodegradable scaffolds. Tissue Eng. 13, 2743–2749 [DOI] [PubMed] [Google Scholar]
- 13. Villalona G. A., Udelsman B., Duncan D. R., McGillicuddy E., Sawh-Martinez R. F., Hibino N., Painter C., Mirensky T., Erickson B., Shinoka T., Breuer C. K. Cell-seeding techniques in vascular tissue engineering. Tissue Eng. B Rev. 16, 341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Di Lorenzo A., Fernandez-Hernando C., Cirino G., Sessa W. C. (2009) Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proc. Natl. Acad. Sci. 106, 14552–14557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones R. J., Collector M. I., Barber J. P., Vala M. S., Fackler M. J., May W. S., Griffin C. A., Hawkins A. L., Zehnbauer B. A., Hilton J., Colvin O. M., Sharkis S. J. (1996) Characterization of mouse lymphohematopoietic stem cells lacking spleen colony-forming activity. Blood 88, 487–491 [PubMed] [Google Scholar]
- 16. Krause D. S., Theise N. D., Collect M. I., Henegariu O., Hwang S., Gardner R., Neutzel S., Sharkis S. J. (2001) Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105, 369–377 [DOI] [PubMed] [Google Scholar]