Abstract
Fibroblastic growth factor 23 (FGF23) is a circulating phosphaturic hormone. Inactivating mutations of the endopeptidase PHEX or the SIBLING protein DMP1 result in equivalent intrinsic bone mineralization defects and increased Fgf23 expression in osteocytes. The mechanisms whereby PHEX and DMP1 regulate Fgf23 expression are unknown. We examined the possibility that PHEX and DMP1 regulate Fgf23 through a common pathway by analyzing the phenotype of compound Phex and Dmp1 mutant mice (Hyp/Dmp1−/−). Compared to single-mutant littermates, compound-mutant Hyp/Dmp1−/− mice displayed nonadditive elevations of serum FGF23 (1912 ± 183, 1715 ± 178, and 1799 ± 181 pg/ml), hypophosphatemia (Pi: 6.0 ± 0.3, 5.8 ± 0.2, and 5.4 ± 0.1 mg/dl), and severity of rickets/osteomalacia (bone mineral density: −36, −36, and −30%). Microarray analysis of long bones identified gene expression profiles implicating common activation of the FGFR pathway in all the mutant groups. Furthermore, inhibiting FGFR signaling using SU5402 in Hyp- and Dmp1−/−-derived bone marrow stromal cells prevented the increase in Fgf23 mRNA expression (129- and 124-fold increase in Hyp and Dmp1−/− vs. 1.3-fold in Hyp+SU5402 and 2.5-fold in Dmp1−/−+SU5402, P<0.05). For all analyses, samples collected from nonmutant wild-type littermates served as controls. These findings indicate that PHEX and DMP1 control a common pathway regulating bone mineralization and FGF23 production, the latter involving activation of the FGFR signaling in osteocytes.—Martin, A., Liu, S., David, V., Li, H., Karydis, A., Feng, J. Q., Quarles, L. D. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway.
Keywords: XLH, ARHR, hypophosphatemia, osteomalacia, microarray
Fibroblastic growth factor 23 (FGF23) is a member of the FGF19 subfamily of FGFs, which have undergone structural modifications during evolution that permit them to function as circulating hormones in a heparin-independent manner (1–3). The N-terminal region of FGF23 contains the FGF homology domain, and the C-terminal allows interaction with the cofactor Klotho, resulting in the selective targeting of organs coexpressing FGFR/Klotho complexes (4–5). Comparative analyses of hereditary hypophosphatemic disorders, including autosomal dominant (6), autosomal recessive (7), X-linked hypophosphatemic rickets (XLH; refs. 8–9), and murine homologues R176Q FGF23 mutant, Dmp1−/−, and Phex-deficient Hyp mice, have identified FGF23 as a novel bone-derived hormone that participates in a bone-kidney axis to regulate renal phosphate and vitamin D metabolism (10). In these contexts, excessive circulating levels of FGF23 are responsible for the overlapping clinical characteristics, including renal phosphate wasting, inappropriately low to normal 1,25(OH)2D levels for the degree of phosphatemia, and impaired mineralization of the extracellular matrix, leading to rickets and osteomalacia (11). Renal-mediated hypophosphatemia is caused by the effects of circulating FGF23 to inhibit NPT2a- and NPT2c-dependent phosphate transport in the proximal tubule (4, 5, 10), and reduction in 1,25(OH)2D results from the inhibition of Cyp27b1 and stimulation of Cyp24 by FGF23 (12–16).
Our understanding of the physiological functions of FGF23 is incomplete. There is compelling evidence that an important function of FGF23 is to act as a counterregulatory hormone for 1,25(OH)2D in a bone-kidney feedback loop. 1,25(OH)2D directly stimulates Fgf23 expression in osteocytes via a vitamin D response element in the Fgf23 promoter (10, 17), and FGF23 inhibits 1,25(OH)2D production and increases its catabolism in the kidney (10). There are inconsistent effects of PTH (10, 18–21) and extracellular phosphate (22–24) to regulate Fgf23 expression. These variable effects may be explained by the actions of PTH and phosphate to indirectly affect Fgf23 expression through alterations in bone turnover and mineralization (25–27).
Inactivating mutations of Phex or Dmp1, two gene products expressed by osteoblasts and osteocytes regulating both the mineralization of extracellular matrix and FGF23 production by bone (28–31), provide additional evidence for linkage between bone mineralization and FGF23 expression. The increased expression of Fgf23 in Hyp- and Dmp1−/−-derived bone marrow stromal cells (BMSCs) in vitro indicates that intrinsic alterations resulting from Phex and Dmp1 inactivation lead to increased FGF23 production in osteocytes (28, 31). However, the proximate mechanisms whereby inactivation of Phex and Dmp1 lead to increased FGF23 production in osteocytes are not known.
Based on the similar phenotypical characteristics observed between Hyp and Dmp1−/− mice (7, 32), we hypothesized that PHEX and DMP1 could regulate extracellular matrix mineralization and Fgf23 expression through a common mechanism intrinsic to the bone microenvironment. To explore this possibility, we transferred Dmp1 deficiency onto the Hyp background and performed comparative analyses of single- and compound-mutant mouse phenotypes. We observed nonadditive effects of the combined Phex and Dmp1 mutations on the expression of FGF23 and defective bone mineralization. In addition, we found that PHEX and DMP1 mutations led to increased FGF23 production through the activation of the canonical FGF/FGFR pathway in bone. These findings provide new insights into how mineralization of extracellular matrix is coupled to the regulation of FGF23, systemic phosphate, and vitamin D homeostasis through local extracellular matrix-derived paracrine factors.
MATERIALS AND METHODS
Animals and genotyping
The Fgf23-eGFP reporter and Dmp1-null mice were created as described previously (28, 33). Hyp mice were originally purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All mice were maintained in our vivarium on a standard diet (7912; Harlan Teklad, Madison, WI, USA). Animal care and protocols were in accordance with the guidelines established by the University of Tennessee Institutional Animal Care and Use Committee as detailed in the Institute on Laboratory Animal Resources Guide for Care and Use of Laboratory Animals (34).
We crossed Dmp1-null males (Dmp1−/−/XY) to female Hyp mice to obtain female Hyp mice heterozygous for Dmp1 (Dmp1+/−/XXHyp). We then bred the Dmp1+/− males to Dmp1+/−/XXHyp females to generate Phex and Dmp1 double-deficient mice and collect samples from wild-type (WT), XHypY (Hyp), Dmp1−/−, and Dmp1−/−/XHypY (Hyp/Dmp1−/−) male littermates. To generate the Phex and Dmp1 double-mutant mouse reporter for Fgf23, we first crossed Dmp1-null to Fgf23 heterozygous mice to obtain double-heterozygous males (Dmp1+/−/Fgf23+/−[eGFP]). We then crossed the double-heterozygous males to Dmp1+/−/XXHyp females to obtain Fgf23+/−[eGFP] (control), XHypY/Fgf23+/−[eGFP] (Hyp), Dmp1−/−/Fgf23+/−[eGFP] (Dmp1−/−), and Dmp1−/−/XHypY/Fgf23+/−[eGFP] (Hyp/Dmp1−/−) male littermates.
Tail or ear biopsies were collected to genotype the mice. REDExtract-N-Amp Tissue PCR Kit (Sigma-Aldrich, St. Louis, MO, USA) was used for DNA extraction and PCR amplification. Mice were genotyped for Phex, Dmp1, and Fgf23 mutations using previously described primers (28, 33).
Dual-energy X-ray absorptiometry (DEXA)
At age 5 wk, mice were anesthetized using a ketamine(120 mg/kg)/xylasine (80 mg/kg) solution to perform a densitometry acquisition using the PIXImus (Lunar Corp., Madison, WI, USA). After image acquisition, the femur bone mineral density (BMD) was measured by adjusting the region of interest (ROI) according to the entire bone size.
Serum biochemistry
Serum samples were collected on 5-wk-old animals by intracardiac exsanguination. Serum calcium was measured using a Calcium CPC Liquicolor Kit (Stanbio Laboratories, Boerne, TX, USA), and serum phosphorus was measured using the phosphomolybdylate-ascorbic acid method, as described previously (28). Serum parathyroid hormone (PTH) levels were measured using the Mouse Intact PTH ELISA kit (Immutopics, Carlsbad, CA, USA). Serum 1,25(OH)2D levels were measured using the 1,25-dihydroxy-vitamin D EIA Kit (Immunodiagnostic Systems, Fountain Hills, AZ, USA). Serum FGF23 levels were measured using the FGF23 ELISA kit (Kainos Laboratories, Tokyo, Japan).
High-resolution 3D microtomography
The femurs from 5-wk-old mice were collected and fixed in 70% ethanol. High-resolution micro-computed tomography (μCT40; Scanco Medical, Basserdorf, Switzerland) was used to scan and evaluate the metaphyseal trabecular bone microarchitecture and the midshaft cortical bone parameters. The entire femurs were scanned in a 12.3-mm-diameter sample holder at 6-μm resolution, energy level of 55 keV, and intensity of 145 μA. The trabecular bone volume (VOX BV/TV) was measured within the secondary spongiosa on a set of 50 sections (0.6 mm) underneath the growth plate at a threshold of 200. Trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), and structure model index (SMI) were calculated without assuming a constant model, as described previously (35). The cortical bone structure was analyzed from 100 sections chosen at the midshaft of each femur at a threshold of 350.
Bone histology and histomorphometry
Evaluation of the bone growth was obtained by measuring the length of the femur of 5-wk-old mice with a slide caliper. Femurs were fixed and dehydrated in 70% ethanol, and embedded in methylmetacrylate at low temperature. Nonserial longitudinal frontal slices (5 μm) were cut from the embedded bones with a microtome (Polycut-S; Reichert-Jung, Wetzlar, Germany) and used for modified Goldner staining. Osteoid thickness (O.Th) and volume (OV/BV) were measured on Goldner sections (36).
RT-PCR and microarray
RT-PCR analyses were performed on kidneys and calvariae collected from 5-wk-old mice and on BMSCs cultured for 3 wk in osteoblast differentiating medium. Microarray analysis was performed on tibia and femur diaphyses (4/mouse) collected from 12-d-old mice, inserted into a pipette tip, and spun in a centrifuge tube at 8000 g for 2 min to remove the bone marrow. Total RNAs were isolated from the kidneys, BMSCs, and cortical bone using TRI-reagent (Molecular Research Center, Cincinnati, OH, USA) according to previously published method (31). First-strand cDNA was synthesized from the kidney RNAs using iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA, USA). The 20-μl reverse transcriptase reaction was based on 1 μg total RNA. The iCycler iQ Real-Time PCR Detection System and iQ SYBR Green Supermix (Bio-Rad) were used for real-time quantitative PCR analysis. The expression was normalized by glyceraldehyde-3-phosphate dehydrogenase (Gapdh) in the same sample and expressed as 100% of the control (WT). Sequences of primers used for real-time quantitative RT-PCR are listed in Supplemental Table S1. The expression of 45,000 genes was tested on the bone samples using the Affymetrix Mouse Genome 430 2.0 Array (Affymetrix Inc., Santa Clara, CA, USA) at the DNA Discovery Core of University of Tennessee Health Science Center.
Cell culture
For BMSC cultures, the epiphyses of the bones (i.e., tibiae and femurs) were removed, and whole marrow was flushed from the diaphyses by centrifugation at 11,500 rpm for 30 s in standard αMEM culture medium (Mediatech Inc., Manassas, VA, USA) supplemented with 10% fetal bovine serum (Mediatech), 10 U/ml penicillin, and 100 μg/ml streptomycin. The supernatant was removed, and the cell pellet was resuspended in fresh standard medium. The cells were plated in standard 25-cm2 flasks and allowed to grow until confluence. They were passaged with 0.25% trypsin solution containing 0.02% EDTA (Sigma-Aldrich, St. Louis, MO, USA). Cells were plated on 12-well plates for eGFP assessment at a concentration of 10 × 104 cells/well. Starting 48 h after seeding, nonadherent cells were discarded, and the medium was changed for an osteoblast differentiating medium (αMEM, 10% FBS, 10 U/ml penicillin, 100 μg/ml streptomycin, 10 mM β-glycerophosphate, and 50 μg/ml ascorbic acid; Sigma-Aldrich). Medium was changed every other day for the 3-wk duration of the culture. Fgf23 promoter activity was evaluated by eGFP detection in the culture, as shown in the past (28, 31). For FGFR1-inhibition experiments, a specific inhibitor of FGFR1 (SU5402; Calbiochem/EMD Biosciences, Gibbstown, NJ, USA) was used at 25 μM concentration, Fgf1 (recombinant human FGF-acidic; PeproTech, Rocky Hill, NJ, USA) was used at 10 or 100 ng/ml concentration, and heparin (heparin sodium salt; Sigma-Aldrich) was used at 10 μg/ml concentration.
Western blot
Total proteins from femur and tibia diaphysis were extracted in 1 ml lysis buffer of T-PER tissue protein extraction reagent (Pierce, Rockford, IL, USA). The protein extraction reagent was supplemented with protease inhibitors (Roche Applied Science, Indianapolis, IN, USA) and phosphatase inhibitors (2 mM EDTA; 10 mM NaF; 1 mM Na3VO4, pH 7.2; and 10 mM sodium glycerophosphate). After centrifugation (10 min, 5000 rpm, 4°C), supernatants were stored at −80°C. Protein concentration was measured using the bicinchoninic acid protein assay kit (Pierce).
Protein lysates (25 μg/sample) were reduced and extracted in LDS sample buffer (Invitrogen) heated for 10 min at 70°C, migrated on NuPAGE Novex 10% Bis-Tris Gels (Invitrogen), and then analyzed by Western blotting using the ECL Advance WB Detection Kit (GE Healthcare, Little Chalfont, UK). After SDS-PAGE, proteins were transferred to nitrocellulose membrane (Bio-Rad). After transfer, membranes were blocked overnight at 4°C using 5% nonfat dried milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T; pH 7.4), and incubated for 1 h at 22°C on a rocking platform with the rabbit anti-FGFR1 Flg (H-76, sc-121; 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-pY FGFR1 (44–1140G; 1:500; Invitrogen), rabbit anti-FRS2 (F9052; 1:500; Sigma), rabbit anti-p-FRS2-α Tyr196 (3864; 1:500; Cell Signaling, Danvers, MA, USA), rabbit anti-PI3 kinase p85 (4292; 1:500; Cell Signaling), rabbit anti-phospho-PI3K p85/p55 (4228, 1:500; Cell Signaling), and goat polyclonal anti-GAPDH (sc-31914; 1:1000; Santa Cruz Biotechnology) antibodies. Membranes were washed 3 times in TBS-T and then incubated with secondary antibodies (peroxidase-conjugated goat anti-rabbit or peroxidase-conjugated rabbit anti-goat; 1:5000) for 1 h at room temperature. After washing 3 times with TBS-T, the immunoreactive bands were visualized using enhanced chemiluminescence detection reagents (GE Healthcare) on a Fluor-S Multi Imager (Bio-Rad). Immunoblots were stripped by using mild antibody stripping solution (Pierce) and reprobed with another antibody. Band intensities were determined by densitometry using ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA).
Statistics
Differences among the 4 groups were tested by 1-way ANOVA followed by a Fisher post hoc test using Statistica software (Statsoft, Tulsa, OK, USA). Differences were considered statistically significant at values of P < 0.05.
Microarray data were analyzed using GeneSpring GX7.3 software (Agilent Technologies, Santa Clara, CA, USA). The Robust Multichip Averaging probe summarization algorithm was used to perform background correction, normalization, and probe summarization. Data were normalized per chip and per gene to the median. Genes were filtered to include only those that were expressed in ≥1 of the 12 samples. The statistical analysis was performed using a 1-way ANOVA followed by Benjamini and Hochberg multiple test correction, assuming variances were equals to minimize the false-positive discovery. Value of P was set at 0.05. Cluster analysis using a gene tree classification, Pearson correlation, and average linkage was then performed to identify groups of genes for which the patterns of expression were similar. Pathway analysis was performed using the Ingenuity program (Ingenuity Systems, Redwood City, CA, USA) to match the identified genes of interest to already known broader networks of genes contained in the literature database.
RESULTS
Gross phenotype in combined Phex and Dmp1 mutations is indistinguishable from single-mutant mice
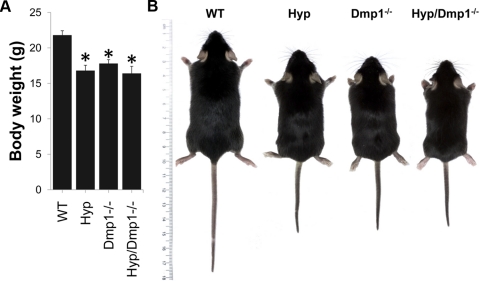
We investigated the role of DMP1 in the pathogenesis of XLH by transferring the Dmp1 deficiency onto the Hyp mouse background. Compound-mutant mice were born at the expected Mendelian frequency. Hyp, Dmp1−/−, and combined Dmp1−/−/Hyp mice had survival rates identical to WT mice. At 5 wk of age, both Hyp and Dmp1−/− mice displayed growth retardation, as previously reported (7, 32). Ablation of Dmp1 in Hyp mice did not further worsen the growth retardation, as evidenced by the similar body weight and size between Hyp, Dmp1−/−, and Hyp/Dmp1−/− mice (Fig. 1).
Figure 1.
Body weight (A) and gross appearance (B) of 5-wk-old WT, Phex-deficient (Hyp), Dmp1-deficient (Dmp1−/−), and compound mutant (Hyp/Dmp1−/−) mice. Values are expressed as means ± se; n = 8 mice/group. *P < 0.05 vs. WT.
Nonadditive effects of combined Dmp1 and Phex deficiencies on FGF23 production and renal effects
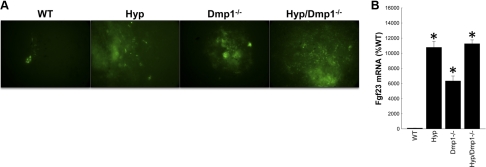
We confirmed prior results that increased FGF23 production in single-mutant Hyp and Dmp1−/− mice is due to an abnormal intrinsic activation of the Fgf23 promoter (28, 31) and further showed nonadditive effects of the combined Phex and Dmp1 mutations on Fgf23 expression. In this regard, we found intrinsic overactivation of the Fgf23 promoter and increased bone Fgf23 mRNA expression in Hyp/Dmp1−/− BMSCs and calvariae, respectively (Fig. 2). In addition, we found that both Hyp and Dmp1−/− mice displayed significantly increased circulating FGF23 levels, PTH levels, hypophosphatemia, and inappropriately normal 1,25(OH)2D levels for the degree of hypophosphatemia compared to WT mice (7, 32). The magnitude of these changes was identical in Hyp and Dmp1−/− mice. More important, the loss of Dmp1 in Hyp/Dmp1−/− mice did not further increase serum FGF23 levels or have additive effects on either the degree of hypophosphatemia or aberrant 1,25(OH)2D levels (Table 1). Consistent with FGF23-mediated reductions in phosphate transport, we measured a 30% decrease in Npt2a expression and a significant 40% decrease in Npt2c expression in the kidney of Hyp, Dmp1−/−, and compound Hyp/Dmp1−/− mice. Klotho, a distal tubular FGF23-regulated gene, was also decreased to a similar degree in the kidney of all 3 mutant groups (Table 2).
Figure 2.
A) Expression of eGFP driven by Fgf23 promoter in BMSCs collected from WT, Hyp, Dmp1−/−, and Hyp/Dmp1−/− mice lacking 1 allele of Fgf23 (Fgf23+/−[eGFP]) and cultured in osteoblast differentiating medium for 3 wk. Expression is reported after 3 wk of culture, showing intrinsic increase of Fgf23 expression by nodule-embedded cells in all 3 mutant groups compared to WT. B) mRNA expression of Fgf23 in calvariae. Values are expressed as mean ± se percentage relative to WT; n = 5 mice/group. *P < 0.05 vs. WT control; 1-way ANOVA and post hoc Fisher test.
Table 1.
Serum biochemistry of WT, Hyp, Dmp1−/−, and Hyp/Dmp1−/− mice
| Parameter | WT | Hyp | Dmp1−/− | Hyp/Dmp1−/− |
|---|---|---|---|---|
| Phosphorus (mg/dl) | 9.5 ± 0.2 | 6.0 ± 0.3* | 5.8 ± 0.2* | 5.4 ± 0.1* |
| Calcium (mg/dl) | 8.5 ± 0.1 | 8.5 ± 0.2 | 8.1 ± 0.1 | 8.2 ± 0.2 |
| PTH (pg/ml) | 21 ± 7 | 111 ± 41* | 96 ± 26* | 85 ± 28* |
| 1,25(OH)2D (pM) | 237 ± 23 | 215 ± 25 | 226 ± 31 | 186 ± 20 |
| FGF23 (pg/ml) | 118 ± 14 | 1912 ± 183* | 1715 ± 178* | 1799 ± 181* |
Values are expressed as means ± se from ≥8 mice/group.
P < 0.05 vs. WT; 1-way ANOVA and post hoc Fisher test.
Table 2.
Kidney-related gene expression measured by RT-PCR
| Gene | Hyp | Dmp1−/− | Hyp/Dmp1−/− |
|---|---|---|---|
| FGFR1c | 0.93 ± 0.04 | 1.05 ± 0.09 | 0.95 ± 0.08 |
| Npt2a | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| Npt2c | 0.6 ± 0.1* | 0.6 ± 0.1* | 0.5 ± 0.1* |
| Klotho | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 |
Values are expressed as means ± se relative to WT (WT=1); n = 3 mice/group.
P < 0.05 vs. WT; 1-way ANOVA and post hoc Fisher test.
Shared bone phenotypic characteristics of compound-mutant Hyp/Dmp1−/− and single-mutant Hyp or Dmp1−/− mice
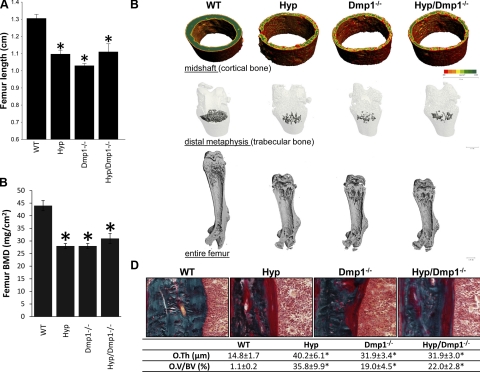
Both mutant models displayed impaired growth, as assessed by the shorter femoral length. The BMD was also significantly impaired in both single-mutant mice, as evidenced by a 30% reduction compared to the WT mice. Three-dimensional microtomographic analysis confirmed the defect of bone mineralization in the trabecular and cortical compartments, showing a smaller mineralized bone volume in both Hyp and Dmp1−/− groups compared to the WT group (P<0.05). Femurs in these two groups display all the classical features of rickets, with smaller size, wider growth plates, and larger diaphysis than a normal bone (Fig. 3 and Table 3). Histologically, the osteoid thickness and volume were also increased in Hyp/Dmp1−/− femurs compared to WT, consistent with impaired mineralization (Fig. 3). Notably, Dmp1 ablation in Hyp mice did not have additive effects on the bone phenotype. The femoral length and BMD were similar between single- and double-mutant mice, as well as the tridimensional architecture (Fig. 3 and Table 3). The osteoid thickness and volume were also similar between the Hyp, Dmp1−/−, and Hyp/Dmp1−/− groups (Fig. 3).
Figure 3.
Bone phenotype of 5-wk-old WT, Hyp, Dmp1−/−, and Hyp/Dmp1−/− mutant mice. A) Entire femur length. B) BMD (DEXA). C) Three-dimensional microCT representation of cortical bone (top), trabecular bone (middle) and entire femur (bottom). Degree of mineralization is represented on the cortical bone using a color scale from less mineralized (red) to more mineralized (green). D) Top panels: modified Goldner staining on femur histological section showing the cortical bone area. Bottom panel: histomorphometric quantification of osteoid thickness (O.Th.) and volume (OV/BV) measured in cortical bone. Values are expressed as means ± se; n ≥ 5 mice/group. *P < 0.05 vs. WT.
Table 3.
Three-dimensional microCT analysis of femoral distal metaphysis trabecular structure and midshaft cortical envelope
| Parameter | WT | Hyp | Dmp1−/− | Hyp/Dmp1−/− |
|---|---|---|---|---|
| Trabecular bone | ||||
| Tb.BMD (mgHA/cm3) | 223.0 ± 13.6 | 50.3 ± 2.7* | 37.1 ± 3.3* | 55.3 ± 7.4* |
| BV/TV (%) | 29.9 ± 2.4 | 5.4 ± 0.3* | 4.2 ± 0.4* | 6.3 ± 0.8* |
| Tb.N. (×1−1) | 7.1 ± 0.3 | 3.6 ± 0.1* | 3.6 ± 0.1* | 3.8 ± 0.1* |
| Tb.Th (μm) | 59.0 ± 2.3 | 50.0 ± 1.7 | 57.8 ± 2.7 | 50.6 ± 1.7 |
| Tb.Sp (μm) | 137.0 ± 8.5 | 277.7 ± 4.2* | 291.6 ± 3.7* | 270.3 ± 7.6* |
| Conn.Dens. | 286.4 ± 30.0 | 17.1 ± 3.5* | 11.9 ± 3.3* | 30.3 ± 6.8* |
| SMI | 1.22 ± 0.13 | 2.96 ± 0.08* | 3.24 ± 0.09* | 2.79 ± 0.11* |
| DA | 1.77 ± 0.05 | 2.19 ± 0.13 | 1.66 ± 0.04# | 1.85 ± 0.1 |
| Cortical bone | ||||
| Ct.BMD (mgHA/cm3) | 1054.3 ± 14.4 | 808.8 ± 17.2* | 773.5 ± 10.3* | 794.9 ± 21.2* |
| Ct.Th. (μm) | 136.5 ± 7.3 | 77.3 ± 3.4* | 78.0 ± 2.3* | 80.2 ± 5.7* |
| CSA (mm2) | 2.64 ± 0.16 | 3.22 ± 0.16* | 3.17 ± 0.11* | 3.40 ± 0.14* |
| Ct.Ar. (mm2) | 0.63 ± 0.03 | 0.37 ± 0.02* | 0.43 ± 0.02* | 0.39 ± 0.02* |
| Ma.Ar. (mm2) | 1.93 ± 0.10 | 2.84 ± 0.16* | 2.75 ± 0.10* | 2.94 ± 0.10* |
Values are expressed as means ± se; 5 mice/group. Tb.BMD, trabecular bone mineral density; BV/TV, bone volume/tissue volume; Tb.N, trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; Conn.Dens, connectivity density; SMI, structure model index; DA, degree of anisotropy; Ct.BMD, cortical bone mineral density; Ct.Th, cortical thickness; CSA, cross-sectional area; Ct.Ar, cortical area; Ma.Ar, marrow area.
P < 0.05 vs. WT;
P < 0.05 vs. Hyp; 1-way ANOVA and post hoc Fisher test.
Identification of the signalization pathways correlated with the inactivation of PHEX and DMP1
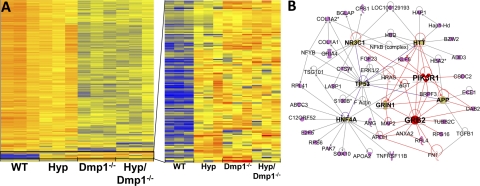
To determine potential common pathways regulated by DMP1 and PHEX in bone, we performed genome-wide microarray analysis of cortical bone isolated from WT, Hyp, Dmp1−/−, and Hyp/Dmp1−/− mice. Clustering analysis of the microarray revealed different clusters corresponding to interactive, additive, and nonadditive effects of both Phex and Dmp1 mutations (Fig. 4 and Supplemental Fig. S1). Given the similar phenotypic characteristics observed in Hyp, Dmp1−/−, and compound Hyp/Dmp1−/− mice, we focused our interest on the cluster showing nonadditive effects of both Phex and Dmp1 mutations, thus representing a possible common regulation pathway. The nonadditive cluster was composed of 150 genes significantly and equally up-regulated in the 3 mutant groups. The top 32 genes are shown in Table 4, and confirmation of a subset of these genes by RT-PCR is provided in Table 5. The most up-regulated gene (10-fold) identified in this cluster was a novel transcript with homology to the Jak3 tyrosine kinase. Fgf23 was the second most increased gene product; its expression was increased by 4.4-fold in Hyp, 7.1-fold in Dmp1−/−, and 7.4-fold in Hyp/Dmp1−/− bones compared to WT. Another transcript previously identified in Hyp bone was Fgf1 (37), expression of which was increased by ∼2-fold compared to WT (Table 4). Genes down-regulated in an additive fashion by Phex and Dmp1 mutations were also identified and reported in Supplemental Table S2.
Figure 4.
A) Left panel: cluster analysis of microarray performed on cortical bone from 12-d-old WT, Hyp, Dmp1−/−, and Hyp/Dmp1−/− mice. Gene expression is represented on the heat map from the less expressed (blue) to the more expressed (red) on 3 samples per group. Right panel: cluster showing nonadditive effects of both Phex and Dmp1 mutations on downstream gene expression, indicating up-regulation through a common pathway. B) Ingenuity pathway analysis of listed genes belonging to the identified cluster. Network is built according the identified interconnected pathways involving the highest majority of genes of the selected cluster. This network represents genes involved in direct interactions only. Genes in pink belong to the cluster. Genes in bold font are central regulators of the identified pathways that do not belong to the cluster [glucocorticoid receptor, also known as nuclear receptor subfamily 3 group C member 1 (NR3C1); Huntingtin (HTT); tumor protein p53 (TP53); phosphatidylinositol 3-kinase regulatory α subunit (PIK3R1); glutamate (NMDA) receptor subunit ζ-1 (GRIN1); amyloid precursor protein (APP); hepatocyte nuclear factor 4 α (HNF4A); growth factor receptor-bound protein 2 (GRB2)]. Genes represented in white are other intermediary regulators that do not belong to the cluster. Genes and pathways directly connected to GRB2 and PIK3R1 are highlighted in red as candidates possibly involved in the regulation of the FGFR activation. Nomenclature of the shapes used for the different genes is provided online (https://analysis.ingenuity.com/pa/info/help/help.htm#legend.htm).
Table 4.
Expression fold change of the top 32 genes up-regulated in cortical bone as a result of Phex, Dmp1, or both mutations
| Gene | Name | Hyp | Dmp1−/− | Hyp/Dmp1−/− |
|---|---|---|---|---|
| BG083989 | Jak3-like tyrosine-protein | 11.7 | 10.1 | 8.9 |
| Fgf23 | Fibroblast growth factor 23 | 4.4 | 7.1 | 7.2 |
| Fcrls | Fc receptor-like S, scavenger receptor | 6.8 | 4.9 | 4.8 |
| F13a1 | Coagulation factor XIII, A1 subunit | 2.8 | 2.4 | 3.8 |
| Lipg | Lipase, endothelial | 4.0 | 4.1 | 3.6 |
| Ctsw | Cathepsin W | 3.4 | 2.8 | 3.6 |
| Cbr2 | Carbonyl reductase 2 | 4.6 | 3.6 | 3.4 |
| Adamtsl2 | ADAMTS-like 2 | 3.7 | 3.2 | 3.3 |
| DBA2 | Gag related peptide | 4.0 | 3.1 | 3.3 |
| Bzw2 | Basic leucine zipper and W2 domains 2 | 3.2 | 2.8 | 2.9 |
| C530028O21Rik | RIKEN cDNA C530028O21 | 3.4 | 1.7 | 2.8 |
| Csdc2 | Cold shock domain containing C2, RNA binding | 1.8 | 2.3 | 2.6 |
| Epcam | Epithelial cell adhesion molecule | 4.1 | 1.8 | 2.4 |
| Elavl2 | Embryonic lethal, abnormal vision, Drosophila-like 2 | 2.9 | 2.0 | 2.4 |
| Coro2b | Coronin, actin binding protein, 2B | 1.8 | 2.2 | 2.4 |
| Cx3cr1 | Chemokine (C-X3-C) receptor 1 | 2.3 | 2.4 | 2.3 |
| Ihh | Indian hedgehog | 1.6 | 1.4 | 2.3 |
| Stab1 | Stabilin 1 | 2.5 | 2.0 | 2.2 |
| Mup1/Mup2 | Major urinary protein 1/2 | 1.7 | 2.4 | 2.2 |
| Igh | Immunoglobulin heavy chain complex | 1.6 | 2.0 | 2.2 |
| S100b | S100 protein, β polypeptide, neural | 2.3 | 2.2 | 2.1 |
| E2f5 | E2F transcription factor 5 | 1.8 | 2.3 | 2.1 |
| Npy | Neuropeptide Y | 1.7 | 1.9 | 2.1 |
| Gpr84 | G-protein-coupled receptor 84 | 1.7 | 1.8 | 2.1 |
| Fgf1 | Fibroblast growth factor 1 | 2.3 | 1.9 | 2.0 |
| Slc6a9 | Solute carrier family 6 (neurotransmitter transporter, glycine), member 9 | 1.5 | 2.2 | 2.0 |
| C1qb | Complement component 1, q subcomponent, β polypeptide | 2.7 | 1.7 | 1.9 |
| Nrxn3 | Neurexin III | 1.4 | 2.1 | 1.9 |
| Add3 | Adducin 3 (γ) | 1.4 | 2.2 | 1.8 |
| Sox10 | SRY-box containing gene 10 | 1.5 | 2.0 | 1.8 |
| EG633640 | Predicted gene, EG633640 | 2.5 | 1.8 | 1.7 |
| 1700049E17Rik1 | RIKEN cDNA 1700049E17 gene 1 | 2.4 | 1.6 | 1.7 |
Values were obtained after clustering analysis on microarray performed in cortical bone of Phex and Dmp1 single-mutant and Phex/Dmp1 double-mutant mice (cluster is represented in Fig. 4A). n = 3 samples/group. Values are expressed as fold change compared to the WT control value. Genes were selected based on a P value threshold of 0.05 and a minimum fold-change absolute value of 2 in ≥1 of the 3 groups.
Table 5.
Expression fold change of selected genes confirmed by RT-PCR
| Gene | Name | Hyp | Dmp1−/− | Hyp/Dmp1−/− |
|---|---|---|---|---|
| Phex | Phosphate regulating endopeptidase homolog, X-linked | NA | 1.1 ± 0.04 | NA |
| Dmp1 | Dentin matrix protein 1 | 1.6 ± 0.1 | NA | NA |
| Fgf23 | Fibroblast growth factor 23 | 372.0 ± 44.1* | 318.3 ± 81.6* | 311.9 ± 95.3* |
| Fcrls | Fc receptor-like S, scavenger receptor | 14.2 ± 1.0* | 9.1 ± 1.5*,# | 12.0 ± 1.9* |
| Lipg | Lipase, endothelial | 7.3 ± 1.1* | 7.7 ± 1.9* | 6.2 ± 1.6* |
| Ctsw | Cathepsin W | 5.1 ± 0.2* | 2.8 ± 0.1*,# | 4.3 ± 0.01*,$ |
| Fgf1 | Fibroblast growth factor 1 | 3.5 ± 0.07* | 3.9 ± 0.3* | 3.1 ± 0.2* |
| Csdc2 | Cold shock domain containing C2, RNA binding | 2.6 ± 0.3* | 2.1 ± 0.7* | 2.7 ± 0.5* |
| S100b | S100 protein, β polypeptide, neural | 2.9 ± 0.1* | 2.0 ± 0.04*,# | 2.1 ± 0.1* |
| Add3 | Adducin 3 (γ) | 1.3 ± 0.05* | 1.8 ± 0.1* | 1.9 ± 0.05*,# |
Values are expressed as fold change compared to the WT value; n = 3 samples/group.
P < 0.05 vs. WT;
P < 0.05 vs. Hyp;
P < 0.05 vs. Dmp1−/−; 1-way ANOVA and post hoc Fisher test.
Next, we performed an ingenuity pathway analysis of the nonadditive cluster to identify molecular interactions networks regulated by both PHEX and DMP1 (Fig. 4). This analysis identified a central role of 8 canonical pathways in the nonadditive effects of Phex and Dmp1 mutations. Two of these, PI3K and Grb2, belong to a common pathway, the FGFs/FGFR pathway, previously reported to increase Fgf23 promoter activity when activated (37). In addition, this analysis identified other pathways of less known significance in FGF23 biology, involving glucocorticoid receptor NR3C1, Huntingtin (HTT), tumor protein 53 (TP53), glutamate NMDA receptor subunit ζ-1 (GRIN1), amyloid precursor protein (APP), and hepatocyte nuclear factor 4 α (HNF4a).
Validating the role of FGFR1 in regulating Fgf23 expression
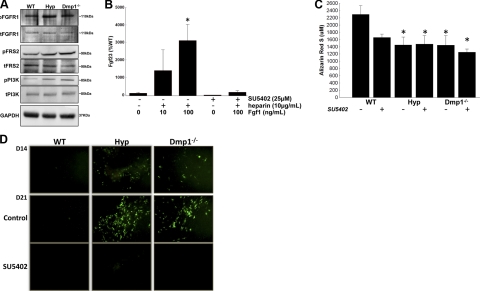
To determine the relevance of the changes in gene expression identified by microarray analysis, we confirmed by Western blot analysis that FGFR1 pathway was activated in Hyp and Dmp1−/− (Fig. 5) by showing increased phosphorylation of FGFR1, FRS2, and PI3K in these bones. We then tested the ability of FGF1 to stimulate Fgf23 expression in WT BMSCs to confirm that activation of FGFR1 pathway leads to increased Fgf23 expression. We found that FGF1 stimulated Fgf23 mRNA expression in a dose-dependent manner and this response was blocked by using a specific inhibitor of FGFR1 (SU5402; ref. 38 and Fig. 5). In addition, SU5402 treatment did not significantly impair in vitro extracellular matrix mineralization as assessed by Alizarin Red S staining after 3 wk of culture in either WT-, Hyp-, or Dmp1−/−-derived BMSCs cultured under osteoblast differentiating conditions (Fig. 5). Similarly, the expression of osteoblastic markers of differentiation was not decreased after treatment. The expression of Phex and Dmp1 mRNA were decreased following SU5402 treatment (Table 6). Notably, we found that inhibition of FGFR1 resulted in decreased Fgf23 promoter activity, as assessed by decreased eGFP fluorescence, in SU5402-treated Hyp and Dmp1−/− BMSCs as compared to nontreated cells (Fig. 5). We confirmed the decrease in Fgf23 promoter activity by separately assessing endogenous Fgf23 mRNA expression. The Hyp- or Dmp1−/−-associated increase in Fgf23 mRNA levels was completely blocked by treatment of BMSCs with SU5402 (Table 6).
Figure 5.
Rescue of the Fgf23 promoter activity in Hyp and Dmp1−/− by inhibition of FGFR1. A) Western blots showing activation of the FGFR1 pathway in Hyp and Dmp1−/− cortical bone compared to WT. B) WT BMSCs were treated with heparin, Fgf1, and/or SU5402 as indicated for the last 24 h of the 3-wk culture period. Graph shows Fgf23 mRNA expression measured by RT-PCR. Values are expressed as percentage of control value (set at 100%). C, D) BMSCs were collected from WT, Hyp, and Dmp1−/− mice lacking 1 allele of Fgf23 (Fgf23+/−[eGFP]) and cultured in osteoblast differentiating medium for 3 wk (control). An FGFR1-specific inhibitor (SU5402) was added to the medium during the third week of culture. C) Spectrophotometric quantification of alizarin red S staining performed at the end of the culture period. D) Expression of eGFP driven by Fgf23 promoter. EGFP expression is reported before (D14) and after (D21) SU5402 treatment. All experiments were performed in triplicate. *P < 0.05 vs. control; 1-way ANOVA and post hoc Fisher test.
Table 6.
Bone-related gene expression measured by RT-PCR
| Gene | WT SU5402 | Hyp |
Dmp1−/− |
||
|---|---|---|---|---|---|
| Control | SU5402 | Control | SU5402 | ||
| Osterix | 0.91 ± 0.02 | 1.05 ± 0.01 | 0.92 ± 0.01 | 1.61 ± 0.36 | 3.73 ± 0.12*,$ |
| Runx2 | 2.64 ± 0.07* | 0.74 ± 0.05* | 2.81 ± 0.37*,# | 3.55 ± 1.99 | 3.31 ± 0.97* |
| Osteocalcin | 2.37 ± 0.39* | 0.86 ± 0.17 | 1.58 ± 0.25,# | 4.52 ± 0.73* | 4.31 ± 1.14* |
| Phex | 0.50 ± 0.08* | NA | NA | 1.73 ± 1.31 | 0.01 ± 0.01* |
| Dmp1 | 0.30 ± 0.04* | 0.74 ± 0.10 | 0.25 ± 0.04*,# | NA | NA |
| Fgfr1 | 2.10 ± 0.10* | 0.53 ± 0.03* | 2.04 ± 0.14*,# | 1.68 ± 0.08* | 2.92 ± 0.06*,$ |
| Fgf23 | 0.08 ± 0.03* | 128.79 ± 52.68* | 1.33 ± 0.49,# | 124.14 ± 22.51* | 2.49 ± 1.28,$ |
WT, Hyp, and Dmp1−/− BMSCs were cultured for 3 wk in osteoblast differentiating medium (control) and treated during the last week with an FGFR1 specific inhibitor (SU5402). Values are expressed as means ± se relative to WT (WT=1); n = 3 wells/group.
P < 0.05 vs. WT;
P < 0.05 vs. Hyp;
P < 0.05 vs. Dmp1−/−; 1-way ANOVA and post hoc Fisher test.
DISCUSSION
Phex and Dmp1 genes encode distinct protein products that regulate the bone mineralization and FGF23 production by osteocytes, but the molecular mechanisms whereby Fgf23 is regulated by these factors is not known. Inactivation of either gene leads to hypophosphatemic rickets and intrinsic elevations of FGF23 in osteocytes (7, 28). In the current study, we created compound-mutant Hyp- and Dmp1-null mice and performed gene expression profiling to identify molecular pathways whereby PHEX and DMP1 regulate Fgf23 expression and mineralization. Our approach is based on the principle that additive effects of PHEX and DMP1 inactivation would indicate distinct mechanisms, whereas nonadditive effects would implicate a common mechanism or an interaction between PHEX and DMP1 (39).
We found that combined Phex and Dmp1 mutations had nonadditive effects on FGF23 production, bone mineralization, and serum FGF23, 1,25(OH)2D, and phosphate levels. In addition, we identified nonadditive gene clusters relevant to common regulatory networks in cortical bone from single- and double-mutant mice and key molecular pathways and downstream genes that are commonly regulated by PHEX and DMP1. However, not all functions of PHEX and DMP1 are shared, as evidenced by 2 additional clusters containing genes with additive down-regulation and discordant effects of Phex and Dmp1 mutations (Supplemental Fig. S1 and Supplemental Table S2).
We initially focused on validating the role of FGFR signaling that was identified as a common pathway regulated by Phex and Dmp1 mutations because of preexisting evidence for FGFR regulation of Fgf23. There is in vitro evidence for Fgf23 promoter stimulation by FGFs (37), FGF2 or FGF7 administration induces hypophosphatemia in vivo (40–42), and elevated FGF23 levels are found in transgenic mice overexpressing high-molecular-weight nuclear isoforms of FGF2 (43), which activate gene transcription through FGFR1-dependent intracrine pathways (44). Also, activating mutations of Fgfr1 causes osteoglophonic dysplasia (OGD), a disorder that exhibits hypophosphatemia and elevated FGF23 levels in some patients (45).
Consistent with the function of the FGFR pathway in the regulation of Fgf23, we found evidence for activation of canonical FGFR1 pathway by Western blot analysis of bones derived from Hyp and Dmp1−/− mice. In addition, we found that FGF1 expression was increased in these bones and confirmed (37) that FGF1 stimulates Fgf23 expression in a dose-dependent manner in WT BMSCs and that this stimulation is inhibited by SU5402. Finally, we could rescue the elevation of FGF23 production observed in Hyp- and Dmp1−/−-mouse-derived BMSCs by inhibiting FGFR1 with SU5402. Prior studies indicate that neither ablation of FGFR3 nor ablation of FGFR4 reduces FGF23 levels in Hyp mice (46), suggesting that either FGFR1 or FGFR2 activation in osteocytes might stimulate Fgf23. At present, we favor FGFR1, since it is more highly expressed in osteocytes than FGFR2 (47).
How PHEX and DMP1 work together to activate FGFR signaling is not defined by our studies, but our results suggest several possible mechanisms. We can exclude a direct effect of FGF23 to stimulate its own production, given the findings that loss of FGF23 increases FGF23-promoter activity in osteocytes of FGF23-null mice (28) and the inability of FGF23 to stimulate FGF23 expression in BMSCs or osteoblasts in vitro (data not shown). Although an initial study suggested that PHEX processes FGF23 (48), subsequent studies failed to establish PHEX-dependent cleavage of FGF23 (29, 49, 50). In addition, DMP1 is not a substrate for PHEX (51, 52), but, similar to MEPE (53), it may be able to bind to PHEX via the acidic serine aspartate-rich MEPE-associated (ASARM) motif present in all SIBLING proteins (54) and bind to αvβ3 integrins via the SIBLING characteristic RGD motif. Moreover, αvβ3 integrins have been shown to increase FGFR activation by binding to FGF1 (55). Thus, DMP1 interactions with both integrins and PHEX through RGD and ASARM motifs explain Fgf23 regulation by PHEX and DMP1 (11). Alternatively, mutations of PHEX and DMP1 have direct effects to inhibit bone mineralization and/or extracellular matrix proteolytic activity, which in turn might release the bioavailability of latent paracrine FGFs stored in the mineralized extracellular matrix (56, 57). Indeed, FGFs are associated with extracellular matrix proteins and bind to glycosaminoglycan motifs, such as those present in the N terminus of DMP1. Moreover, heparan sulfate and FGF signaling can be regulated by either extracellular matrix proteins or proteolytic enzymes (58). Proteolytic activity is also increased in Hyp bone, and inhibitors of proteases result in a partial rescue of the Hyp phenotype (59).
We identified several additional pathways in the shared networks showing nonadditive regulation by PHEX and DMP1. In this regard, we identified pathways implicating a possible role for the NR3C1 glucocorticoid receptor in regulating Fgf23. Interestingly, glucocorticoid treatment results in osteocytic osteolysis (a demineralization process surrounding osteocytes) and increased Fgf23 expression in animal models (unpublished results). These data, along with the known effects of calcitriol to stimulate Fgf23 expression through the vitamin D responsive element (10), suggest that nuclear receptor pathways are important in regulating the Fgf23 expression. We also identified Hnf4α, a transcription factor involved in liver and pancreas development, known to regulate genes involved in the control of lipid homeostasis (60), as one of the central connectors of the genes present in the 3 identified clusters, with >10 downstream connections to these genes. A majority of the genes belonging to these clusters are also known to be involved in the regulation of energy and lipid metabolism. Interestingly, we have previously reported the presence of a putative HNF4α binding site in the sequence of the Fgf23 promoter (10). Third, the most highly upregulated gene commonly found in Hyp, Dmp1−/−, and Hyp/Dmp1−/− mutant mice is a novel transcript with some homology to the Jak3 tyrosine kinase. Recently, it was shown that activation of the leptin receptor, which signals through JAK1/2/STAT3, MAPK, and/or PI3K/AKT pathways, stimulates Fgf23 (61). The up-regulation of a Jak3-like transcript as a consequence of PHEX and DMP1 inactivations raises the possibility of additional putative regulatory pathway. Additional studies will be needed to determine whether these pathways are directly linked to Phex and Dmp1 mutations in bone or to possible secondary effects of these mutations.
In summary, we established a common mechanism linking paracrine/autocrine effects of PHEX and DMP1 on Fgf23 expression in osteocytes via regulation of FGFR signaling. Further studies are needed to identify how PHEX and DMP1 cooperate to control the activation of the FGFR signalization pathways and to define the role of other possible regulators of Fgf23 expression. Regardless, it appears that FGFRs are involved in both the regulation of FGF23 production in bone and the end organ effects of FGF23 in the kidney, which likely represents an evolutionary extension of FGFR function to not only mediate local autocrine/paracrine effects in bone but to regulate FGFR in the kidney through the secretion of FGF23 (46), thereby permitting local signals in bone to be relayed to the kidney. The physiological function of the FGF23 bone-kidney axis may be to coordinate bone mineralization and remodeling with renal handling of phosphate and vitamin D metabolism.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health grant RO1-AR45955 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The authors thank Drs. Ivan Gerling, Weikuan Gu, and Yan Jiao for their help and advice on microarray analysis and Jianping Zhou for his technical expertise.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Suzuki M., Uehara Y., Motomura-Matsuzaka K., Oki J., Koyama Y., Kimura M., Asada M., Komi-Kuramochi A., Oka S., Imamura T. (2008) βKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol. Endocrinol. 22, 1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Itoh N., Ornitz D. M. (2004) Evolution of the Fgf and Fgfr gene families. Trends Genet. 20, 563–569 [DOI] [PubMed] [Google Scholar]
- 3. Yamashita T., Yoshioka M., Itoh N. (2000) Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem. Biophys. Res. Commun. 277, 494–498 [DOI] [PubMed] [Google Scholar]
- 4. Kurosu H., Ogawa Y., Miyoshi M., Yamamoto M., Nandi A., Rosenblatt K. P., Baum M. G., Schiavi S., Hu M. C., Moe O. W., Kuro-o M. (2006) Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281, 6120–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Urakawa I., Yamazaki Y., Shimada T., Iijima K., Hasegawa H., Okawa K., Fujita T., Fukumoto S., Yamashita T. (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–77417086194 [Google Scholar]
- 6. ADHR Consortium (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 26, 345–348 [DOI] [PubMed] [Google Scholar]
- 7. Feng J. Q., Ward L. M., Liu S., Lu Y., Xie Y., Yuan B., Yu X., Rauch F., Davis S. I., Zhang S., Rios H., Drezner M. K., Quarles L. D., Bonewald L. F., White K. E. (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 38, 1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brownstein C. A., Adler F., Nelson-Williams C., Iijima J., Li P., Imura A., Nabeshima Y., Reyes-Mugica M., Carpenter T. O., Lifton R. P. (2008) A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc. Natl. Acad. Sci. U. S. A. 105, 3455–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. HYP Consortium (1995) A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat. Genet. 11, 130–136 [DOI] [PubMed] [Google Scholar]
- 10. Liu S., Tang W., Zhou J., Stubbs J. R., Luo Q., Pi M., Quarles L. D. (2006) Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 17, 1305–1315 [DOI] [PubMed] [Google Scholar]
- 11. Quarles L. D. (2008) Endocrine functions of bone in mineral metabolism regulation. J. Clin. Invest. 118, 3820–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomoe Y., Segawa H., Shiozawa K., Kaneko I., Tominaga R., Hanabusa E., Aranami F., Furutani J., Kuwahara S., Tatsumi S., Matsumoto M., Ito M., Miyamoto K. (2010) Phosphaturic action of fibroblast growth factor 23 in Npt2 null mice. Am. J. Physiol. Renal Physiol. 298, F1341–1350 [DOI] [PubMed] [Google Scholar]
- 13. Bai X., Miao D., Li J., Goltzman D., Karaplis A. C. (2004) Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology 145, 5269–5279 [DOI] [PubMed] [Google Scholar]
- 14. Larsson T., Marsell R., Schipani E., Ohlsson C., Ljunggren O., Tenenhouse H. S., Juppner H., Jonsson K. B. (2004) Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology 145, 3087–3094 [DOI] [PubMed] [Google Scholar]
- 15. Shimada T., Mizutani S., Muto T., Yoneya T., Hino R., Takeda S., Takeuchi Y., Fujita T., Fukumoto S., Yamashita T. (2001) Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl. Acad. Sci. U. S. A. 98, 6500–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimada T., Yamazaki Y., Takahashi M., Hasegawa H., Urakawa I., Oshima T., Ono K., Kakitani M., Tomizuka K., Fujita T., Fukumoto S., Yamashita T. (2005) Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am. J. Physiol. Renal Physiol. 289, F1088–F1095 [DOI] [PubMed] [Google Scholar]
- 17. Kolek O. I., Hines E. R., Jones M. D., LeSueur L. K., Lipko M. A., Kiela P. R., Collins J. F., Haussler M. R., Ghishan F. K. (2005) 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G1036–G1042 [DOI] [PubMed] [Google Scholar]
- 18. Samadfam R., Richard C., Nguyen-Yamamoto L., Bolivar I., Goltzman D. (2009) Bone formation regulates circulating concentrations of fibroblast growth factor 23. Endocrinology 150, 4835–4845 [DOI] [PubMed] [Google Scholar]
- 19. Tebben P. J., Singh R. J., Clarke B. L., Kumar R. (2004) Fibroblast growth factor 23, parathyroid hormone, and 1alpha,25-dihydroxyvitamin D in surgically treated primary hyperparathyroidism. Mayo Clin. Proc. 79, 1508–1513 [DOI] [PubMed] [Google Scholar]
- 20. Kawata T., Imanishi Y., Kobayashi K., Miki T., Arnold A., Inaba M., Nishizawa Y. (2007) Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J. Am. Soc. Nephrol. 18, 2683–2688 [DOI] [PubMed] [Google Scholar]
- 21. Saji F., Shiizaki K., Shimada S., Okada T., Kunimoto K., Sakaguchi T., Hatamura I., Shigematsu T. (2009) Regulation of fibroblast growth factor 23 production in bone in uremic rats. Nephron. Physiol. 111, p59–66 [DOI] [PubMed] [Google Scholar]
- 22. Perwad F., Azam N., Zhang M. Y., Yamashita T., Tenenhouse H. S., Portale A. A. (2005) Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146, 5358–5364 [DOI] [PubMed] [Google Scholar]
- 23. Ferrari S. L., Bonjour J. P., Rizzoli R. (2005) Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J. Clin. Endocrinol. Metab. 90, 1519–1524 [DOI] [PubMed] [Google Scholar]
- 24. Nishida Y., Taketani Y., Yamanaka-Okumura H., Imamura F., Taniguchi A., Sato T., Shuto E., Nashiki K., Arai H., Yamamoto H., Takeda E. (2006) Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 70, 2141–2147 [DOI] [PubMed] [Google Scholar]
- 25. Murshed M., Harmey D., Millan J. L., McKee M. D., Karsenty G. (2005) Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Devel. 19, 1093–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lotinun S., Sibonga J. D., Turner R. T. (2002) Differential effects of intermittent and continuous administration of parathyroid hormone on bone histomorphometry and gene expression. Endocrine 17, 29–36 [DOI] [PubMed] [Google Scholar]
- 27. Poole K. E., Reeve J. (2005) Parathyroid hormone - a bone anabolic and catabolic agent. Curr. Opin. Pharmacol. 5, 612–617 [DOI] [PubMed] [Google Scholar]
- 28. Liu S., Zhou J., Tang W., Jiang X., Rowe D. W., Quarles L. D. (2006) Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 291, E38–49 [DOI] [PubMed] [Google Scholar]
- 29. Liu S., Guo R., Simpson L. G., Xiao Z. S., Burnham C. E., Quarles L. D. (2003) Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J. Biol. Chem. 278, 37419–37426 [DOI] [PubMed] [Google Scholar]
- 30. Guo R., Quarles L. D. (1997) Cloning and sequencing of human PEX from a bone cDNA library: evidence for its developmental stage-specific regulation in osteoblasts. J. Bone Miner. Res. 12, 1009–1017 [DOI] [PubMed] [Google Scholar]
- 31. Liu S., Zhou J., Tang W., Menard R., Feng J. Q., Quarles L. D. (2008) Pathogenic role of Fgf23 in Dmp1-null mice. Am. J. Physiol. Endocrinol. Metab. 295, E254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu S., Brown T. A., Zhou J., Xiao Z. S., Awad H., Guilak F., Quarles L. D. (2005) Role of matrix extracellular phosphoglycoprotein in the pathogenesis of X-linked hypophosphatemia. J. Am. Soc. Nephrol. 16, 1645–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng J. Q., Huang H., Lu Y., Ye L., Xie Y., Tsutsui T. W., Kunieda T., Castranio T., Scott G., Bonewald L. B., Mishina Y. (2003) The Dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J. Dent. Res. 82, 776–780 [DOI] [PubMed] [Google Scholar]
- 34. Institute on Laboratory Animal Resources (1996) Guide for Care and Use of Laboratory Animals. National Research Council. Department of Health and Human Services Publication NIH 86-23, National Academy Press, Washington, DC [Google Scholar]
- 35. Martin A., David V., Vico L., Thomas T. (2008) Impaired energetic metabolism after central leptin signaling leads to massive appendicular bone loss in hindlimb-suspended rats. J. Bone Miner. Res. 23, 2040–2047 [DOI] [PubMed] [Google Scholar]
- 36. David V., Martin A., Hedge A., Rowe P. S. (2009) MEPE is a new bone renal hormone and vascularization modulator. Endocrinology 150, 4012–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu S., Tang W., Fang J., Ren J., Li H., Xiao Z., Quarles L. D. (2009) Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol. Endocrinol. 23, 1505–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mohammadi M., McMahon G., Sun L., Tang C., Hirth P., Yeh B. K., Hubbard S. R., Schlessinger J. (1997) Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science (New York) 276, 955–960 [DOI] [PubMed] [Google Scholar]
- 39. Wells J. A. (1990) Additivity of mutational effects in proteins. Biochemistry 29, 8509–8517 [DOI] [PubMed] [Google Scholar]
- 40. Carpenter T. O., Ellis B. K., Insogna K. L., Philbrick W. M., Sterpka J., Shimkets R. (2005) Fibroblast growth factor 7: an inhibitor of phosphate transport derived from oncogenic osteomalacia-causing tumors. J. Clin. Endocrinol. Metab. 90, 1012–1020 [DOI] [PubMed] [Google Scholar]
- 41. Nauman E. A., Sakata T., Keaveny T. M., Halloran B. P., Bikle D. D. (2003) bFGF administration lowers the phosphate threshold for mineralization in bone marrow stromal cells. Calcif. Tissue Int. 73, 147–152 [DOI] [PubMed] [Google Scholar]
- 42. Liang H., Pun S., Wronski T. J. (1999) Bone anabolic effects of basic fibroblast growth factor in ovariectomized rats. Endocrinology 140, 5780–5788 [DOI] [PubMed] [Google Scholar]
- 43. Xiao L., Naganawa T., Lorenzo J., Carpenter T. O., Coffin J. D., Hurley M. M. (2010) Nuclear isoforms of fibroblast growth factor 2 are novel inducers of hypophosphatemia via modulation of FGF23 and KLOTHO. J. Biol. Chem. 285, 2834–2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dunham-Ems S. M., Lee Y. W., Stachowiak E. K., Pudavar H., Claus P., Prasad P. N., Stachowiak M. K. (2009) Fibroblast growth factor receptor-1 (FGFR1) nuclear dynamics reveal a novel mechanism in transcription control. Mol. Biol. Cell 20, 2401–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. White K. E., Cabral J. M., Davis S. I., Fishburn T., Evans W. E., Ichikawa S., Fields J., Yu X., Shaw N. J., McLellan N. J., McKeown C., Fitzpatrick D., Yu K., Ornitz D. M., Econs M. J. (2005) Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am. J. Hum. Genet. 76, 361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li H., Martin A., David V., Quarles L. D. (2011) Compound deletion of Fgfr3 and Fgfr4 partially rescues the Hyp mouse phenotype. Am. J. Physiol. Endocrinol. Metab. 300, E508–E517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stevens D. A., Harvey C. B., Scott A. J., O'Shea P. J., Barnard J. C., Williams A. J., Brady G., Samarut J., Chassande O., Williams G. R. (2003) Thyroid hormone activates fibroblast growth factor receptor-1 in bone. Mol. Endocrinol. 17, 1751–1766 [DOI] [PubMed] [Google Scholar]
- 48. Bowe A. E., Finnegan R., Jan de Beur S. M., Cho J., Levine M. A., Kumar R., Schiavi S. C. (2001) FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem. Biophys. Res. Commun. 284, 977–981 [DOI] [PubMed] [Google Scholar]
- 49. Benet-Pages A., Lorenz-Depiereux B., Zischka H., White K. E., Econs M. J., Strom T. M. (2004) FGF23 is processed by proprotein convertases but not by PHEX. Bone 35, 455–462 [DOI] [PubMed] [Google Scholar]
- 50. Guo R., Liu S., Spurney R. F., Quarles L. D. (2001) Analysis of recombinant Phex: an endopeptidase in search of a substrate. Am. J. Physiol. Endocrinol. Metab. 281, E837–E847 [DOI] [PubMed] [Google Scholar]
- 51. Zhang B., Sun Y., Chen L., Guan C., Guo L., Qin C. (2010) Expression and distribution of SIBLING proteins in the predentin/dentin and mandible of hyp mice. Oral Dis. 16, 453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lu Y., Qin C., Xie Y., Bonewald L. F., Feng J. Q. (2009) Studies of the DMP1 57-kDa functional domain both in vivo and in vitro. Cells Tissues Organs 189, 175–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu S., Rowe P. S., Vierthaler L., Zhou J., Quarles L. D. (2007) Phosphorylated acidic serine-aspartate-rich MEPE-associated motif peptide from matrix extracellular phosphoglycoprotein inhibits phosphate regulating gene with homologies to endopeptidases on the X-chromosome enzyme activity. J. Endocrinol. 192, 261–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Martin A., David V., Laurence J. S., Schwarz P. M., Lafer E. M., Hedge A. M., Rowe P. S. (2008) Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology 149, 1757–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mori S., Wu C. Y., Yamaji S., Saegusa J., Shi B., Ma Z., Kuwabara Y., Lam K. S., Isseroff R. R., Takada Y. K., Takada Y. (2008) Direct binding of integrin alphavbeta3 to FGF1 plays a role in FGF1 signaling. J. Biol. Chem. 283, 18066–18075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu S., Tang W., Zhou J., Vierthaler L., Quarles L. D. (2007) Distinct roles for intrinsic osteocyte abnormalities and systemic factors in regulation of FGF23 and bone mineralization in Hyp mice. Am. J. Physiol. 293, E1636–1644 [DOI] [PubMed] [Google Scholar]
- 57. Xiao Z. S., Crenshaw M., Guo R., Nesbitt T., Drezner M. K., Quarles L. D. (1998) Intrinsic mineralization defect in Hyp mouse osteoblasts. Am. J. Physiol. 275, E700–708 [DOI] [PubMed] [Google Scholar]
- 58. Taipale J., Keski-Oja J. (1997) Growth factors in the extracellular matrix. FASEB J. 11, 51–59 [DOI] [PubMed] [Google Scholar]
- 59. Rowe P. S., Matsumoto N., Jo O. D., Shih R. N., Oconnor J., Roudier M. P., Bain S., Liu S., Harrison J., Yanagawa N. (2006) Correction of the mineralization defect in hyp mice treated with protease inhibitors CA074 and pepstatin. Bone 39, 773–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hayhurst G. P., Lee Y. H., Lambert G., Ward J. M., Gonzalez F. J. (2001) Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 21, 1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tsuji K., Maeda T., Kawane T., Matsunuma A., Horiuchi N. (2010) Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J. Bone Miner. Res. 25, 1711–1723 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.