Abstract
Fibrocytes are circulating mesenchymal progenitor cells that participate in tissue responses to injury and invasion. Accumulating knowledge from animal models regarding the differentiation, trafficking, and function of these cells implicates them in the development of diseases characterized by chronic inflammation and excessive collagen deposition. Recent data obtained from the clinical setting suggests that the enumeration of circulating fibrocytes may be a biomarker for disease progression in chronic lung diseases including asthma and pulmonary fibrosis. A greater understanding of the immunologic mediators that influence fibrocyte biology suggests new opportunities for therapeutic manipulation of these cells in fibrogenesis. This review integrates new developments in the cellular and molecular biology of fibrocytes with current concepts regarding the etiopathogenesis of fibrosing disorders.
INTRODUCTION
Tissue integrity is maintained by the coordinated activation of cellular responses that counter pathogen invasion and maintain normal architecture. In most circumstances, host immunity acts to restore tissue homeostasis and function. However, in the setting of persistent invasion, injury, immunodeficiency, vascular insufficiency or metabolic abnormalities, reparative processes do not proceed normally and pathologic remodeling occurs 1. This process, which results in the replacement of normal tissue components with inflammatory cells and connective tissue scar, is the basis for the pathologic changes of asthma 2, lung fibrosis 3, chronic kidney disease 4, liver fibrosis 4, vasculopathies such as atherosclerosis 5, and skin abnormalities such as keloids and hypertrophic scarring 6. Similar reactive stromal responses to neoplasia also promote tumor progression and metastasis 7. While the current paradigm of tissue repair describes a sequential process that proceeds from injury to inflammation followed by resolution and repair, it is clear that cells recruited during the early response to invasion and injury play a critical role in the ensuing progression of the repair response.
Until recently, it was considered that both normal and dysregulated repair responses originated from the recruitment, proliferation, and activation of local connective tissue cells 4. Research performed over the last several years however, now supports the contention that connective-tissue/extracellular matrix (ECM) producing cells also develop by alternate pathways such as the reactivation of an embryonic epithelial-mesenchymal pathway 8 or the recruitment of bone marrow derived progenitor cells 9. Interestingly, the notion of monocytic fibroblast precursors derived from the circulation was first proposed over 150 years ago following the careful microscopic observations of James Paget. Bloodborne, connective tissue cell precursors are described in the writings of Cohnheim, Metchnikov, Fischer, and Maximow 10–12 and this area has been an active focus of debate for many years, and in many respects it remains so.
FIBROCYTE CHARACTERISTICS
Fibrocyte Discovery and Initial Characterization
The “fibrocyte” was described in 1994 as a circulating, bone marrow derived cell with the ability to adopt a mesenchymal phenotype 13. This cell bears features of both fibroblasts and monocytes, and this combination of connective tissue cell and myeloid features allows its identification by a number of markers. Fibrocytes express the stem cell marker CD34, the pan-hematopoietic marker CD45, monocyte markers such as CD14 and CD11, and they produce components of the connective tissue matrix including collagen-1, collagen-III and vimentin 14. Originally identified by CD34 and collagen-1 co-expression 13, fibrocytes may be identified by dual positivity of CD34 or CD45 and collagen-1 or pro-collagen-1. The expression of the progenitor cell marker CD34 engendered the notion that fibrocytes constitute a bone marrow-derived fibroblast population that circulates in the peripheral blood 14. Human and murine fibrocytes are easily isolated by the expansion of adherent peripheral blood mononuclear on either plastic or fibronectin-coated plates 15. While enrichment for CD14+ peripheral blood cells increases fibrocyte outgrowth 15, fibrocytes also are found in stromal cultures of bone marrow, splenocytes, and solid organs 14. In the normal host, fibrocytes constitute approximately 0.5% of circulating leukocytes 13. This quantity increases in response to certain cytokines and chemokines or the presence of underlying fibrotic or inflammatory conditions 16,17. Fibrocytes exit the circulation at sites of injury and contribute to the formation of granulomas, scars, and remodeled tissue 15.
The co-expression of CD34 or CD45 with collagen-1 or pro-collagen-1 is considered sufficient to identify bloodborne fibrocytes in most settings 17. Fibrocytes also produce the matrix components fibronectin and vimentin, and prolyl-hydroxylase has also been a useful marker in some studies 15,18. When used in conjunction with pro-collagen detection, leukocyte specific protein-1, which is a p38 MAPK target, identifies fibrocytes in the post-burn hypertrophic scar 19. Other hematopoietic markers expressed by these cells include CD11b, CD13 and MHCII 15. Fibrocytes lack lymphocyte markers such as CD3, CD4, CD8, CD19, and CD25 13.
In a more recent study, the expression of CD45RO, 25F9 (a marker of mature macrophages), S100A8/A9 (calprotectin) and a lack of PM-2K (which identifies mature macrophages) identified fibrocytes in the lungs of patients with IPF 18 both in vivo and after in vitro culture. The combination of CD34/CD45/pro-col-1 identified both fibrocytes and a subpopulation of macrophages; this may indicate a common progenitor population. Further work is needed to determine the differentiation pathway of fibrocytes from monocytic precursors. It is noteworthy that fibrocytes may share certain phenotypic features with alternatively activated macrophages, which are also considered to contribute to reparative processes 20.
Physiologic Role in Wound Repair
Fibrocytes display many properties that are important for wound repair. Upon entry into diseased tissue, fibrocytes can adopt the phenotype of myofibroblasts as evidenced by the loss of CD34 and the acquisition of αsmooth muscle action (α-SMA) expression 15,21; however the extent to which this occurs in different settings of tissue repair remains unclear. Fibrocytes secrete proinflammatory cytokines (TNF, IL-6, IL-8, IL-10, MIP1α/β and metalloproteinases such as and MMP-9 in response to IL-1β stimulation 22. In addition, fibrocytes may demonstrate a pro-angiogeneic phenotype. They promote endothelial tube formation in vitro and neoangiogenesis within implanted matrigel in vivo. Fibrocytes secrete the pro-angiogenic factors: VEGF, PDGF-A, M-CSF, HGF, GM-CSF, b-FGF and CN-TGF, IL-8 and IL-1β22,23. Fibrocytes also express a number of chemokine receptors that regulate the recruitment and trafficking of inflammatory cells. Lastly, fibrocytes express MHC Class II and demonstrate antigen-presenting capabilities in vitro and in vivo 24. These functions may be expected to clearly influence the progression of wound healing and tissue remodeling responses. Further insight into fibrocyte function is provided by studies that have examined their differentiation, cell surface markers, and chemokine receptors.
Fibrocyte Differentiation and Trafficking
Fibrocytes have been described to differentiate from a precursor population present within the CD14+ monocyte fraction of peripheral blood 15. Further studies have determined that this precursor expresses the Fcγ receptor (CD16) 25 and that inhibition of this receptor significantly reduces fibrocyte outgrowth. Fibrocyte differentiation from CD14+ precursors is augmented by the Th2 cytokines IL-4 and IL-13 and inhibited by the Th1 cytokines IFNγ, TNF, and IL-12 26. A very recent study indicates that fibrocytes differentiate from the CD11b(+) CD115(+) Gr1(+) cells within the CD14+ peripheral blood leukocyte fraction, and that activated T cell subsets enhance fibrocyte outgrowth in culture 27. However, careful lineage tracing studies will be required to confirm the monocyte origin of fibrocytes under different fibrogenic stimuli.
Signaling pathways implicated in fibrocyte outgrowth include ITIMs, mTOR-PI3 kinase, and ATR2. Treatment of fibrocyte precursors with the Fc receptor antagonist serum amyloid P (SAP), with the mTOR inhibitor rapamycin 28, or with the ATR inhibitor valsartan 29 attenuates fibrocyte accumulation in mouse models of fibrosis. Our own work indicates that the neuronal guidance protein and immunoregulatory surface molecule Semaphorin 7a critically regulates fibrocyte differentiation in a β1 integrin-dependent manner and that there is a requirement for apoptosis derived signals in this process (Herzog et al, submitted). In these studies, genetic deletion of the GPI anchored membrane protein Semaphorin 7a significantly attenuated intrapulmonary fibrocyte appearance in a TGFβ1 dependent mouse model of lung fibrosis. This effect was mimicked by disruption of β1 integrin function (a receptor for Semaphorin 7a expressed by fibrocytes) and by blocking apoptosis with the caspase inhibitor Z-VAD/fmk. Fibrocyte outgrowth also was observed to be increased three-fold in a β1 integrin-dependent, caspase-dependent manner when human CD14+ cells were cultured in the presence of recombinant Semaphorin 7a.
It has been postulated that the ultimate phenotype of fibrocytes is the contractile myofibroblast 15,30–32. This idea is based on the finding that cultured fibrocytes respond to TGFβ1 by expressing α- SMA and contracting collagen gels in vitro 15,30–32. However, the ability of fibrocytes to differentiate into myofibroblasts in vivo is less clear. Studies using bone marrow transplantation show only a minimal contribution of fibrocytes to α-SMA production in some models 33–35, implying that this differentiation pathway may not necessarily be a dominant feature of fibrocytes in the tissue remodeling response.
Fibrocyte Recruitment into Tissues
Murine fibrocytes express the chemokine receptors CCR2, CCR7, and CXCR4; these molecules mediate the recruitment of fibrocytes to injured tissue 30,36,37. Human fibrocytes also express the chemokine receptors CCR3 (eotaxin receptor) and CCR5 (MCP-1 receptor) 31. While there are only limited data regarding the circulating mediators that effect fibrocyte recruitment in humans, our own work demonstrates an association between circulating concentrations of MCP-1 and high levels of CD45/Pro-Col-1+ve fibrocytes in the circulation of scleroderma patients with interstitial lung disease ILD) or in healthy aging (S. Mathai, submitted). These data suggest that MCP-1 may be involved in mobilization of fibrocytes into the peripheral blood. In addition, high levels of CXCL12, which is the ligand for CXCR4, have been found in the lungs and blood of patients with idiopathic pulmonary fibrosis (IPF) and these levels correlate with circulating fibrocyte concentrations 16. Nevertheless, the relationship between chemokine production and fibrocyte differentiation and trafficking is an active area of investigation with the potential to lead to new therapies for fibrosing diseases.
ROLE IN PATHOLOGIC DISORDERS AND FIBROSING DISEASES
Fibrocytes in Lung Disease
Fibrotic lung disease can affect the airway (asthma) and the parenchyma (pulmonary fibrosis). In both of these disorders the progressive destruction of normal lung tissue leads to lung remodeling and persistent gas exchange abnormalities. Fibrocytes have been implicated in the pathogenesis of both asthma and pulmonary fibrosis.
Asthma
The lungs of patients with chronic asthma demonstrate persistent inflammation and remodeling of the airways. The latter leads to progressive airway obstruction and a permanent impairment in respiratory function. Pathologic examination of these tissues demonstrate subepithelial fibrosis and myofibroblast accumulation. A number of studies have demonstrated fibrocytes in these lesions 21,38. The first evidence for fibrocytes in asthma came in 2003 when Schmidt et al demonstrated that the airways of patients with asthma contained fibrocytes (characterized by CD34/pro-collagen-1a expression) and that these cells increased in number following allergen exposure. Concurrent studies in a mouse model of antigen-induced asthma demonstrated that circulating fibrocytes trafficked to the lungs of aerosolized antigen-challenged mice. Once in the lungs, fibrocytes quickly lost CD34 expression and acquired expression of α-SMA, which is consistent with the differentiation of these cells into myofibroblasts 21. Subsequent studies have found that CD34+/45+/α-SMA+ cells recovered from the bronchoalveolar lavage fluid of patients with asthma display characteristics of fibrocytes; moreover, the presence of fibrocytes correlated with airway basement membrane thickness on histologic sections 39. Circulating fibrocytes also may be associated with chronic asthma; in one study patients with chronic persistent asthma showed an increase in circulating fibrocytes enumerated by in vitro culture 39. Interestingly, the presence of increased numbers of circulating fibrocytes displayed a positive correlation with the rate of FEV1 decline. The appearance of fibrocytes in the culture of asthmatic PBMCs was attenuated by the addition of normal serum 38, thus implying that serum factors are responsible, at least in part, for the increased number of fibrocytes in the circulation of asthma patients. Increased concentrations of fibrocytes also have been detected by direct measurement of these cells in the lungs and the blood of patients with severe chronic asthma 40.
Pulmonary Fibrosis
The term “pulmonary fibrosis” encompasses a heterogeneous group of disorders characterized by progressive replacement of the lung parenchyma with collagen and ECM components. The most common form of the disease, Idiopathic Pulmonary Fibrosis (IPF), differs from most other types of the disease in both its lack of known etiology and unresponsiveness to therapy.
Intrapulmonary fibrocytes have been detected in several experimental models of lung fibrosis 9,30,35,36,41. The earliest suggestions of a contribution of blood-borne cells to fibrotic lung disease was obtained in rodent studies of mesenchymal cells derived from transplanted bone marrow 9,41. In 2004, Hashimoto et al utilized the transplantation of transgenic, green fluorescent protein (GFP) labeled whole bone marrow to demonstrate that a large percentage of collagen expressing cells in the bleomycin damaged lung was derived from a bone marrow source, presumably fibrocytes 35. Follow up studies further showed that the accumulation of CD45/Col-1+ fibrocytes was mediated by specific chemokine pathways, including SDF-1/CXCL4 in a bleomycin lung injury model 30 and CCR2/5/MCP-1 in an inhaled fluorescein isothiocyante (FITC) model 36. In both of these studies, interruption of the chemokine axis attenuated both fibrocyte accumulation and pulmonary fibrosis.
Several independent research groups now have identified fibrocytes in different forms of fibrotic human lung disease. Reliable methodologies have been developed, and an increase in the absolute quantity or percentage of fibrocytes has been reported 16,17. In an initial study, circulating fibrocytes expressed CXCR4 and both lung and plasma levels of CXCL12 were elevated in IPF patients 16. Moeller et al studied blood from patients with IPF or late stage ARDS and found that those with IPF showed increased percentages of CD45/Col-1+ve fibrocytes. Circulating fibrocyte percentages did not correlate with disease severity; however, especially high percentages were predictive of poor clinical outcome 17. This report was the first to suggest that fibrocyte measurements may be a useful biomarker in this otherwise intractable and difficult to manage disease. Immunofluorescence analysis of human lungs also has shown that the lungs of patients with IPF contain increased numbers of fibrocytes. These cells cluster in the region of fibroblastic foci and correlate with the number of such foci, which are a pathognomic finding in IPF 42. Moreover there is an accompanying increase in CXCL12 levels in the blood and BAL fluid of IPF patients who show increased numbers of intrapulmonary fibrocytes 43. While these studies provide strong support for a role of fibrocytes in the pathogenesis of IPF, it is important to note that there is evidence that additional processes, such as epithelial to mesenchymal transition 8 or the proliferation of post-embryonic fibroblasts 44, may also contribute importantly to the progression of lung parenchymal fibrosis.
Fibrocytes in Skin Disease
An association between fibrocytes and aberrant wound healing has been described in the studies of Tredgett and colleagues 19. Murine experiments using subcutaneously implanted wound chambers demonstrated a rapid and robust recruitment of CD34+ vimentin + Col-1+ fibrocytes during the initial inflammatory phase of cutaneous injury 13. A time-dependent increase in fibrocytes has been noted in multiple forms of human wounds, with scant fibrocytes detected prior to 3 days but a linear increase noted thereafter 45. Fibrocytes are found at the expanding edge of hypertrophic scars and keloids, and their expression of CD34 decreases over time 46,47. High levels of fibrocytes also have been reported in the circulation of burn patients 48 and it has been hypothesized that these cells regulate the activity of surrounding fibroblasts through their secretion of TGF-β1 49. In burn injury, fibrocyte appearance in wounds tends to peak approximately 33 weeks post injury 50. Fibrocytes also have been identified in the skin of patients with cutaneous fibrosing diseases such as scleroderma and nephogenic systemic fibrosis, as described further below.
Fibrocytes in Scleroderma and Nephrogenic Systemic Fibrosis
Scleroderma is an autoimmune connective tissue disease characterized by progressive dermal and visceral fibrosis. Scleroderma skin contains increased numbers of connective tissue cells, and a role for fibrocytes in this disorder has been an attractive hypothesis. An early human study examining the presence of CD34+/proline-4-hydroxylase-positive fibrocytes in the skin lesions of scleroderma patients found scant fibrocytes compared to normal controls [reference 52 and unpublished data]. If fibrocytes are involved in the cutaneous manifestations of scleroderma, then these data suggest either a rapid downregulation of CD34 or the selective in situ depletion of this cell type. It also is possible that fibrocytes play a role in the maintenance of normal skin homeostasis and that their activation, differentiation, or loss promotes the accelerated remodeling seen in SSc.
Serum from patients with scleroderma also promotes fibrocyte outgrowth in vitro. This propensity was found in some patients to be associated with a low concentration of SAP 25. Our own recent work demonstrates that, in accord with what has been reported in IPF, the blood of scleroderma patients with ILD contains increased concentrations of CD45/pro-Col-1+ fibrocytes. This elevation in circulating fibrocytes also is accompanied by increased levels of MCP-1 (Mathai et al, submitted). Moreover, in one reported study of patients with limited scleroderma, which is a clinical subtype in which internal organ involvement is spared, an increase in circulating fibrocytes was not detected 51. Further work is needed to define the relationship between soluble mediators and fibrocytes, and to determine whether this association may hold prognostic significance for scleroderma patients at risk for developing lung disease, which has become the most frequent cause of mortality in this disease.
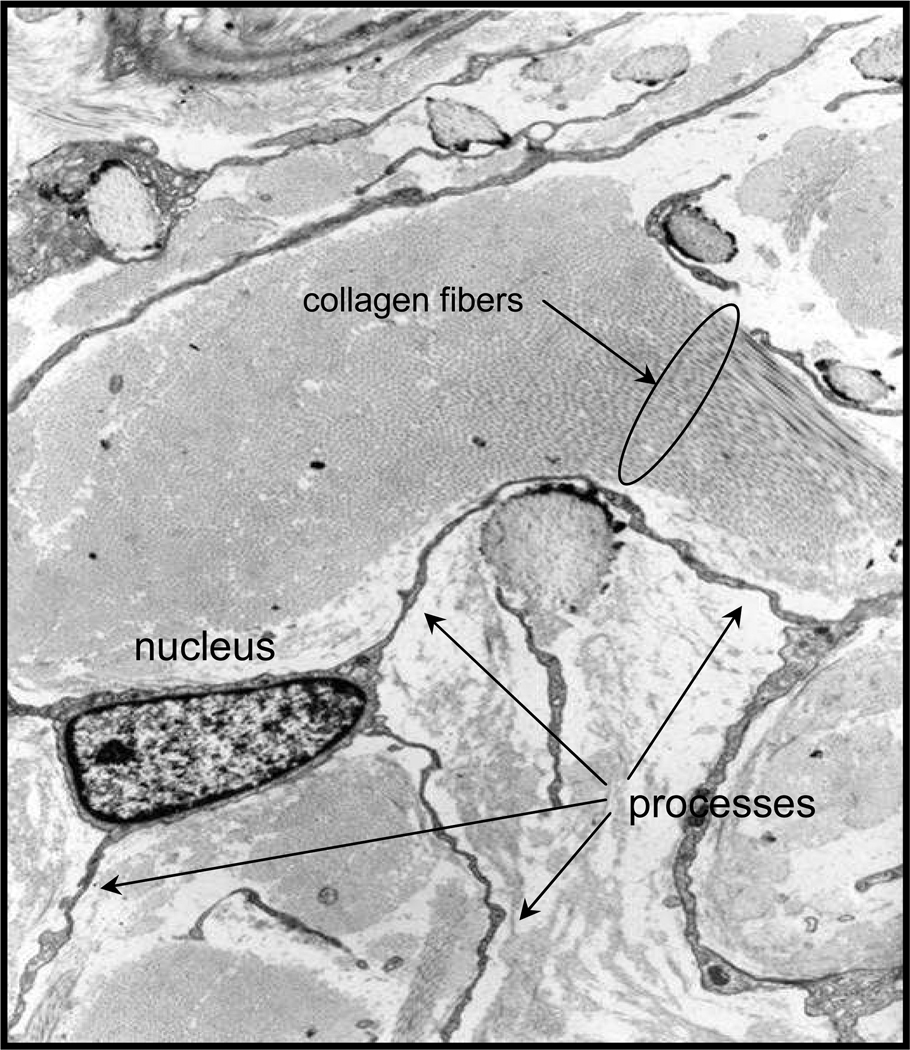
Nephrogenic systemic fibrosis (NSF) is a recently described cutaneous fibrosing disorder that exhibits pathologic similarities with scleroderma but occurs exclusively in patients with renal insufficiency who have received gadolinium containing magnetic resonance contrast agents 52. Skin biopsies from patients with this disease have revealed a striking accumulation of CD34, pro-Col-1+ fibrocytes in the dermis with abundant collagen and connective tissue matrix production 2,53 (Fig. 1). In vitro studies have revealed that gadolinium may decrease the ability of endogenous mediators such as SAP and IL-12 to inhibit fibrocyte outgrowth. In addition, monocytes from some patients with NSF demonstrate a gadolinium-induced proclivity to fibrocyte differentiation that is resistant to the regulatory effects of SAP 54. Whether this property is an intrinsic feature of fibrocytes from those patients destined to develop NSF, or reflects an ongoing disease process remains to be determined. The reason for why fibrocytes are present in such high numbers and are such a prominent feature of the dermatopathology of NSF but not scleroderma remains unclear, but may be due to the acute and abrupt development of skin fibrosis in NSF, or to a greater role for autoimmune pathways in scleroderma.
Figure 1.
Electron micrograph of a fibrocyte in the dermis of a patient with nephrogenic systemic fibrosis (image provided by Shawn Cowper, MD).
Fibrocytes in Cardiac Disease
A number of studies have supported the contribution of bone marrow progenitor cells to myocardial remodeling after ischemic injury. Haudek et al used repetitive ischemia reperfusion events to induce global left ventricular dysfunction in mice transplanted with Lac-Z+ bone marrow. β-Galactosidase staining demonstrated a robust contribution of Lac-Z+/ CD34+/αSMA+/Col-1+ fibrocytes to the remodeled left ventricle. A high level of circulating MCP-1, which engages the fibrocyte CCR2 receptor, also was observed. Interestingly, pretreatment of the mice with SAP attenuated fibrocyte accumulation and remodeling, thus suggesting a regulatory role for this acute phase protein in the myocardial response to injury 55. Fibrocytes also have been postulated to contribute to familial hypertrophic obstructive cardiomyopathy but there is little mechanistic evidence at present to support this hypothesis 56. CD34+ fibrocyte-like cells are detectable in normal mitral valves. Gross morphologic abnormalities of fibrocytes in the myxomatous mitral valve have been reported 57, suggesting an involvement of fibrocytes in the maintenance or degeneration of mitral valve anatomy.
Fibrocytes in Liver Fibrosis
The final common pathway for most forms of liver injury, whether infectious, autoimmune, or toxin-induced, terminates with the accumulation of type I collagen and the obliteration of normal liver architecture by fibrosis. This process involves activated myofibroblasts that arise from local fibroblast precursors such stellate cells and portal fibroblasts 58, and a minor contribution of hepatocyte to fibroblast transition 59. Fibrocytes also contribute to hepatic fibrosis, at least in part, as bone marrow transplantation studies demonstrate that bone marrow derived fibrocytes which express CD45 and Col-1 appear in the recipient liver. Most of these cells do not co-localize with α-SMA in vivo, however, so their contribution to hepatic myofibroblast accumulation remains unclear 34. Interestingly, an examination of liver tissue obtained from human bone marrow transplants with cirrhosis revealed that >10% of α-SMA expressing cells are of donor origin, indicating a bone marrow source for hepatic myofibroblasts. These cells did not meet strict immunochemical criteria for fibrocytes (CD45 expression) but their donor origin suggests a role for bone marrow derived cells 60.
Fibrocytes in Renal Fibrosis
As in other organ specific fibroses, the contribution of fibrocytes to kidney repair and remodeling is an ongoing field of investigation. Chronic kidney disease is a major public health burden that results from a variety of acute or chronic conditions. It is characterized pathologically by glomerulosclerosis and interstitial fibrosis with increased leukocytes and ECM accumulation 61. Animal modeling studies also have implicated circulating fibrocytes in these processes. In a model of unilateral ureteral obstruction (UUO), fibrocytes appeared in injured parenchyma in a time dependent fashion and the accumulation of these cells in renal tissue was inhibited by blockade of the CCR7/SLC axis. This intervention also decreased fibrosis 37. Fibrocytes also have been identified in a model of renal ischemia-reperfusion injury and up to 20% of the myofibroblast pool identified 28 days after injury was of bone marrow origin 62. While interventions that affect fibrocyte recruitment or accumulation are ameliorative in renal fibrosis, the precise mechanisms by which fibrocytes promote renal fibrosis are not clear. Bone marrow transplantation studies using either Lac-Z 63 or GFP labeled bone marrow 33 followed by kidney injury found that fibrocyte infiltration does not co-localize with collagen deposition, suggesting that other cell types are the predominant source of collagen in this model. Conclusions from the bone marrow transfer studies are necessarily qualified by the experimental limitations of such models; for instance transgenes may sometimes fail to identify donor derived cells 64. Alternatively, fibrocytes may infiltrate sites of tissue injury but contribute indirectly to collagen production, perhaps by inducing the recruitment or activation of other connective tissue cell types. There is also histologic evidence for fibrocytes in the tubulointerstitial lesions of patients with chronic kidney disease 65; however, their pathologic significance remains to be clarified.
Fibrocytes in Aging
Most work examining fibrocytes has investigated their role in different fibrosing disorders. However two recent studies show that circulating fibrocytes also increase in number during normal aging. One study, which used a murine model of accelerated aging (senescence-accelerated prone mice) found increased levels of CXCR-expressing fibrocytes in the blood of these mice when compared to wild type controls. The senescence-prone mice also displayed increased lung fibrosis when exposed to intratracheal bleomycin suggesting that the increased fibrocytes contributed to disease 66. Our own work demonstrates that the blood of healthy aged individuals contain increased concentrations of CD45+/Col-1+ fibrocytes and high circulating levels of MCP-1 and IL-13 (Mathai et al, submitted). Whether such an increase in circulating fibrocytes is due to an increase in the efflux of fibrocytes from the circulation or to an elevated expression of chemokine recruitment signals from aged or injured but clinically silent tissue sites remains to determined. These data suggest that fibrocytes may be associated with certain aging phenomena. Fibrocytes may have a physiologic role in the maintenance of tissue integrity or a pathologic role in the development of age-associated sequelae.
Fibrocyte T Cell- Interactions and Immune Mediated Fibrosis
While it was reported some years ago that a monocyte to fibrocyte transition is facilitated by contact with lymphocytes 15, a specific mechanism has been defined only recently. Studies in a murine model of UUO demonstrate that Gr-1+, CD11b+ monocytes require direct contact with naïve CD4+ T cells to differentiate into collagen expressing fibrocytes. This transition is inhibited by T cell activation both in vitro and in vivo. Furthermore, treatment with calcineurin inhibitors promoted fibrocyte differentiation and tissue fibrosis while treatment with IL-2 and TNF significantly reduced these outcomes 27. This study raises the possibility that lymphocytes play a critical role in the pathogenesis of different fibrosing disorders by modulating fibrocyte function in situ. Given the emerging role of regulatory T cells (T regs) in the development of such diverse diseases as asthma 67, IPF 68, and renal fibrosis 69 it is intriguing to speculate that specific T cell subpopulations regulate fibrocyte outgrowth from monocytes. These investigations add an important new dimension to studies of the pathogenesis and maintenance of the fibrotic response that could have both preventative and therapeutic implications for immune mediated fibrosis.
PROSPECTS FOR THERAPEUTIC INTERVENTION
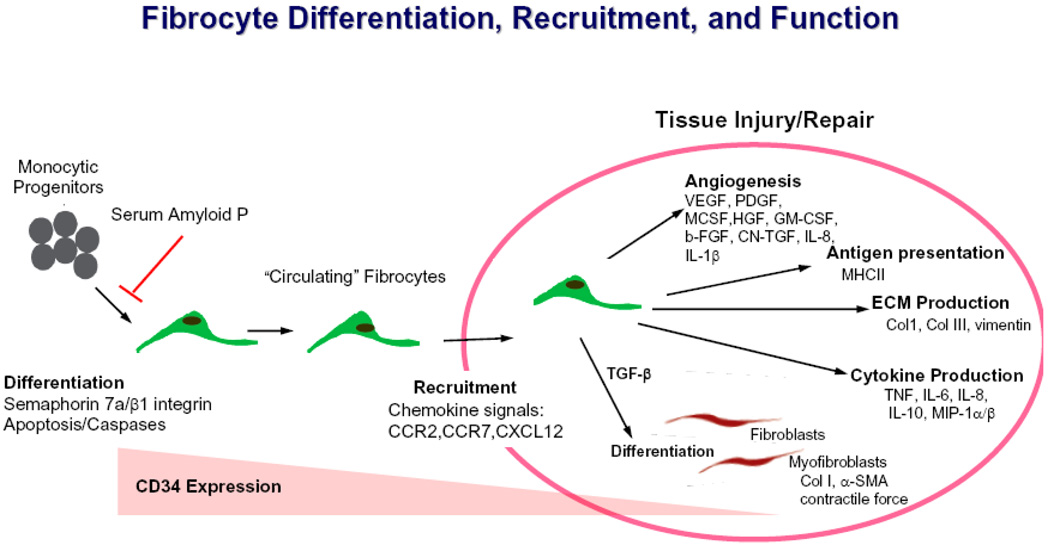
Fibrocyte directed therapies may target several checkpoints including differentiation, trafficking, and function (Fig. 2). SAP, which inhibits fibrocyte outgrowth in vitro and fibrocyte accumulation in vivo, has shown encouraging results in preclinical studies of lung, heart, and renal fibrosis and a phase I clinical trial for the prevention of corneal scarring has commenced (M. Lupher, personal communication). Modulation of the PI3 kinase-mTOR pathway, as supported by the studies of Neidermeier et al27, may have therapeutic potential as may approaches that target the Semaphorin-7a-β1 integrin axis and/or caspase activation. Interventions that target the receptors and chemokines mediating fibrocyte trafficking and accumulation such as MCP-1, SLC and SDF-1 also may be beneficial in selected disorders. Finally, further delineation of the regulatory role of T cells and the adaptive immune response in fibrocyte biology may lead to novel forms of immunotherapy targeting the lymphocyte-fibrocyte interaction. Given the lack of therapeutic options currently available for the treatment of systemic or organ specific fibrosis, it is hoped that such approaches ultimately find clinical utility in these progressive and inexorable disorders.
Figure 2.
Fibrocyte origin, differentiation, migration and function.
CONCLUSION
Fibrocytes are mesenchymal progenitor cells that have been shown to contribute to many forms of tissue fibrosis in both experimental and clinical settings. They have the potential to function as biomarkers or even therapeutic targets in human disease. Investigation of how chemokines, semaphorin 7a, and apoptosis promote fibrocyte accumulation may lead to novel therapies for such diverse diseases as IPF, scleroderma, and asthma. While fibrocytes may be considered a component of the innate response to tissue injury, the regulatory role of T cells in fibrocyte differentiation is an emerging area warranting further investigation. Further understanding of the factors that control fibrocyte biology may unveil new opportunities for the therapeutic manipulation of these cells in fibrotic disease.
Acknowledgements
K08 HL079066, Edward Mallinckrodt, Jr, Scholar Award (Herzog); Funds from the Department of Internal Medicine (Herzog and Bucala)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure:
Dr. Bucala is on the Scientific Advisory Board of Promedior, Inc., which is developing agents to inhibit fibrosis.
REFERENCES
- 1.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CG, Homer RJ, Cohn L, et al. Transgenic overexpression of interleukin (IL)-10 in the lung causes mucus metaplasia, tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J Biol Chem. 2002;277:35466–35474. doi: 10.1074/jbc.M206395200. [DOI] [PubMed] [Google Scholar]
- 3.Noble PW, Homer RJ. Idiopathic pulmonary fibrosis: new insights into pathogenesis. Clin Chest Med. 2004;25:749–758. vii. doi: 10.1016/j.ccm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 5.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47:C7–C12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 6.Tuan TL, Nichter LS. The molecular basis of keloid and hypertrophic scar formation. Mol Med Today. 1998;4:19–24. doi: 10.1016/S1357-4310(97)80541-2. [DOI] [PubMed] [Google Scholar]
- 7.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Kim KK, Wei Y, Szekeres C, et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira RF, Halford KW, O'Hara MD, et al. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohnheim J. Ueber Entzundung und Eiterung (About inflammation and suppuration) Path Anat Physiol Klin Med. 1867;40:1–79. [Google Scholar]
- 11.Fischer AC. Transformation outside of organism of momonuclears into fibroblasts. Compt rend Soc de biol. 1925;92:109–112. [Google Scholar]
- 12.Maximow A. Cultures of blood leucocytes. From lymphocyte and monocyte to connective tissue. Arch exp Zellforsch. 1928;5:169–268. [Google Scholar]
- 13.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 14.Medbury H. Role of fibrocytes in atherogenesis. In: Bucala R, editor. Fibrocytes: New Insights Into Tissue Repair and Systemic Fibroses. Singapore: World Scientific Publishing Co. Pte. Ltd; 2007. [Google Scholar]
- 15.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 16.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 17.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, et al. Circulating fibrocytes are an indicator for poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 18.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L, Scott PG, Dodd C, Medina A, Jiao H, Shankowsky HA, et al. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen. 2005;13:398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- 20.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 22.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- 23.Hartlapp I, Abe R, Saeed RW, et al. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J. 2001;15:2215–2224. doi: 10.1096/fj.01-0049com. [DOI] [PubMed] [Google Scholar]
- 24.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A. 1997;94:6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171:5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niedermeier M, Reich B, Rodriguez Gomez M, et al. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci U S A. 2009;106:17892–17897. doi: 10.1073/pnas.0906070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol. 2009;41:1708–1718. doi: 10.1016/j.biocel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakai N, Wada T, Matsushima K, et al. The renin-angiotensin system contributes to renal fibrosis through regulation of fibrocytes. J Hypertens. 2008;26:780–790. doi: 10.1097/HJH.0b013e3282f3e9e6. [DOI] [PubMed] [Google Scholar]
- 30.Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol. 2007;82:449–456. doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- 33.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kisseleva T, Uchinami H, Feirt N, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakai N, Wada T, Yokoyama H, et al. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci U S A. 2006;103:14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang CH, Huang CD, Lin HC, et al. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med. 2008;178:583–591. doi: 10.1164/rccm.200710-1557OC. [DOI] [PubMed] [Google Scholar]
- 39.Nihlberg K, Larsen K, Hultgårdh-Nilsson A, Malmström A, Bjermer L, Westergren-Thorsson G. Tissue fibrocytes in patients with mild asthma: a possible link to thickness of reticular basement membrane? Respir Res. 2006;7:50. doi: 10.1186/1465-9921-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saunders R, Siddiqui S, Kaur D, et al. Fibrocyte localization to the airway smooth muscle is a feature of asthma. J Allergy Clin Immunol. 2009;123:376–384. doi: 10.1016/j.jaci.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 43.Andersson-Sjöland A, de Alba CG, Nihlberg K, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishida Y, Kimura A, Takayasu T, Eisenmenger W, Kondo T. Detection of fibrocytes in human skin wounds and its application for wound age determination. Int J Legal Med. 2009;123:299–304. doi: 10.1007/s00414-009-0320-4. [DOI] [PubMed] [Google Scholar]
- 46.Aiba S, Tagami H. Phorbol 12-myristate 13-acetate can transform monocyte-derived dendritic cells to different cell types similar to those found in dermatofibroma. A possible in vitro model of the histogenesis of dermatofibroma. J Cutan Pathol. 1998;25:65–71. doi: 10.1111/j.1600-0560.1998.tb01692.x. [DOI] [PubMed] [Google Scholar]
- 47.Aiba S, Tagami H. Inverse correlation between CD34 expression and proline-4-hydroxylase immunoreactivity on spindle cells noted in hypertrophic scars and keloids. J Cutan Pathol. 1997;24:65–69. doi: 10.1111/j.1600-0560.1997.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Scott PG, Giuffre J, Shankowsky HA, Ghahary A, Tredget EE. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82:1183–1192. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 49.Wang JF, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15:113–121. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 50.Yau FM, Scott PG, Ghahary A, et al. Peripheral blood fibrocytes and scar formation. UAHSJ. 2005;2:4–7. [Google Scholar]
- 51.Russo R, Medbury H, Guiffre A, Englert H, Manolios N. Lack of increased expression of cell surface markers for circulating fibrocyte progenitors in limited scleroderma. Clin Rheumatol. 2007;26:1136–1141. doi: 10.1007/s10067-006-0461-5. [DOI] [PubMed] [Google Scholar]
- 52.Cowper SE, Bucala R, LeBoit PE. Case 35–2004: nephrogenic fibrosing dermopathy. N Engl J Med. 2005;352:1723–1724. Author reply 1723–1724. [PubMed] [Google Scholar]
- 53.Cowper SE, Bucala R. Nephrogenic fibrosing dermopathy: suspect identified, motive unclear. Am J Dermatopathol. 2003;25:358. doi: 10.1097/00000372-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 54.Vakil V, Sung JJ, Piecychna M, et al. Gadolinium-containing magnetic resonance image contrast agent promotes fibrocyte differentiation. J Magn Reson Imaging. 2009;30:1284–1288. doi: 10.1002/jmri.21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haudek SB, Xia Y, Huebener P, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberts R, Sigwart U. Current concepts of the pathogenesis and treatment of hypertrophic cardiomyopathy. Circulation. 2005;112:293–296. doi: 10.1161/01.CIR.0000146788.30724.0A. [DOI] [PubMed] [Google Scholar]
- 57.Barth PJ, Koster H, Moosdorf R. CD34+ fibrocytes in normal mitral valves and myxomatous mitral valve degeneration. Pathol Res Pract. 2005;201:301–304. doi: 10.1016/j.prp.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Guyot C, Lepreux S, Combe C, et al. Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int J Biochem Cell Biol. 2006;38:135–151. doi: 10.1016/j.biocel.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 59.Zeisberg M, Yang C, Martino M, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–23347. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 60.Forbes SJ, Russo FP, Rey V, et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–9639. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 61.Schoolwerth AC, Engelgau MM, Hostetter TH, et al. Chronic kidney disease: a public health problem that needs a public health action plan. Prev Chronic Dis. 2006;3:A57. [PMC free article] [PubMed] [Google Scholar]
- 62.Broekema M, Harmsen MC, van Luyn MJ, et al. Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J Am Soc Nephrol. 2007;18:165–175. doi: 10.1681/ASN.2005070730. [DOI] [PubMed] [Google Scholar]
- 63.Roufosse C, Bou-Gharios G, Prodromidi E, et al. Bone marrow-derived cells do not contribute significantly to collagen I synthesis in a murine model of renal fibrosis. J Am Soc Nephrol. 2006;17:775–782. doi: 10.1681/ASN.2005080795. [DOI] [PubMed] [Google Scholar]
- 64.Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- 65.Okon K, Szumera A, Kuzniewski M. Are CD34+ cells found in renal interstitial fibrosis? Am J Nephrol. 2003;23:409–414. doi: 10.1159/000074298. [DOI] [PubMed] [Google Scholar]
- 66.Xu J, Gonzalez ET, Iyer SS, et al. Use of senescence-accelerated mouse model in bleomycin-induced lung injury suggests that bone marrow-derived cells can alter the outcome of lung injury in aged mice. J Gerontol A Biol Sci Med Sci. 2009;64:731–739. doi: 10.1093/gerona/glp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–746. doi: 10.1016/j.jaci.2009.02.030. quiz 747–748. [DOI] [PubMed] [Google Scholar]
- 68.Kotsianidis I, Nakou E, Bouchilou I, et al. Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:1121–1130. doi: 10.1164/rccm.200812-1936OC. [DOI] [PubMed] [Google Scholar]
- 69.Mittal SK, Sharma RK, Gupta A, Naik S. Increased interleukin-10 production without expansion of CD4+CD25+ T-regulatory cells in early stable renal transplant patients on calcineurin inhibitors. Transplantation. 2009;88:435–441. doi: 10.1097/TP.0b013e3181af20fd. [DOI] [PubMed] [Google Scholar]