Abstract
Prion diseases are infectious neurodegenerative disorders associated with the misfolded prion protein (PrPSc), which appears to be the sole component of the infectious agent (termed prion). To produce disease, prions have to be absorbed into the body and reach sufficient quantities in the brain. Very little is known about the biological mechanisms controlling the initial fate of prions. Here, we studied the systemic pharmacokinetics and biodistribution of PrPSc in vivo. After an intravenous injection of highly purified radiolabeled or native unlabeled PrPSc, the protein was eliminated rapidly from the serum (half-life of 3.24 h), mostly through tissue uptake. The quantity of intact PrPSc reaching the brain was ∼0.2% of the injected dose per gram of brain tissue (ID/g). The highest levels were found in liver (∼20% ID/g), spleen (∼13% ID/g), and kidney (∼7.4% ID/g). Cell surface PrPC does not appear to play a role in PrPSc pharmacokinetics, since the infectious protein distributed similarly in wild-type and PrP-null mice. To measure tissue uptake kinetics and biodistribution accurately, vascular space in tissues was measured with radioactively labeled albumin coinjected with radioactively labeled PrPSc. Our results provide a fundamental pharmacokinetic characterization of PrPSc in vivo, which may be relevant to estimate tissue risks and mechanisms of prion neuroinvasion and to identify novel therapeutic strategies.—Urayama, A., Morales, R., Niehoff, M. L., Banks, W. A., Soto, C. Initial fate of prions upon peripheral infection: half-life, distribution, clearance, and tissue uptake
Keywords: PrPSc, pharmacokinetics, blood-brain barrier
Prion diseases are a group of infectious neurodegenerative disorders affecting animals and humans. The neuropathological features of prion disease include spongiform brain degeneration, neuronal loss, synaptic alterations, glial activation, and the cerebral deposition of misfolded prion protein (PrPSc) (1). PrPSc appears to be the main or sole component of the infectious agent (termed prion) that has the surprising ability to propagate the disease in the absence of nucleic acid by inducing the autocatalytic conversion of the natively folded host prion protein (PrPC). To produce the pathology, prions have to be absorbed into the body and reach the brain in quantities sufficient to induce the conversion of brain PrPC into PrPSc.
Prions can infect an individual by various routes, including ingestion, and remain silently replicating in various tissues for a long time before causing a devastating disease in the brain (2). The infectious agent is largely confined to the central nervous system (CNS), although small amounts of PrPSc have been detected consistently in peripheral tissues and biological fluids (1, 3). Lymphoid organs have long been known to be involved in early steps of prion diseases (4, 5). In particular, the spleen and lymph nodes have been demonstrated to be the first sites of PrPSc replication after infection by peripheral routes and also to be affected significantly following intracerebral challenge. Several results suggest that prion transport from the lymphoid system to the CNS occurs along peripheral nerves in a manner that seems to depend on PrPC expression (6, 7). The innervation pattern of lymphoid organs is mainly sympathetic, and sympathectomy delays the transport of prions from lymphatic organs to the thoracic spinal cord (7), which is the entry site of sympathetic nerves to the CNS. However, in these experiments, although the onset of the disease was delayed, animals still became ill (7). This and other findings suggest that an alternative direct route of prion entry to the brain exists (8). After first appearing in the brain, the quantity of PrPSc increases rapidly and exponentially in the entire brain (9, 10). This finding is more easily explained if prions enter the brain through the blood-brain barrier (BBB), because in that case, multiple entry points would exist, which would probably not be the case in the peripheral nerve model. In a previous study, we reported that purified PrPSc can reach the brain exclusively by crossing the BBB, without the involvement of peripheral nerves, and PrPSc appeared in both the brain parenchyma and the cerebrospinal fluid (CSF) (11). For these experiments, mice received short-term perfusions through the cerebrovasculature, so that the solution containing labeled PrPSc was exposed to the brain through the BBB without opportunity for circulation from tissues to brain. The results indicated that a significant amount of the protein reached the brain intact, providing a possible route for prion neuroinvasion. In addition, circumventricular organs (CVOs) in the brain have capillary vessels with fenestrae allowing open communications between vascular and neural components (12, 13). Although free diffusion of blood-borne materials in the milieu are limited by the cellular barrier of tanycytes and polarized local flow of CSF toward the perivascular area, the presence of PrPSc in CVOs, such as median eminence including arcuate nucleus, was demonstrated at the presymptomatic phase of the disease (14). These findings further support the dissemination of prions through vascular and CSF routes. Thus, both peripheral nerve and vascular pathways may be hypothesized on the basis of existing findings, and these possibilities are not mutually exclusive.
Most studies that have attempted to track the course of prions from the periphery into the CNS have relied on infectivity bioassays. However, in such cases, it is not possible to distinguish PrPSc present in the original infectious material from the newly generated protein as part of the early replication of the agent. Very little is known about the regional variation in the mechanisms that control the rate and amount of conversion of PrPc to PrPSc. Thus, regional differences in PrPSc concentrations in established disease may reflect differences in conversion kinetics rather than transport routes. Given the enormous economical and public health consequences of prion transmission by infection and the unprecedented features of this protein-only infectious agent, it is highly surprising that this very important aspect of the disease pathogenesis has been almost completely neglected. Currently, the field has no information regarding the half-life, bioavailability, clearance rate, or distribution profile of PrPSc. Systemic uptake profiling of PrPSc at the initial stage of infection will provide greater understanding of the route and mechanism of infection, preferential tissues for the distribution of PrPSc, and the metabolism and elimination of the misfolded protein. This study aims to elucidate the fundamental pharmacokinetic profiles of infectious PrPSc in the initial phase of infection, including the determination of serum half-life, volume of distribution, clearance rate, and quantitative tissue uptake of infectious prion proteins in vivo.
MATERIALS AND METHODS
Ethics statement
All animal experiments were approved by and conducted in strict accordance with guidelines of the Animal Care and Use Committee of the University of Texas Medical Branch in Galveston and the University of Texas Health Science Center in Houston and complied with the recommendations in the Guide for the Care and Use of Laboratory Animals of the U.S. National Institutes of Health.
Purification of PrPSc from infected mouse brain
PrPSc was purified from mice infected with Rocky Mountain Laboratory (RML) scrapie strain as described previously (15, 16). Briefly, brain tissue was homogenized at 10% w/v in phosphate-buffered saline containing 10% sarkosyl and subjected to a series of differential centrifugations employing a Beckman TL-100 ultracentrifuge (OptimaMAX Ultracentrifugep; Beckman-Coulter, Fullerton, CA, USA) with the final step consisting of a sucrose gradient. The material was then treated with proteinase K (PK; 50 μg/ml) for 2 h, followed by ultracentrifugation to precipitate PrPSc. The purity of PrPSc was confirmed by silver staining and estimated to be >95%. PrPSc concentration was measured by microBCA protein assay reagent (Pierce, Rockford, IL, USA). This preparation was fully infectious when injected into wild-type mice. For some experiments, native PrPSc partially purified from RML-infected brain was used to evaluate the pharmacokinetics of full-length PrPSc. Sarkosyl precipitate of infected brain homogenate without PK treatment was washed 3 times with sterile saline by ultracentrifugation to remove the detergent, and the pellet was resuspended by sonication at the amount of 1 brain equivalent of native PrPSc per 250 μl of saline.
Radiolabeling of PrPSc
Highly purified PrPSc was radioactively labeled by the iodobead method (Pierce) with [131I]Na or [125I]Na (Perkin-Elmer, Wellesley, MA, USA), as described in our previous reports (11, 17). Briefly, radioactive sodium iodine (2 mCi) was incubated with 1 iodobead for 15 min in 250 mM chloride-free sodium phosphate buffer (pH 7.4). Thereafter, ultrapurified PrPSc (10 μg) was added to the mixture and incubated for 5 min. The labeling reaction was terminated by removing the bead from the mixture. Albumin (fraction G, bovine serum albumin; Sigma, St. Louis, MO, USA) was labeled with [125I]Na by the chloramine-T method (18, 19). Briefly, albumin (5 μg) was mixed with [125I]Na (1 mCi) in 250 mM chloride-free sodium phosphate buffer (pH 7.4), and the radioactive labeling was initiated by adding chloramine-T (10 μg/reaction) to the mixture. After 1 min, the reaction was terminated by adding sodium metabisulfite (100 μg/reaction). Each radioactively labeled agent was purified by Sephadex G-10 chromatography to remove free iodine. Radioactively labeled PrPSc was diluted with phosphate-buffered saline containing 0.1% SB3-14 and further centrifuged in albumin-precoated Microcon filtration tube (MW cutoff 10 kDa; Millipore, Billerica, MA, USA) at 12,000 rpm for 30 min to further remove free radioactive iodine from the G-10 eluate. Radiolabeled PrPSc was washed extensively by this procedure. [131I]PrPSc had a specific radioactivity of 8.5 × 104 cpm/ng. The radioactively labeled PrPSc preparations we used had >95% precipitation with trichloroacetic acid (TCA). For some experiments, PrPSc was labeled with nonradioactive iodine (127I). Each agent was freshly prepared on the day of the experiment.
Animal procedures
For pharmacokinetics and biodistribution studies, male C57BL/6 mice (Charles River, Wilmington, MA, USA) were studied at 8 to 10 wk of age (24.4±1.0 g body weight). Mice anesthetized with urethane (40%) received an intravenous (i.v.) injection of [131I]PrPSc (1×106 cpm; 11.8 ng) with [125I]albumin (5×105 cpm), or 1 brain equivalent of unlabeled full-length PrPSc (250 μl), into the jugular vein. At 1, 3, 5, 10, 20, 30, 60, 120, and 180 min after injection, mice were sacrificed, and the blood, brain, heart, lung, liver, spleen, kidney, and skeletal muscle from the left hindlimb were immediately collected and weighed. The urine was also sampled from the urinary bladder with a 30-gauge needle. Serum from mouse blood was isolated by centrifugation. The packed cell volume in whole blood was also measured by centrifuging the whole-blood sample (100 μl) in a capillary tube, and the length of the column of blood cells relative to the length of the column of the whole specimen was measured to determine the hematocrit (%). Radioactivity was measured by a γ counter (Packard Instrument Co., Meriden, CT, USA). TCA precipitation of radioactively labeled PrPSc in each sample was also measured after injection, and results were normalized by the precipitation ratio (%).
Immunodetection of unlabeled PrPSc in serum
Serum samples were precipitated by centrifugation at 100,000 g for 1 h with 10% sarkosyl followed by washing 3 times, and the pellet was resuspended in 1 ml of PBS using sonication. The serum extract was then lyophilized, reconstituted in 30 μl of PBS, and treated with PK (12.5 μg/ml) at 37°C for 1 h. The samples were subsequently used for SDS-PAGE under reducing conditions, then blotted, and immunoreactive bands were probed with 6D11 antibody. Western blot data was analyzed by densitometry compared to standard of partially purified protein.
Systemic pharmacokinetics
Serum concentration-time profiles were fitted with Eq. 1, a biexponential equation using the nonlinear least-squares method (20):
| (1) |
The distribution and elimination half-lives were estimated as ln2/α and ln2/β, respectively. The initial distribution volume Vc was calculated as ID/(A+B), and steady-state distribution volume Vss was calculated as ID(Aβ2+Bα2)/(Aβ+Bα)2, where injected dose ID is the total dose (cpm) injected intravenously. The area under the serum-time curve AUC0–t was calculated by integrating Eq. 1 from 0 to t. The total body clearance rate CLtot was calculated as ID/AUC0–∞.
Multiple time-regression analysis
The blood-to-tissue unidirectional influx rate CLin of the radioactively labeled agent was calculated according to our previous study (19). The tissue/serum ratios were plotted against exposure time, which was calculated as the area under the serum concentration-time curve (the integral part in Eq. 2) divided by the serum concentration at time t in Eq. 2:
| (2) |
where Am and Cp(t) are counts per minute per gram of wet tissue and counts per minute per microliter of serum at time t, respectively. CLin was measured as the slope for the linear portion of the curve between the tissue/serum ratios and respective exposure times. The y intercept of the line represents Vi, the initial volume of distribution in the tissue at t = 0.
The percentage of the intravenously injected dose taken up per gram of tissue at time t (%ID/g) was calculated from Eq. 3:
| (3) |
HPLC analysis
The stability of [125I]PrPSc in serum, brain, heart, lung, liver, spleen, kidney, skeletal muscle, and urine was examined using HPLC. Mice received an i.v. injection of [125I]PrPSc of 8 × 106 cpm/mouse. The samples from animals sacrificed at 20 or 180 min were used for the study. Serum (10 μl) was diluted with 90 μl of mobile phase. The tissues were mechanically homogenized in a 9-fold volume of mobile phase and centrifuged at 20,000 g for 20 min, and the supernatant was collected. Each tissue extract was injected onto an HPLC (LC-10AT; Shimadzu, Kyoto, Japan) using the size-exclusion column BioSep-SEC-S2000 (7.8×300 mm; Phenomenex, Torrance, CA, USA). The mobile phase consisted of 25 mM sodium phosphate buffer (pH 7.4). Fractions were collected at 1-min intervals for 20 min at a flow rate of 1.0 ml/min, and the radioactivity in each fraction was detected using a γ counter. TCA precipitation of fractions was also performed.
SDS-PAGE and Western blotting
Samples were fractionated by SDS-PAGE under reducing conditions, electroblotted into nitrocellulose membranes, and probed with 6D11 antibody (dilution 1:5000). The immunoreactive bands were visualized by enhanced chemiluminescence assay (Amersham, Piscataway, NJ, USA), and the signal was processed by a digital imaging analyzer (UVP EpiChemi3 imaging system; UVP, Upland, CA, USA).
PK sensitivity study
Nonradioactively iodinated PrPSc was incubated with PK (0–3000 μg/ml) at 45°C for 1 h. Following SDS-PAGE under reducing conditions and Western blotting of PK-treated samples, the density of immunoreactive bands detected by 6D11 antibody was analyzed by densitometry to determine the PK concentration required to digest 50% of PrPSc.
Guanidine denaturation study
Nonradioactively iodinated PrPSc was incubated with various concentrations (0–3.5 M) of guanidine hydrochloride for 2 h at room temperature. Thereafter, samples were centrifuged at 100,000 g for 1 h, and the pellet was resuspended in PBS containing electrophoresis sample buffer. Following SDS-PAGE under reducing conditions and Western blotting, the denaturation pattern of iodinated PrPSc by guanidine was compared to that of noniodinated PrPSc.
PMCA procedure
Aliquots of purified PrPSc, either iodinated or not, were used to trigger conversion of mouse PrPC present in 10% normal mouse brain homogenate. Tubes were positioned on an adaptor placed on the plate holder of a microsonicator (Misonix Model 4000; Miosonix, Inc., Farmdale, NY, USA) programmed to perform 96 cycles of 30 min incubation at 37°C, followed by a 20 s pulse of sonication set at an amplitude of 70. The detailed protocol for PMCA, including reagents, solutions, and troubleshooting, has been published elsewhere (21, 22).
Statistical analysis
Results are presented as means ± se and compared by 1-way analysis of variance (ANOVA) followed by Newman-Keuls multiple comparison test or by 2-tailed unpaired t test with Welch's correction. Statistical analysis was done with the Prism 5.0 program (GraphPad, San Diego, CA, USA).
RESULTS
Systemic pharmacokinetics of PrPSc
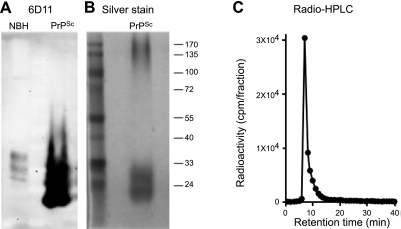
For systemic pharmacokinetic study, we have prepared ultrapurified PrPSc from RML-infected mouse brain following a widely used protocol (15) that has been further optimized in our laboratory to achieve high purity (16). Purity was analyzed by Western blot (Fig. 1A) and silver staining (Fig. 1B). The result showed a single set of bands corresponding to di-, mono-, and unglycosylated PrP at the molecular mass expected for the PrP monomer (Fig. 1A, B). We also detected a signal corresponding to high molecular mass (>170 kDa) in silver-stained gels. This signal also corresponds to PrP, since it is reactive against anti-PrP antibodies when blots are exposed longer. It is not surprising that the ratio of the signal for monomer vs. oligomers is much higher in Western blot than in silver staining, since large-molecular-mass aggregates do not blot efficiently. Purified PrPSc was radiolabeled by the iodobead procedure as described in Materials and Methods. This methodology has been designed to obtain a high yield and specific iodination of proteins (ref. 18 and unpublished results). The specificity of radiolabeling for PrPSc was further supported by size-exclusion chromatography, which showed radioactivity associated exclusively with the PrPSc aggregates without any signal in lower-molecular-mass fractions (Fig. 1C).
Figure 1.
Preparation and radioactive labeling of ultrapurified PrPSc. A) Western blotting of ultrapurified PrPSc probed by 6D11 anti-PrP antibody. B) Silver staining of ultrapurified PrPSc. C) Size-exclusion radio-HPLC chromatogram of radiolabeled [131I]PrPSc. NBH, normal brain homogenate without proteinase K treatment. Clear PrPSc bands were observed as well as some aggregation forms in both Western blotting and silver stain. Radiolabeled PrPSc was eluted as a single peak at 7 min through a size-exclusion column. No reactivity was found associated with lower-molecular-mass fractions.
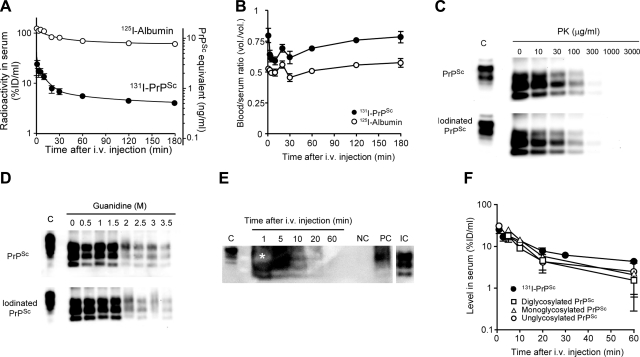
The amount of PrPSc injected (1×106 cpm/mouse) was equivalent to 11.8 ng/mouse, which is similar to the quantity typically used for infectivity assays. The serum concentration-time profiles of [131I]PrPSc and [125I]albumin were compared after i.v. injection in mice (Fig. 2A). The concentrations of [131I]PrPSc in serum decreased biphasically with time. The pharmacokinetic parameters for [131I]PrPSc and [125I]albumin in the initial phase of the infection were estimated (Table 1). Distribution half-lives of [131I]PrPSc and [125I]albumin were 5.44 ± 1.16 and 13.8 ± 5.1 min, respectively. Elimination half-lives of [131I]PrPSc and [125I]albumin were 3.24 ± 0.45 and 22.6 ± 7.9 h, respectively. The rates for [125I]albumin were consistent with those found previously in several studies (23, 24), suggesting that we have successfully estimated the pharmacokinetic profiles of [131I]PrPSc and [125I]albumin in living animals. The surprisingly short half-life of PrPSc indicates that the level of PrPSc in serum 24 h after i.v. injection is <1% of ID. However, it is possible that a very slow rerelease of PrPSc from tissues of initial uptake back into blood may contribute to the longer terminal half-life of PrPSc. The initial volume of distribution is the theoretical volume in which the agent is homogenously distributed in the body at t0 and is basically dependent on the properties of the agent, especially its permeation of the vasculature. The initial volume of distribution Vc for [131I]PrPSc (4.12±1.31 ml) suggests that PrPSc is being taken up efficiently by tissues in the body, whereas the majority of [125I]albumin remains in the serum space (0.78±0.04 ml), which in mice is ∼0.8 ml. Large steady-state volumes of distribution Vss indicate that significant amounts of the protein have been removed from the bloodstream. The Vss value of [131I]PrPSc (Table 1) indicates that PrPSc is rapidly sequestered by various tissues. Systemic clearance of [131I]PrPSc was 17.2-fold faster than that of albumin and resulted in differences in the AUC.
Figure 2.
Pharmacokinetics of PrPSc after i.v. injection in mice. A, B) Serum concentration time curves (A) and whole-blood/serum ratios (B) were estimated after injection of [131I]PrPSc and [125I]albumin in wild-type mice. Mice received [131I]PrPSc (1×106 cpm) and [125I]albumin (5×105 cpm) intravenously and were then killed at various time points (1–180 min after injection). Points represent mean ± se of 3–5 mice. C, D) Biochemical properties of iodinated PrPSc compared to noniodinated protein were studied by PK sensitivity (C) and conformational stability tested by guanidine denaturation (D). Highly purified PrPSc, either iodinated or noniodinated, was used. All samples were treated with PK, except the normal brain homogenate that was used to mark the electrophoretical migration of full-length PrPC. Pharmacokinetic studies were also carried out using partially purified, full-length, unlabeled PrPSc. E) PrPSc was detected in serum by Western blot using 6D11 antibody (1:5000). Lane NC (negative control) represents serum from a control animal that was injected with buffer. Lane PC (positive control) corresponds to normal serum spiked with partially purified PrPSc, which was subjected to the same processing as the experimental samples. Lane IC (input control) represents partially purified PrPSc sample used to inject the animals (1:250 dilution of amount injected). Lanes 1, 5, 10, 20, and 60 represent representative results of serum from animals injected with partially purified PrPSc, which were killed at the respective times. Asterisk indicates an oversaturated band. Mice received i.v. full-length PrPSc at the dose of 1 brain equivalent of the brain of RML-infected animals. All samples were treated with PK before Western blot, except the normal brain homogenate that was used to mark the electrophoretical migration of full-length PrPC. F) Western blot results were quantitated by densitometry, using blots with different exposition to accurately estimate oversaturated and very faint signals. Quantity of each glycosylation form in serum was estimated independently and plotted together with the data obtained by using radiolabeled PrPSc. Points represent means ± se of 2–3 mice.
Table 1.
Pharmacokinetic parameters for [131I]PrPSc and [125I]albumin after i.v. injection
| Parameter | [131I]PrPSc | [125I]albumin |
|---|---|---|
| C0 (%ID/ml) | 34.1 ± 9.6 | 130.0 ± 5.9 |
| Distribution t1/2 (min) | 5.44 ± 1.16 | 13.8 ± 5.1 |
| Elimination t1/2 (h) | 3.24 ± 0.45 | 22.6 ± 7.9 |
| Vc (ml) | 4.12 ± 1.31 | 0.78 ± 0.04 |
| Vss (ml) | 11.5 ± 2.5 | 1.32 ± 0.08 |
| CLtot (ml/min) | 0.048 ± 0.010 | 0.0011 ± 0.0004 |
| AUC0-∞ (×103 %ID min/ml) | 2.36 ± 0.33 | 146 ± 50 |
Pharmacokinetic parameters were estimated by fitting a biexponential function (Eq. 1) to serum concentration time curves of each agent. Initial concentration C0 was estimated by the y intercept of the fitted equation and represents the concentration of the agent at time 0. Distribution and elimination serum half-lives t1/2 are estimated by the slopes of the fast (α phase) and slow (β phase) portions of the serum concentration time curve. The fast phase typically corresponds to the distribution of the agent, and the slow phase generally involves the elimination of the agent after distribution. Initial volume of distribution Vc is used to quantify the distribution of the substance throughout the body relatively soon after administration and prior to the material reaching a steady-state equilibrium. Intuitively, the Vc for albumin, the vascular marker, registers the vascular space in tissues. Steady-state volume of distribution Vss estimates the capacity of distribution when the serum concentration of the agent is at constant levels; i.e., when equilibrium of tissue distribution and elimination is reached. Total body clearance CLtot describes how quickly drugs are eliminated, metabolized, or distributed throughout the body. The area under the serum concentration time curve AUC0-∞ estimates total bodily exposure of the agent.
The whole-blood/serum ratios of [131I]PrPSc averaged 0.68 (Fig. 2B). The ratio for [125I]albumin was close to 0.5, which is consistent with albumin being retained mostly in serum, and with an estimated hematocrit of 47.9 ± 2.4% (n=3). This result indicates that ∼76% of [131I]PrPSc in whole blood is localized in the serum component, and only ∼24% is sequestered by the cellular fraction.
To assess whether iodination changes PrPSc characteristics, we compared the biochemical properties of iodinated and noniodinated PrPSc. For this study, we used nonradioactive iodine instead of radioactive iodine. PK sensitivity of iodinated PrPSc was compared to that of noniodinated PrPSc (Fig. 2C). The band intensity of PrPSc probed by 6D11 antibody decreased with increasing amounts of PK, and both iodinated and noniodinated PrPSc showed similar digestion patterns. The PK concentrations required to degrade 50% of iodinated and noniodinated PrPSc were 19.2 ± 1.2 and 19.7 ± 1.2 μg/ml, respectively, indicating that iodination did not affect PK sensitivity of PrPSc. The PK concentrations used in this experiment are a bit lower than those conventionally used for PK resistance of PrPSc, since our samples consist of highly purified PrPSc, and thus no competition exists for PK digestion from the bulk of brain proteins. Conformational stability assay by guanidine denaturation of PrPSc showed identical patterns between iodinated and noniodinated PrPSc (Fig. 2D). In both samples, a 50% denaturation was obtained with a guanidine concentration of ∼1.7 M, in accordance with previously published studies (25). To assess further whether iodination altered the properties of PrPSc, we studied whether iodinated PrPSc could induce the autocatalytic conversion of PrPC into PrPSc in vitro. For these experiments, we used the PMCA technology that mimics prion replication (26), producing infectious material that maintains the strain and species barrier characteristics (25, 27, 28). The results showed that both iodinated and noniodinated purified PrPSc could convert PrPC at a similar rate (data not shown).
Taken together, these results suggest that PrPSc iodination did not affect the major biochemical properties of PrPSc. To further rule out that not only iodination but also purification of PrPSc (which requires PrP truncation by PK) may alter the pharmacokinetic properties of the protein, initial distribution-phase pharmacokinetics of full-length PrPSc was determined using partially purified unlabeled PrPSc. For this experiment, large amounts of unlabeled protein were injected, and the quantity of PrPSc in serum was detected by Western blot at 1, 5, 10, 20, and 60 min after the i.v. injection (Fig. 2E). Immunodetection of serum PrPSc later than 60 min was too low to make an accurate determination. The densitometry of immunoreactive PrPSc revealed that initial disappearance of PrPSc from serum was consistent with the time curve seen with radiolabeled [131I]PrPSc (Fig. 2F). Significant correlations (R2 values for linear regression: 0.901 to 0.958) were found in the distribution phase of serum concentration time curves between radioactive detection and immunodetection of PrPSc (Fig. 2F). Interestingly, determination of PrPSc by Western blot aided the assessment of the fate of the individual glycosylation isoforms of PrPSc. The results showed that the serum concentration-time profiles and the distribution half-life for each glycosylation form of PrPSc (average t1/2 5.36±0.34 min; n=3) did not differ significantly (Fig. 2F). These findings indicate that glycosylation does not affect distribution-phase pharmacokinetics of PrPSc. Our results indicated that distribution-phase pharmacokinetics of truncated iodinated PrPSc and native full-length PrPSc were similar, supporting again the conclusion that radioactive labeling did not affect the pharmacokinetics and biodistribution of PrPSc.
Tissue uptake kinetics and retention of PrPSc
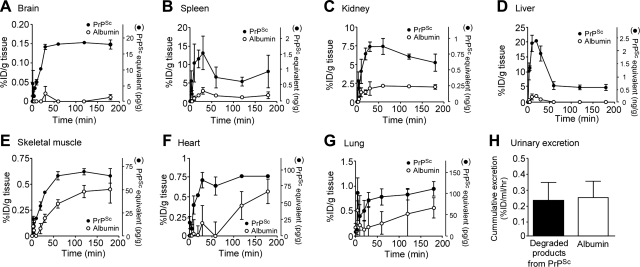
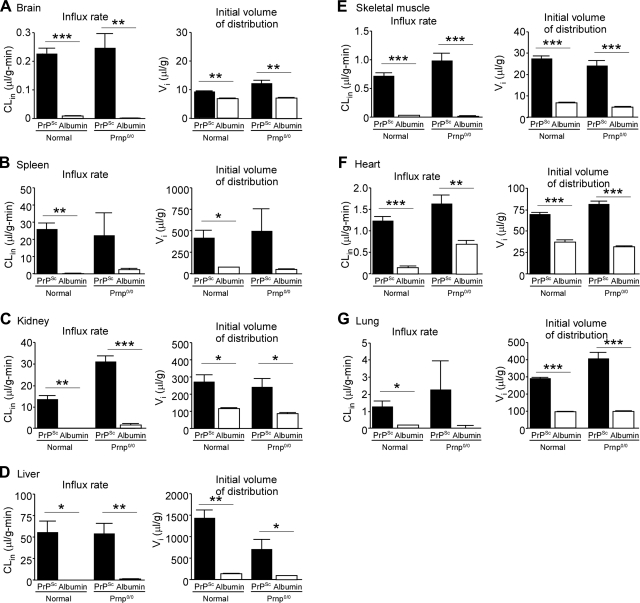
We investigated the quantitative biodistribution of [131I]PrPSc and [125I]albumin in the CNS and peripheral tissues (Fig. 3) as well as the uptake kinetics of [131I]PrPSc, involving tissue CLin rates and Vi distributions in various tissues (Fig. 4). The initial vascular space for each tissue, estimated by Vi values of [131I]PrPSc and [125I]albumin, was subtracted so that net uptake in tissues was more accurately estimated. The uptake of [131I]PrPSc in the brain increased with time and reached equilibrium 30 min after i.v. injection (Fig. 3A). Thereafter, the brain concentrations of [131I]PrPSc were sustained during the course of the experiment. At 3 h after the injection, the absolute amount found in brain tissue was 17.5 ± 1.4 pg/ml, which was 3.2% of the serum PrPSc level (529.4±20.3 pg/ml) at the same time point. The percentage of the i.v. injected dose of PrPSc taken up by brain tissue was 0.15 ± 0.01% ID/g (Fig. 3A). Multiple time-regression analysis found that the CLin (0.23±0.02 μl/g/min) of [131I]PrPSc from blood to brain was significantly higher than that of [125I]albumin (0.008±0.002 μl/g/min), which was measured simultaneously (Fig. 4A). This result strongly suggests that [131I]PrPSc is crossing the BBB. In contrast, the CLin of [125I]albumin was not significantly different from 0, indicating no measurable influx of albumin into the brain during this time period, which is consistent with previous studies (19, 29). This result also indicates that PrPSc interaction with the BBB did not alter the tightness of the barrier. The Vi of [131I]PrPSc was significantly larger than that of [125I]albumin, suggesting a rapid equilibrium between blood-borne PrPSc and brain microvasculature.
Figure 3.
PrPSc tissue uptake. Mice received [131I]PrPSc (1×106 cpm; 11.8 ng) and [125I]albumin (5×105 cpm) intravenously and were then killed 1 to 180 min after the injection. Brain (A), spleen (B), kidney (C), liver (D) skeletal muscle (E), heart (F), and lung (G) tissues were collected immediately. Cumulative excretion of degraded PrPSc in urine (H) was measured by collecting urine directly from the bladder. Points represent means ± se of 3–5 mice. Two-way ANOVA revealed that time curves of [131I]PrPSc and [125I]albumin differed significantly over the study period (A–G). There was no statistical difference in urinary excretion rate (H).
Figure 4.
PrPSc influx clearance and volume of distribution in various tissues. Tissue influx clearance CLin and initial volume of distribution Vi in tissues for [131I]PrPSc and [125I]albumin were estimated by multiple time-regression analysis of tissue/serum ratios of the agents. CLin and Vi values were estimated for the brain (A), spleen (B), kidney (C), liver (D), skeletal muscle (E), heart (F), and lung (G). CLin represents the unidirectional influx rate of the agent into tissues from the vasculature, which represents efficiency of tissue uptake. Vi indicates immediate vascular association of the agent with the tissue, suggesting the existence of binding sites including both specific and nonspecific sites in tissue vasculature. Bars represent means ± se of 3–5 determinations. *P < 0.05, **P < 0.01, ***P < 0.001; 2-tailed unpaired t test with Welch's correction.
Compelling evidence has shown that spleen and other lymphoid tissues participate in the peripheral replication of prions, which appear to play an essential role in prion neuroinvassion (4, 5). PrPSc uptake in spleen reached the highest level (13.1±4.5% ID/g; 1.54±0.53 ng/g) at 30 min after injection (Fig. 3B), and thereafter the concentrations decreased to 8.1 ± 4.4% ID/g (0.95±0.52 ng/g) at 3 h. The CLin (25.6±4.0 μl/g/min) of [131I]PrPSc in spleen was significantly higher than that of albumin (0.15±0.08 μl/g/min) (Fig. 4B), and the level of [131I]PrPSc was 111-fold higher when compared to that in the brain. Splenic levels of 0.95 ng/g at 3 h were higher than that of the serum concentration, showing blood-borne PrPSc is concentrated in the spleen. These results are consistent with the idea that spleen may be a reservoir of PrPSc in the body of infected mice.
Other tissues that are usually considered as having lower quantities of prions, including liver, kidney, muscle, heart, and lung (30) were also investigated (Fig. 3C–G). The [131I]PrPSc taken up by kidney reached a maximal level (7.4±0.6% ID/g; 0.87±0.08 ng/g) 30 min after injection and thereafter decreased to 5.3 ± 1.2% ID/g (0.62±0.14 ng/g) at 3 h (Fig. 3C). The influx rate in kidney was 13.6 ± 1.8 μl/g/min (Fig. 4C), a value significantly higher than for albumin (0.19±0.07 μl/g/min). Interestingly, degraded products from [131I]PrPSc were clearly detected in urine (Fig. 5). The cumulative amount of degraded products from [131I]PrPSc in urine (0.24±0.13% ID/ml/h) by 3 h after injection was comparable to the albumin level (0.25±0.13% ID/ml/h), suggesting that passive glomerular filtration mediates urinary excretion of PrPSc metabolites (Fig. 3H).
Figure 5.
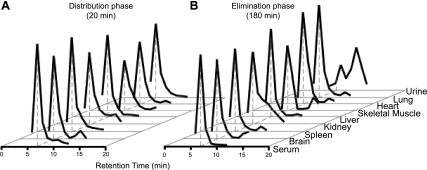
PrPSc metabolism in biological samples. Size-exclusion radio-HPLC chromatograms after correction by TCA precipitation in samples from different tissues in distribution phase (A) and elimination phase (B). Peak at 7 min represents intact [125I]PrPSc. Radioactivity in urine at the distribution phase was not detectable. Degraded PrPSc protein product was found in kidney and urine in elimination phase. Authentic radiolabeled PrPSc appears at 7 min in the size-exclusion chromatogram shown in Fig. 1.
Liver showed the highest influx rate (55.1±13.7 μl/g/min) of [131I]PrPSc from systemic circulation (Fig. 4D). [131I]PrPSc was rapidly taken up by liver, reached the highest concentration of 20.3 ± 0.3% ID/g (2.39±0.04 ng/g) 20 min after injection, and thereafter the levels biphasically decreased to 4.9 ± 1.0% ID/g (0.85±0.12 ng/g) at 3 h. Negligible uptake of [125I]albumin in the liver was found. Thus, such high concentrations of liver [131I]PrPSc immediately after injection and the rapid decrease may partially account for the transit of PrPSc through hepatic tissues and capillaries. Alternatively, hepatic metabolism may play a role in this phenomenon, or the liver could participate in the clearance of the prion protein.
PrPSc levels in other peripheral tissues, such as skeletal muscle, heart, and lung, gradually increased after injection, with values of 68.8 ± 8.4, 90.5 ± 5.8, and 111.7 ± 17.9 pg/g at 3 h, respectively, which corresponded to 13–21% of the serum concentration (Fig. 3E–G). The CLin values in these tissues were 0.71 ± 0.06, 1.2 ± 0.1, and 1.3 ± 0.4 μl/g/min, respectively (Fig. 4E–G).
Following i.v. injection of [131I]PrPSc and [125I]albumin, the CLin values of [131I]PrPSc in tissues were significantly higher than those of [125I]albumin (Fig. 4). The ranking of the CLin for [131I]PrPSc (relative to the influx rate into the brain) was liver (×240) > spleen (×111) > kidney (×59) > lung (×5.5) = heart (×5.3) > skeletal muscle (×3.1) > brain (×1.0), where values in parentheses represent the fold increase of the influx rate compared to that of brain. The Vi values of [131I]PrPSc were also higher than [125I]albumin values in all the tissues.
To investigate the role of PrPC on the uptake of circulating PrPSc from exogenous origin, we performed pharmacokinetic experiments in prnp0/0 mice. The results showed that the influx rates of [131I]PrPSc in most of the tissues (except for kidney) were not statistically different from those in wild-type mice (Fig. 4), suggesting that PrPC expression does not play a role in the uptake of PrPSc. The serum concentration-time profiles for [131I]PrPSc in wild-type or PrP-knockout animals were also indistinguishable (data not shown).
In vivo stability of [125I]PrPSc in serum and tissues
The intactness of injected [125I]PrPSc was measured by HPLC at the end of the distribution phase (20 min) and the elimination phase (180 min) in serum and tissues (Fig. 5). Under the conditions tested, the retention time for purified [125I]PrPSc was 7 min, and free iodine was eluted at 12 min. Fractions from 6 to 13 min in serum and tissues were analyzed further after TCA precipitation. Retention time of PrPSc recovered from tissues after distribution remained the same as the protein prior to injection (Fig. 1). The results show that the large majority of radioactivity was associated to intact PrPSc in all samples examined (except for urine), suggesting that the dissemination of PrPSc throughout the body via systemic blood flow did not result in substantial degradation of the protein.
The percentages of intact [125I]PrPSc in the distribution and the elimination phases were as follows: 95.3 ± 0.3 and 96.8 ± 0.8% in serum, 87.3 ± 1.2 and 79.9 ± 2.2% in brain, 94.5 ± 1.3 and 92.4 ± 1.2% in spleen, 80.1 ± 0.5 and 73.3 ± 3.3% in kidney, 96.6 ± 0.2 and 93.6 ± 0.8% in liver, 81.7 ± 0.6 and 71.0 ± 1.7% in skeletal muscle, 89.8 ± 1.0 and 87.1 ± 0.4% in heart, and 96.0 ± 0.2 and 94.7 ± 0.5% in lungs (Fig. 5). The radioactivity in urine at the latest time points of our study was in the form of low-molecular-mass fragments of PrPSc. However, since the radioactivity was TCA precipitable, we conclude that it corresponds to protein. Free iodine, lipid, and sugar chain products do not precipitate with TCA. The nature and mechanism of such degradation is currently unknown and remains to be investigated further.
DISCUSSION
Although prion diseases have been a public health concern for decades, the lack of detailed knowledge about how prions are taken up, distributed, metabolized, and cleared from the body has complicated understanding of what biological processes regulate the fate of the infectious agent. It is important to highlight that most cases of infectious prion disease are transmitted by ingestion of prion-contaminated food or exposure across a mucosal surface (i.e., nasal, oral, and gastrointestinal pathways). Prions can infect an individual after ingestion, silently replicating in various tissues for a long time before producing substantial brain damage and the onset of the clinical disease (1). In natural infections, the quantity of infectious agent to which individuals are exposed is very low, and clearance, metabolism and biodistribution of the infectious agent likely plays a major role in determining whether or not exposure will progress to full-blown disease. Systemic uptake profiling of PrPSc at the initial stage of infection enables understanding the route and mechanism of infection, preferential tissues for the distribution of PrPSc and the metabolism and elimination of the misfolded protein.
In the present study, we performed pharmacokinetic studies in mice using highly purified radioactively labeled PrPSc. Biochemical studies showed that purification and labeling with iodine did not alter the key characteristics of PrPSc, including conformational stability, PK sensitivity, and ability to convert PrPC into PrPSc in vitro. The validity of iodinated material was supported further by studies in which full-length, unlabeled PrPSc was used to estimate the pharmacokinetic parameters, and Western blots were used to measure the amount of PrPSc in serum at different times after injection. Results comparing both sources of materials and methodologies were indistinguishable. Notably, the comparison between these studies indicated that PrPSc behavior in vivo was not dependent on the PrPSc quantity injected, the procedure used for PrPSc purification, or the chemical modifications introduced during labeling with iodine. Since PrPSc purification for the radioactive labeling studies involved protease treatment, leading to the removal of the N-terminal fragment of PrP spanning residues 1 to ∼90, our results indicate that this PrP region does not affect PrPSc pharmacokinetics. This is not entirely surprising, considering that this PrP fragment is mostly unordered and is not necessary for propagation of infectivity (31, 32). However, the N-terminal PrP fragment has been associated with some of the putative biological functions of PrPC, such as the binding and transport of divalent metals (33, 34). In the pharmacokinetic studies done with radioactivity, the quantity of [131I]PrPSc injected was 11.8 ng/animal. In comparison, the amount of unlabeled PrPSc injected was ∼35 μg; i.e., almost 3000-fold higher than in the radioactivity experiments. The large quantity used in the experiments performed with unlabeled PrPSc was necessary to permit detection by Western blot. The fact that the pharmacokinetic parameters obtained by the two methodologies were virtually the same strongly suggests that the spread of PrPSc from the vasculature throughout the body is mainly a nonsaturable process. Finally, the studies that used immunodetection, although less sensitive than those that used radioactivity, permitted analysis of each individual PrPSc glycoform. PrP has 2 glycosylation sites, and typically both PrPC and PrPSc are composed of a mixture of un-, mono-, and diglycosylated isoforms (35). The proportion of each of the forms varies in different species and strains of the infectious agent (36). Our results showing that the 3 glycosylation isoforms exhibited the same pharmacokinetic features may be interpreted as an indication that glycosylation does not alter distribution-phase pharmacokinetic behavior of PrPSc. Alternatively, although glycosylation may have a profound influence on PrPSc pharmacokinetic properties, the differences cannot be observed, because PrPSc polymers may consist of heterogeneous mixtures of the 3 isoforms.
Our results indicate that PrPSc distributed rapidly in the body, with a t1/2 of 5.44 min. The elimination half-life for PrPSc from serum (3.24 h) was unexpectedly short. This result suggests that the large majority of injected PrPSc (>99%) disappears from serum 24 h after the i.v. injection. Several biological processes are typically involved in controlling the serum elimination half-life of a substance, including metabolic degradation, excretion, and tissue uptake. Our analysis of the intactness of PrPSc over time by HPLC analysis clearly shows that degradation is virtually not occurring in the majority of tissues and blood. Similarly, PrPSc excretion either through renal or hepatic clearance is very limited. Indeed, only low levels of degradation products from PrPSc were observed in kidney and urine (Figs. 3H and 5), and we could not detect any measurable amount in bile (data not shown). In contrast, our results clearly show a rapid and large tissue uptake of PrPSc, indicating that the short serum half-life is mostly determined by the efficient tissue uptake of PrPSc. In blood, the originally administered PrPSc remains mostly in serum. Our estimation, based on whole-blood/serum ratios and the hematocrit value, is that 76.4% of PrPSc was present in serum. This is also surprising, considering that both infectivity experiments and determination of PrPSc in blood by PMCA have shown that the majority of PrPSc is associated with white blood cells in the buffy coat fraction (37, 38). However, our recent studies have shown that the distribution of PrPSc in blood may depend on its origin (39, 40). Indeed, during the middle stage of the disease, PrPSc in blood is mostly cell associated and likely comes from the peripheral replication in lymphoid tissues. In this phase, lymphocytes may acquire prions while they reside in spleen and later bring the infectious agent to the blood with them. In contrast, at the symptomatic stage of the disease, PrPSc is circulating mainly in serum and likely comes from brain extravasation, either mediated by the bulk flow of CSF or by the disruption of the BBB (39). In the same way, our current results suggest that at the initial infection, prions exogenously getting into the blood would likely remain in the serum fraction until they are taken up into tissues.
Eventually, PrPSc reached the tissues through arterial blood, from which it diffused out of the capillary bed into the interstitial and cellular structures of the organs. We found that PrPSc crossed the BBB at a rate of 0.23 μl/g/min, which was similar to the rate previously estimated for circulating PrPC (17). PrPSc penetration across the BBB is significantly higher than the rate for albumin, which is considered a vascular marker that does not cross the BBB. However, the brain uptake rate of PrPSc is lower compared to other amyloidogenic proteins, such as Aβ1–40, Aβ1–42, amylin, and insulin, which are known to transverse the BBB, typically at rates ranging from 0.4 to 0.8 μl/g/min (41–43). However, the comparison is complicated by the unknown aggregation stage of the proteins used in the previous studies. When PrPSc is solely exposed to brain vasculature by in situ brain perfusion, which negates the effects of peripheral components such as degradation, binding proteins, cellular interactions, and sympathetic nerve transmission, the transport rate across the BBB (11) is ∼19-fold higher compared to the rate observed in the current study, which was done under more physiological conditions. In our previous study, we showed that aggregated PrPSc was detected in the CSF compartment after crossing the BBB (11). The complete penetration of PrPSc to the brain parenchyma was demonstrated further by a capillary depletion method that removes any contribution to the signal from PrPSc that might be trapped in cerebral capillaries (11). In the present study, we estimate that the total amount of PrPSc that entered the brain under our experimental conditions was ∼10 pg. This estimation is based on our data showing that 0.15% of the injected dose reaches the brain parenchyma per gram of tissue. This number already excludes the blood present in the brain. Since the injected dose of PrPSc was ∼12 ng and the brain weight is ∼0.5 g, we calculate that 10 pg of PrPSc reached the brain. This quantity is ≥1 order of magnitude higher than the minimum amount of material necessary to produce disease by direct intracerebral injection. The later conclusion is based on the fact that a dilution of a scrapie-infected brain equivalent to 10−8 is still sufficient to induce the disease. Since the total PrPSc concentration in brain is ∼67 ng/μl (21), we estimate that an entire brain has ∼35 μg of PrPSc. Thus, a 108 dilution, which when injected directly into the brain produces disease, contains ∼0.35 pg of PrPSc. Therefore, our data indicate that PrPSc in the vasculature can reach the brain through the BBB in amounts sufficient to induce the disease.
The most accepted pathway for prion neuroinvasion proposes that PrPSc acquired by peripheral routes is first taken up by the spleen and other lymphoid tissues, where it undergoes amplification, and is later transported to the brain via peripheral nerves (2, 44). In vivo, it is likely that both neural and vascular pathways concurrently mediate the transmission to the CNS, and these mechanisms may not be mutually exclusive. The high uptake by spleen observed in our study supports an important role for spleen as a major site of PrPSc enrichment and replication. In fact, we found that circulating PrPSc was taken up rapidly by spleen, and the uptake rate was 3.5-fold slower than splenic blood flow rate (45, 46). The PrPSc level in spleen observed at 3 h after injection was ∼1.8-fold higher than that in serum, demonstrating the high capacity of spleen for sequestering PrPSc from the circulation, which may contribute substantially to subsequent replication and disease progression. It remains to be determined the exact contribution of vascular vs. retrograde neuronal transport and their relevance for prion infection. It seems that the vascular pathway of transmission may be an acute route to reach the brain at the initial phase of infection, whereas the splenic-nerve transmission of PrPSc may provide a more systematic and constant influx into the brain.
Despite very low expression of endogenous PrPC in kidney (47), the concentrations of PrPSc showed a rapid increase to 7.5% ID/g by 60 min, and thereafter decreased with time. The uptake rate was 299-fold slower than the blood flow rate in kidney, suggesting that the uptake was independent from blood flow rate (45, 46). HPLC analysis found that a small portion of PrPSc was degraded in the kidney by 3 h. The nature of the metabolites and the metabolic mechanism are unclear. It will be of great importance to further investigate the metabolism of PrPSc in vivo, which is a totally unexplored area of research. Interestingly, the cumulative amount of degradation products from PrPSc excreted into urine was similar to albumin, suggesting passive urinary excretion of PrPSc metabolites.
We quantified the amount of intact PrPSc in liver, skeletal muscle, heart, and lung in the initial phase of infection. Although the influx rates of PrPSc were also slower than blood flow rates in these tissues, the rate was faster than brain entry of PrPSc. Initial vascular association of PrPSc in these tissues was significantly higher than the albumin levels even though these tissues also express little PrPC.
The autocatalytic replication of PrPSc implies that PrPSc binds PrPC in tissues to propagate the disease. It has been proposed that PrPC can act as a receptor for PrPSc (48). To test whether PrPC participates in PrPSc uptake, we measured CLin and Vi of PrPSc in prnp0/0 mice and compared with the values obtained in wild-type animals. There was little difference in either entry rate or initial distribution between normal and prnp0/0 mice, indicating that cell surface PrPC is not involved in PrPSc uptake.
Understanding the in vivo processes responsible for the fate of prions during the initial infection may provide novel strategies for treatment. From our results, it is possible to propose several new targets for intervention. Our observation that PrPSc is taken up by liver and kidney in large quantities, despite little or no replication occurring in these organs, suggests that increasing liver or kidney uptake of PrPSc may reduce the amount of the protein available to reach tissues of replication and disease expression (i.e., spleen and brain). Further understanding of the mechanisms responsible for the efficient uptake of PrPSc by the spleen may provide important information to reduce the flux of the protein toward this important organ for peripheral prion amplification. Although enzymatic degradation of PrPSc is, in general, low, it does seem to occur under some conditions (49–51); thus, understanding how to enhance the metabolism of PrPSc would reduce the PrPSc load in the body. Since PrPSc can be directly taken up by the brain from the blood, blockade of BBB transport may prove beneficial to decreasing prion neuroinvasion. Finally, as most cases of infectious prions are transmitted by ingestion of prion-contaminated food or exposure across a mucosal surface, it is important to assess the distribution of PrPSc by other exposure routes, including nasal, oral, and gastrointestinal pathways.
Acknowledgments
This study was supported by U.S. National Institutes of Health grant R01 NS050547 (to W.A.B. and C.S.) and P01 AI77774 (to C.S.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. A.U. designed the study, performed the bulk of the experiments, analyzed all data, wrote the manuscript, and managed the peer review process. R.M.L. helped on the characterization of iodinated PrPSc. M.L.N. contributed to HPLC analysis. W.A.B. analyzed all data and critically reviewed the manuscript. C.S. developed the hypothesis, wrote the manuscript, and supervised the entire research project and the peer review process.
REFERENCES
- 1. Aguzzi A., Calella A. M. (2009) Prions: protein aggregation and infectious diseases. Physiol. Rev. 89, 1105–1152 [DOI] [PubMed] [Google Scholar]
- 2. Aguzzi A., Heppner F. L., Heikenwalder M., Prinz M., Mertz K., Seeger H., Glatzel M. (2003) Immune system and peripheral nerves in propagation of prions to CNS. Br. Med. Bull. 66, 141–159 [DOI] [PubMed] [Google Scholar]
- 3. Brown P. (2005) Blood infectivity, processing and screening tests in transmissible spongiform encephalopathy. Vox Sang. 89, 63–70 [DOI] [PubMed] [Google Scholar]
- 4. Kimberlin R. H., Walker C. A. (1989) The role of the spleen in the neuroinvasion of scrapie in mice. Virus Res. 12, 201–211 [DOI] [PubMed] [Google Scholar]
- 5. Brandner S., Klein M. A., Frigg R., Pekarik V., Parizek P., Raeber A., Glatzel M., Schwarz P., Rülicke T., Weissmann C., Aguzzi A. (2000) Neuroinvasion of prions: insights from mouse models. Exp. Physiol. 85, 705–712 [DOI] [PubMed] [Google Scholar]
- 6. Blattler T., Brandner S., Raeber A. J., Klein M. A., Voigtlander T., Weissmann C., Aguzzi A. (1997) PrP-expressing tissue required for transfer of scrapie infectivity from spleen to brain. Nature 389, 69–73 [DOI] [PubMed] [Google Scholar]
- 7. Glatzel M., Heppner F. L., Albers K. M., Aguzzi A. (2001) Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron 31, 25–34 [DOI] [PubMed] [Google Scholar]
- 8. Baldauf E., Beekes M., Diringer H. (1997) Evidence for an alternative direct route of access for the scrapie agent to the brain bypassing the spinal cord. J. Gen. Virol. 78, 1187–1197 [DOI] [PubMed] [Google Scholar]
- 9. Bolton D. C. (1998) Prion distribution in hamster lung and brain following intraperitoneal inoculation. J. Gen. Virol. 79 (Pt. 10), 2557–2562 [DOI] [PubMed] [Google Scholar]
- 10. Soto C., Anderes L., Suardi S., Cardone F., Castilla J., Frossard M. J., Peano S., Saa P., Limido L., Carbonatto M., Ironside J., Torres J. M., Pocchiari M., Tagliavini F. (2005) Pre-symptomatic detection of prions by cyclic amplification of protein misfolding. FEBS Lett. 579, 638–642 [DOI] [PubMed] [Google Scholar]
- 11. Banks W. A., Niehoff M. L., Adessi C., Soto C. (2004) Passage of murine scrapie prion protein across the mouse vascular blood-brain barrier. Biochem. Biophys. Res. Commun. 318, 125–130 [DOI] [PubMed] [Google Scholar]
- 12. Banks W.A. (2001) Cytokines, CVOs, and the blood-brain barrier . In Psychoneuroimmunology (Ader R., Felten D. L., Cohen N. eds) pp. 483–497, Academic Press, San Diego, CA, USA [Google Scholar]
- 13. Rodríguez E. M., Blázquez J. L., Guerra M. (2010) The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides 31, 757–776 [DOI] [PubMed] [Google Scholar]
- 14. Sisó S., González L., Jeffrey M. (2010) Neuroinvasion in prion diseases: the roles of ascending neural infection and blood dissemination. Interdiscip. Perspect. Infect. Dis. 747–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diringer H., Gelderblom H., Himert H., Ozel M., Edelbluth C., Kimberlin R. H. (1983) Scrapie infectivity and low molecular weight protein. Nature 306, 476–478 [DOI] [PubMed] [Google Scholar]
- 16. Hetz C., Russelakis-Carneiro M., Maundrell K., Castilla J., Soto C. (2003) Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 22, 5435–5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Banks W. A., Robinson S. M., Diaz-Espinoza R., Urayama A., Soto C. (2009) Transport of prion protein across the blood-brain barrier. Exp. Neurol. 218, 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Markwell M. A. (1982) A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal. Biochem. 125, 427–432 [DOI] [PubMed] [Google Scholar]
- 19. Urayama. A., Grubb J. H., Sly W. S., Banks W. A. (2004) Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc. Natl. Acad. Sci. U. S. A. 101, 12658–12663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Allen L., Kimura K., MacKichan J., Ritschel W. A. (1982) Committee for Pharmacokinetic Nomenclature of the American College of Clinical Pharmacology: manual of symbols, equations and definitions in pharmacokinetics. J. Clin. Pharmacol. 22, 1S–23S [PubMed] [Google Scholar]
- 21. Saa P., Castilla J., Soto C. (2006) Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J. Biol. Chem. 281, 35245–35252 [DOI] [PubMed] [Google Scholar]
- 22. Castilla J., Saa P., Morales R., Abid K., Maundrell K., Soto C. (2006) Protein misfolding cyclic amplification for diagnosis and prion propagation studies. Methods Enzymol. 412, 3–21 [DOI] [PubMed] [Google Scholar]
- 23. Poduslo J. P., Curran G. L., Berg C. T. (1994) Macromolecular permeability across the blood-nerve and blood-brain barriers. Proc. Natl. Acad. Sci. U. S. A. 91, 5705–5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gitlin D., Klinenberg J. R., Hughes W. L. (1958) Site of catabolism of serum albumin. Nature 181, 1064–1065 [DOI] [PubMed] [Google Scholar]
- 25. Castilla J., Gonzalez-Romero D., Saa P., Morales R., De Castro J., Soto C. (2008) Crossing the species barrier by PrP(Sc) replication in vitro generates unique infectious prions. Cell 134, 757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saborio G. P., Permanne B., Soto C. (2001) Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411, 810–813 [DOI] [PubMed] [Google Scholar]
- 27. Castilla J., Saá P., Hetz C., Soto C. (2005) In vitro generation of infectious scrapie prions. Cell 121, 195–206 [DOI] [PubMed] [Google Scholar]
- 28. Castilla J., Morales R., Saa P., Barria M., Gambetti P., Soto C. (2008) Cell-free propagation of prion strains. EMBO J. 27, 2557–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Urayama A., Grubb J. H., Banks W. A., Sly W. S. (2007) Epinephrine enhances lysosomal enzyme delivery across the blood brain barrier by up-regulation of the mannose 6-phosphate receptor. Proc. Natl. Acad. Sci. U. S. A. 104, 12873–12878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Head M. W., Ritchie D., Smith N., McLoughlin V., Nailon W., Samad S., Masson S., Bishop M., McCardle L., Ironside J. W. (2004) Peripheral tissue involvement in sporadic, iatrogenic, and variant Creutzfeldt-Jakob disease: an immunohistochemical, quantitative, and biochemical study. Am. J. Pathol. 164, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riek R., Hornemann S., Wider G., Glockshuber R., Wuthrich K. (1997) NMR characterization of the full-length recombinant murine prion protein, mPrP(23–231). FEBS Lett. 413, 282–288 [DOI] [PubMed] [Google Scholar]
- 32. Bolton D. C., Meyer R. K., Prusiner S. B. (1985) Scrapie PrP 27–30 is a sialoglycoprotein. J. Virol. 53, 596–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aronoff-Spencer E., Burns C. S., Avdievich N. I., Gerfen G. J., Peisach J., Antholine W. E., Ball H. L., Cohen F. E., Prusiner S. B., Millhauser G. L. (2000) Identification of the Cu2+ binding sites in the N-terminal domain of the prion protein by EPR and CD spectroscopy. Biochemistry 39, 13760–13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hetz C., Maundrell K., Soto C. (2003) Is loss of function of the prion protein the cause of prion disorders? Trends Mol. Med. 9, 237–243 [DOI] [PubMed] [Google Scholar]
- 35. Caughey B., Race R. E., Ernst D., Buchmeier M. J., Chesebro B. (1989) Prion protein biosynthesis in scrapie-infected and uninfected neuroblastoma cells. J. Virol. 63, 175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Collinge J., Clarke A. R. (2007) A general model of prion strains and their pathogenicity. Science 318, 930–936 [DOI] [PubMed] [Google Scholar]
- 37. Brown P., Rohwer R. G., Dunstan B. C., MacAuley C., Gajdusek D. C., Drohan W.N. (1998) The distribution of infectivity in blood components and plasma derivatives in experimental models of transmissible spongiform encephalopathy. Transfusion 38, 810–816 [DOI] [PubMed] [Google Scholar]
- 38. Castilla J., Saa P., Soto C. (2005) Detection of prions in blood. Nat. Med. 11, 982–985 [DOI] [PubMed] [Google Scholar]
- 39. Saa P., Castilla J., Soto C. (2006) Presymptomatic detection of prions in blood. Science 313, 92–94 [DOI] [PubMed] [Google Scholar]
- 40. Chen B., Morales R., Barria M. A., Soto C. (2010) Estimating prion concentration in fluids and tissues by quantitative PMCA. Nat. Methods 7, 519–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Banks W. A., Kastin A. J. (1998) Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides 19, 883–889 [DOI] [PubMed] [Google Scholar]
- 42. Zlokovic B. V., Ghiso J., Mackic J. B., McComb J. G., Weiss M. H., Frangione B. (1993) Blood-brain barrier transport of circulating Alzheimer's amyloid beta. Biochem. Biophys. Res. Commun. 197, 1034–1040 [DOI] [PubMed] [Google Scholar]
- 43. Poduslo J. F., Curran G. L., Sanyal B., Selkoe D. J. (1999) Receptor-mediated transport of human amyloid beta-protein 1–40 and 1–42 at the blood-brain barrier. Neurobiol. Dis. 6, 190–199 [DOI] [PubMed] [Google Scholar]
- 44. Aguzzi A. (1997) Neuro-immune connection in spread of prions in the body? Lancet 349, 742–743 [DOI] [PubMed] [Google Scholar]
- 45. Davies B., Morris T. (1993) Physiological parameters in laboratory animals and humans. Pharm. Res. 10, 1093–1095 [DOI] [PubMed] [Google Scholar]
- 46. Dedrick R. L., Forrester D. D., Cannon J. N., el-Dareer S. M., Mellett L. B. (1973) Pharmacokinetics of 1-beta-D-arabinofuranosylcytosine (ARA-C) deamination in several species. Biochem. Pharmacol. 22, 2405–2417 [DOI] [PubMed] [Google Scholar]
- 47. Ford M. J., Burton L. J., Morris R. J., Hall S. M. (2002) Selective expression of prion protein in peripheral tissues of the adult mouse. Neuroscience 113, 177–192 [DOI] [PubMed] [Google Scholar]
- 48. Brown D. R., Herms J., Kretzschmar H. A. (1994) Mouse cortical cells lacking cellular PrP survive in culture with a neurotoxic PrP fragment. Neuroreport 5, 2057–2060 [DOI] [PubMed] [Google Scholar]
- 49. Kruger D., Thomzig A., Lenz G., Kampf K., McBride P., Beekes M. (2009) Faecal shedding, alimentary clearance and intestinal spread of prions in hamsters fed with scrapie. Vet. Res. 40, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luhr K. M., Wallin R. P., Ljunggren H. G., Low P., Taraboulos A., Kristensson K. (2002) Processing and degradation of exogenous prion protein by CD11c(+) myeloid dendritic cells in vitro. J. Virol. 76, 12259–12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scherbel C., Pichner R., Groschup M. H., Mueller-Hellwig S., Scherer S., Dietrich R., Maertlbauer E., Gareis M. (2006) Degradation of scrapie associated prion protein (PrP(Sc)) by the gastrointestinal microbiota of cattle. Vet. Res. 37, 695–703 [DOI] [PubMed] [Google Scholar]