Abstract
Introduction
A variable repertoire of coagulation protein expression is observed in different cancers. We evaluated expression of thrombin in prostate tissue.
Methods
Detection of thrombin was performed using quantitative real-time PCR in fresh tissue and in situ hybridization (ISH) in archival prostate tissue and by immunohistochemistry of prostate tissue microarrays.
Results
(Pro)thrombin mRNA expression was detected in cancer tissue and localized to prostatic epithelium and stroma by ISH. Thrombin protein was detected in stroma of benign and malignant epithelium (p <.05).
Conclusions
Prostate tissue is a rich reservoir of thrombin. This may have potential for developing antithrombin-based cancer therapy.
Keywords: Prostatic neoplasms, Prothrombin, Coagulation
INTRODUCTION
The role of thrombin, a trypsin-like serine protease generated fromits inactive precursor prothrombin, after enzymatic cleavage by factor Xa is critical in coagulation activation. Its generation is a central event in coagulation activation that is followed by thrombin catalyzed cross-linked insoluble fibrin formation of soluble fibrinogen. Thrombin expression and function in tumor microenvironment is heterogeneous and like other coagulation proteins also may vary according to tumor type. For example, thrombin localizationon tumor epithelium distant from stromal fibrin/fibrinogen has been observed in small cell lung cancer, renal (clear cell) cancer, and multiplemyeloma, while in adenocarcinoma lung, colon cancer, and squamous cell carcinoma of the lung expression is absent on tumor epithelium but found localized to tumor associated host macrophages (1). Thrombin’s cellular effects are pluripotent and not limited to coagulation activation alone in the tumor microenvironment, as even low thrombin concentrations result in activation of the protease-activated receptors (PARs), which are G-protein coupled transmembrane family of receptors. In the interplay between coagulation and malignancy, the cellular effects of thrombin function via PAR activation are known to include regulation of microvascular permeability by formation of interendothelial gaps, induction of mitogenesis of several cell types like smooth muscle, fibroblasts, and malignant cells and pro-inflammatory actions by the stimulation of transvascular leukocyte migration (2, 3). These effects result in thrombin associated tumor growth, progression, andmetastasis through the activation of PARs, independent of the well known procoagulant effects of thrombin.
In prostate cancer, while PAR-1 expression has been reported previously onmalignant epitheliumand appears to be stage dependent (4), expression of its ligand thrombin and/or its precursor prothrombin has not been previously evaluated and therefore any role the thrombin-PAR axis may play in prostate cancer physiology or coagulation activation remains undetermined. The purpose of the current study was to identify expression of prothrombin and thrombin in benign and malignant prostate tissue in order to hypothesize any role of this axis in prostate cancer pathophysiology and the potential to therapeutically target using novel anticoagulants as anticancer agents.
MATERIALS AND METHODS
We evaluated (pro)thrombin expression in prostate tissue using several methods including quantitative real-time PCR of fresh cancer tissue lysates for mRNA level detection, prothrombin RNA transcript localization using in situ hybridization (ISH) in randomly selected archival specimens, and finally protein detection by immunohistochemistry (IHC) in archival tissue microarrays.
Real-time PCR for prothrombin gene expression
Freshly resected tissue was collected from 18 patients diagnosed with prostate cancer in two different stages on an institutional review board approved study after obtaining informed consent (University of Arkansas for Medical Sciences). The patients with early stage cancer (n = 10) had no clinical or radiological evidence of regional or systemic lymph node or bone metastasis. In contrast, those with advanced stage disease (n = 8) had clinical evidence of systemic disease and were receiving androgen deprivation therapy. Of the 18 tissue specimens, 16 were available with adequate tissue for prothrombin gene expression studies. Nine patients with early stage prostate cancer undergoing radical prostatectomy (RP) and seven patients with advanced stage cancer undergoing transurethral resection (TURP) for the bladder outlet obstruction consented to participate in tissue collection. The fresh tissue was snap frozen in liquid nitrogen and processed total RNA extraction as described previously (5, 6). Quantitative real-time PCR was performed as described previously using cDNA synthesized from total RNA (4). The primers sequences used were based on thrombin mRNA (5′-TGGAGGACAAAACCGAAAGAGA-3′ and 5′-CATCCGAGCCCTCCACAA-3′) and 18s rRNA (5′-TTCGGACGTCTGCCCTATCAA-3′ and 5′-ATGGTAGGCACGGCGACTA-3′). For thrombin and 18s respectively, the specific PCR products displayed melting temperatures of 80.2°C and 78.5°C, assays were optimized at primer concentrations of 110 nM and 300 nM resulting in 93% and 100% amplification efficiency, and relative gene expression was measured for each sample using 16 ng and 1 ng RNA equivalents of cDNA. Expression was using standard curves (Log of the ng RNA-equivalents of cDNA versus cycle number) generated from four-fold serial dilutions of pooled sample cDNAs. Thrombin data was normalized to 18s.
In situ methods
ISH for prothrombin transcripts was performed on paraffin embedded archival prostate tissue specimen set. Total RNA was isolated from human liver tissue and reversed transcribed using random primers and transcriptor reverse transcriptase (Roche North America, USA). A 283 base-pair segment corresponding to nucleotides 834–1116 of the prothrombin cDNA, Genbank accession NM_000506 was amplified from the cDNA using GoTaq DNA polymerase. Primer sequences, ATGAGGAGGGCGTGTGGTGCTATGT and CCGTCGATGTAGGATTCCAGGAGC were those used by Arai et al. (2006) for RT-PCR of the prothrombin cDNA. The product was cloned into the pCR2.1-TOPO (Clontech, CA, USA) plasmid according to the manufacturer’s instructions. Plasmid DNA with prothrombin cDNA inserts in sense and antisense orientations were selected for generating sense and antisense in situ hybridrization probes. Plasmids were linearized by digesting with BamH1 restriction endonuclease and RNA synthesized using T7 RNA polymerase in the presence of digoxigenin labeled UTP. The probes were titered, and incorporation of the digoxigenin label was verified by spotting a serial dilution on nitrocellulose membrane, probing with antidigoxigenin antibody labeled with alkaline phosphatase and detection with chemiluminescent substrate. All aqueous solutions used in the ISH proecedure were prepared with nuclease-free water. Paraffin embedded tissues were sectioned at 5-µm thickness and floated on distilled water at 45°C. Sections were mounted on charged slides followed by drying at room temperature until opaque and placed in the oven at 57°C overnight. Sections were deparaffinized in two changes of fresh xylene for 30 min, rehydrated in ethanol to 70% followed by two changes of water, and two changes of phosphate buffered saline (PBS) for 5 min. The slides were incubated in proteinase K solution, 10 µg/ml in TE buffer (100 mM Tris-HCl, 50 mM EDTA, pH 8.0) for 5 min at 37°C then rinsed with PBS. Tissues were postfixed in fresh 4% paraformaldehyde for 5 min at 4°C, rinsed in PBS and then acetylated in 0.25% acetic anhydride in 0.1 M triethanolamine, pH 8.0 with constant stirring for 10 min. Sections were incubated in a humid chamber with prehybridization solution, 4× SSC in 50% formamide at 37°C for 10 min. Sufficient hybridization solution (40% formamide, 10% dextran sulfate, 1× Denhardts solution, 4× SSC, 10 mM dithiothreitol, 1 mg/ml yeast t-RNA, and 1 mg/ml sheared salmon sperm DNA) with sense or antisense probe was applied to each section, sealed under a glass coverslip with rubber cement, and incubated overnight at 42°C. Probes were titered on control slides to a final dilution of 1–40. Following hybridization, slides were washed in 2× SSC for 15 min, two changes each of 1× SSC (15 min), and 0.1× SSC (30 min) all at 50°C.
Detection of bound probe began with two rinses of 100 mM Tris-HCl, pH 7.5, for 10 min. Slides were blocked for 30 min with 1% normal rabbit serum, and then incubated for 2 hr in a humid chamber with antidigoxigenin–alkaline phosphatase in Tris-HCl with 0.1% Triton x-100 (diluted 1:100). Unbound antibody was removed by washing twice in 100 mM Tris-HCl for 10 min and then two washes with Tris-HCL, pH 9.5, for 10 min. NBT/BCIP substrate was applied to the slides and incubated at room temperature for 20 min. The reaction was stopped in 100 mM Tris-HCl with 50 mM EDTA, pH8.1. Slides were rinsed in distilled water and counterstained with nuclear fast red (7).
Immunohistochemistry
For thrombin and prothrombin protein expression by IHC, a separate archival tissue microarray was constructed from 256 prostate tissue specimens, representing benign prostatic hyperplasia (BPH, n = 117), prostate intraepithelial neoplasia (PIN) (n = 27), early stage disease (n = 69) and advanced-stage prostate cancer (n = 112) was used. Some slides did not contain all cores within each group but no more than n = 1 were missing from any given study herein. IHC was performed on formalin-fixed, paraffin-embedded tissue sections using a rabbit polyclonal antibody against antihuman thrombin (American Diagnostica Inc., Stanford, CT 06911, Cat. No. 4702, used at 1:1–400) and a sheep polyclonal against antihuman prothrombin (US Biological, Swampscott, MA 01907, Cat. No. P9115–06, used at 1:800). Paraffin embedded tissues were sectioned at 5 mm thickness and floated on distilled water at 45°C. Sections were mounted on chemically charged slides followed by drying at room temperature until opaque and placed in the oven at 57°C overnight. Sections were deparaffinized according to established procedures and quenched with 3% hydrogen peroxide for 6 min. They were then cleared in running water followed by Tris-buffered saline (TBS) (50 mM Tris-hydrogen chloride, 150 mM sodium chloride, and 0.05% Tween 20 at pH 7.6).
Slides were then rinsed with TBS for 5 min and mounted in the DAKO Autostainer. Slides were covered with fresh TBS to prevent drying of sections during mounting. Sections were digested with proteinase K (Dako, Carpinteria, CA) for 4 min followed by a TBS rinse. The sections were incubated with the primary antibodies at room temperature for 60 min. Followed by a 30-minute incubation in either goat antirabbit IgG-Biotin (Thrombin) (Vector Laboratories, Inc., Burlingame, Ca) or rabbit antisheep IgG-Biotin (Prothrombin) (Vector Laboratories, Inc.) and both are followed by 30-minute incubation with Streptavidin-HRP (Jackson Labs). Slides were developed with AEC+ (Dako) for 10 min, rinsed in running distilled water, counterstained in Modified Mayer’s Hematoxylin, blued in 0.3% ammonia water followed by a tap water rinse. Slides were mounted using an aqueous media and viewed with a light microscope.
Immunohistochemical scoring and statistical methods
Immunohistochemical scoring was performed using the German immunoreactive score as described previously (8), which is calculated by combining the percentage of immunoreactive cells (quantity score) with an estimate of the staining intensity (staining intensity score) as follows: no staining is scored as 0, 1–10% of cells stained scored as 1, 11%–50% as 2, 51%–80% as 3, and 81%–100% as 4. Staining intensity was rated on a scale of 0–3, with 0 = negative, 1 = weak, 2 = moderate, and 3 = strong. The raw data are converted to the immunoreactive score (IS) by multiplying the quantity and staining intensity scores. Cores with a score of IS of 0 or 1 are considered negative and those with IS ≥2 are considered positive. Each core was examined under a light microscope and separately scored. Cores that had <50% of designated tissue present were disregarded.
Thrombin localization and expression were analyzed using Fisher’s exact test to determine statistically significant difference between benign hyperplasia, PIN and prostate cancer.
RESULTS
Immunolocalization of prothrombin and thrombin by IHC in prostate tissue
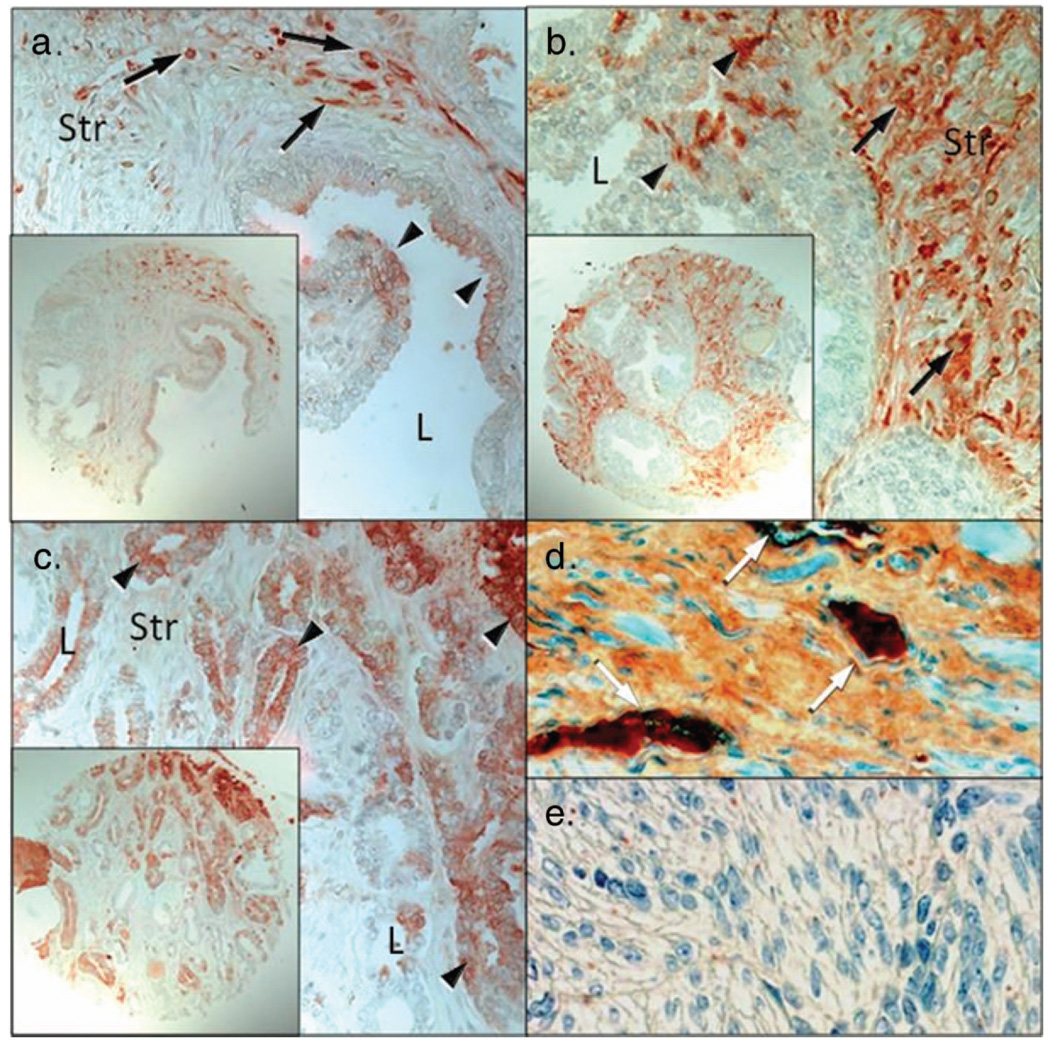
Prothrombin and thrombin immunoreactivity was observed in benign and malignant prostate tissue with stromal thrombin expression observed in 84 of 116 benign cores, 25 of 27 PIN samples, and 89 of 112 carcinoma cores. Within the prostatic stroma, from all groups evaluated there were numerous cells positive for thrombin (Table 1; no cells are mentioned in Table 1). Thrombin protein expression was observed in benign (normal and BPH), PIN, and malignant prostate specimens (Figure 1). Stromal staining appeared to be more intense in PIN lesions when compared with the staining of benign and malignant glands. Little thrombin was localized to prostatic epithelial cells from benign and PIN specimens, however increased epithelial staining was observed in malignant specimens [Figure 1(c)]. Thrombin protein was also observed within endothelial cells of the prostatic vasculature [Figure 1(d)]. No immunostaining was detected when thrombin protein was added to the antibody prior to incubation on the tissue (negative control, data not shown) or in uterine stroma (negative control).
Table 1.
Summary of thrombin expression in human prostate tissues
| Thrombin IHC | Benign | Stroma PIN |
Carcinoma | Benign | Epithelium PIN |
Carcinoma |
|---|---|---|---|---|---|---|
| % positive cores | 72 | 93* | 79 | 35 | 50 | 42 |
| Number of cores | 117 | 27 | 112 | 117 | 26 | 112 |
p <.05 for percentage of positive PIN cores compared with percentage of positive cores in benign or carcinoma.
Figure 1.
Immunohistochemical localization of thrombin in normal prostate, prostatic intraepithelial neoplasia, and prostate cancer specimens. (a) Thrombin protein was localized primarily to prostatic stroma (Str) with little thrombin localized to the epithelium (arrowheads). Arrows denote intense stromal cell positivity within a stromal cell. (b) Localization of thrombin in PIN samples demonstrated increased expression within the stromal layer and little thrombin expression was observed within the epithelium (arrowheads). (c) Localization of thrombin in prostate adenocarcinoma. Prostatic carcinoma cells (arrowheads) whereas stromal cells were diffuse and of moderate intensities for thrombin. (d) prostate specinmen containing thrombin rich vascular (white arrows; positive control). (e) Uterine smooth muscle serves as a negative control (L = lumen; Str = stroma; Bar = 50 µm; insets are at high magnification).
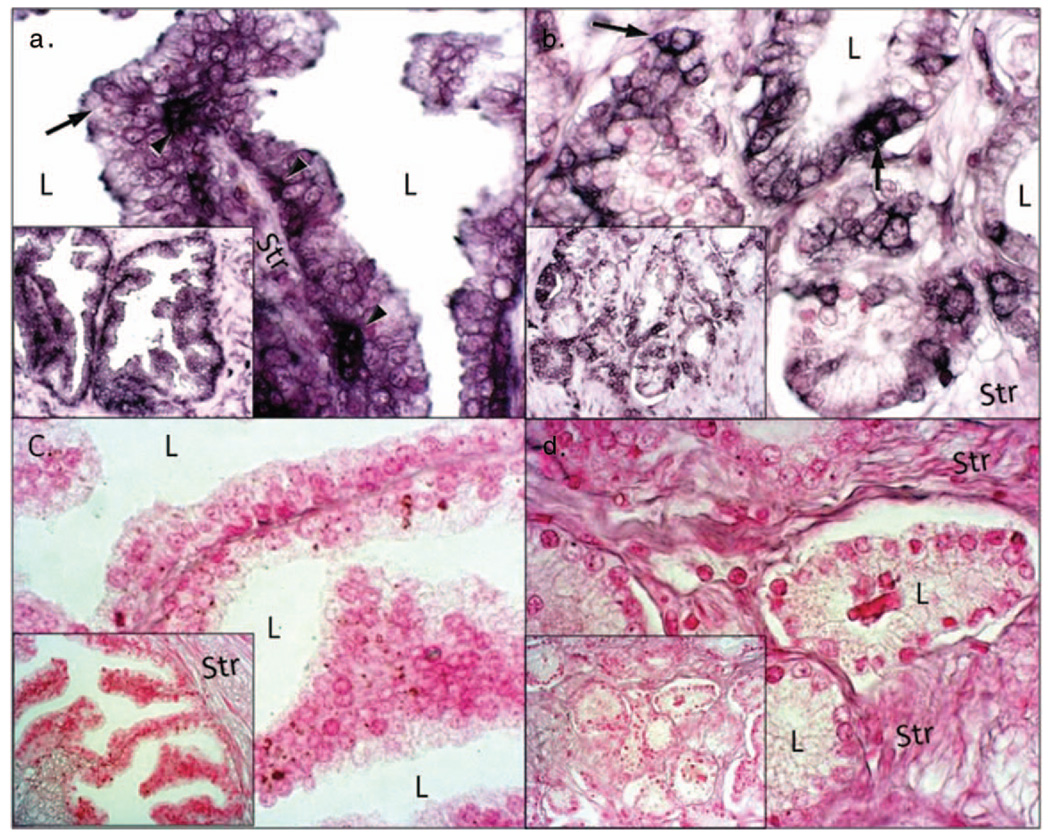
In situ hybridization histochemistry of prothrombin
ISH studies detected prothrombin mRNA to be localized to both the prostatic epithelium and stroma [Figures 2(a)–(c)]. Strong prothrombin mRNA staining was observed with use of antisense probes, whereas no staining was observed with the sense probe. Prothrombin mRNA was present within both the basal and luminal epithelial cells in the benign tissue, and especially strong staining was observed within the basal cell layer. Stromal tissue immediately adjacent to the tumor cells displayed decreased mRNA signal than that observed in epithelium of benign and tumor tissue indicating prostate epithelium as a source for prothrombin.
Figure 2.
Detection of thrombin mRNA using in situ hybridization. We evaluated the presence of thrombin mRNA in () benign prostate (a, c) and prostate cancer (b, d). Antisense probe for thrombin (a, b) and sense probes (c, d) for thrombin. Note localization of thrombin RNA (dark purple color) in epithelial cells (arrows) and stroma (str) as well as intense expression of thrombin mRNA in basal layer (arrowheads). Also note thrombin RNA localization in cancerous prostate tissue, especially within the epithelia (arrows). Additionally note that only counterstain (pink color) is observed in sense strand negative controls (L = lumen, Str = stroma; Bar = 50 µm; insets are at high magnification).
Quantitative PCR for thrombin gene expression
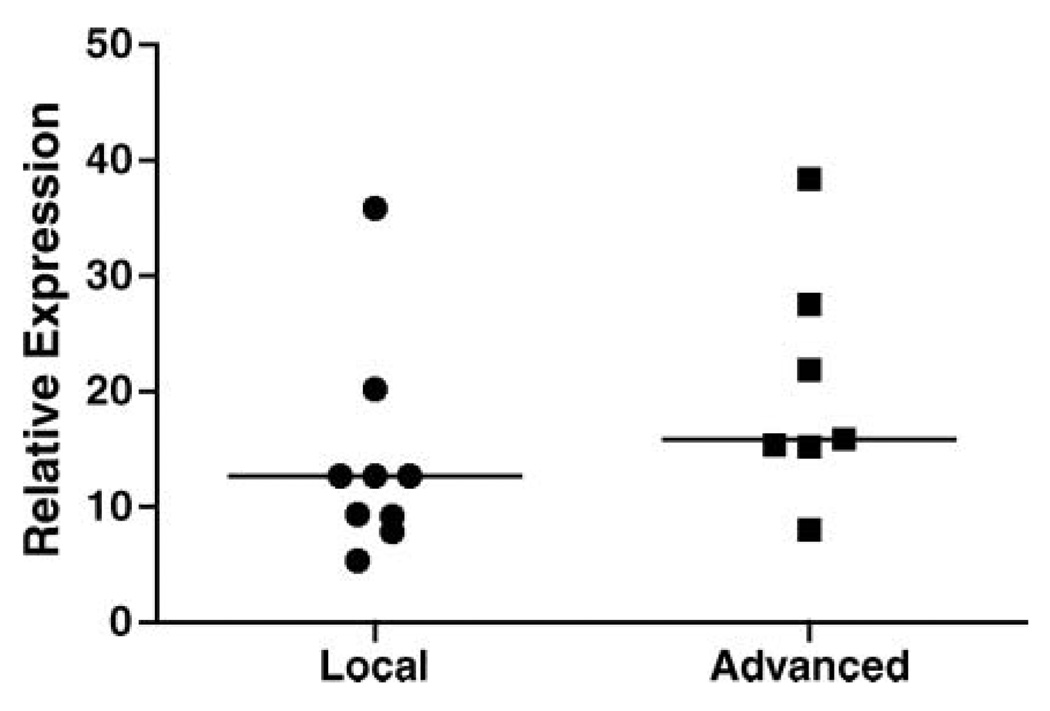
To determine thrombin gene expression in early and hormonally treated advanced prostate cancer stage patients, QPCR was performed on freshly resected prostate tissues collected from localized and advanced stage patients. Thrombin expression was observed in all specimens of both groups and while increased normalized signal was noted in advanced stage specimens it was not significantly (p = .09) different from localized stage specimens (Figure 3).
Figure 3.
Comparison of thrombin mRNA expression between local (N = 9) and advanced (N = 7) prostate cancer specimens. Median levels (line) were not significantly different between groups (p = .09, Mann–Whitney test). Expression was measured by quantitative real-time PCR. Expression was calculated using standard curves and normalized using 18s rRNA as a control.
DISCUSSION
Thrombin’s link between a tumorigenic role and as a procoagulant has been observed in preclinical models (9) and from induction of a direct mitogenic effect on tumor cells to activation of several integrins and adhesion molecules that promote angiogenesis and/or provide a scaffolding for the growingmalignant clone by increasing fibrin deposition (10). Clinically thrombin’s role in cancer biology has been suspected on the basis of several observations. A persistent activation of prothrombin to thrombin in the general population has been found strongly associated with an increased incidence of developing cancer in previously undiagnosed cancer patients, implicating an association of thrombin with carcinogenesis (11). Cancer patients with new thromboembolic events are known to have an inferior survival compared with matched cancer populations without thrombotic events (12, 13). More recently in prospective clinical trials of cancer patients undergoing specific antithrombotic treatments increased cancer specific survivals have been observed compared with cancer patients not administered the same antithrombotic treatments suggesting an anticancer effect of these interventions (14, 15).
However, tumor cell associated with thrombin expression either in stroma or epithelium is not uniform and is a feature of some but not all tumor types (1, 16), and since its expression in prostate adenocarcinoma remains unclear, we evaluated its expression pattern in prostate tissue and found that prostate tissue is an abundant reservoir of thrombin expression. Real-time PCR for prothrombin gene detected expression in freshly excised prostate cancer tissue from patients some of whom had received hormonal therapy (n = 7). Since these tissue lysates are rich in epithelia, stroma, and a host of inflammatory cells and as prothrombin generation is known to occur from activated host macrophages/monocytes in several tumor types, we attempted to spatially localize prothrombin transcripts by performing ISH and protein by IHC in malignant and benign prostate tissue. Cellular localization patterns of the RNA transcript and protein results and thrombin protein IHC in our study confirms thrombin generation in prostatic tissue. This is suggested by the localization of RNA transcripts to both benign and malignant prostatic stromal and epithelial tissue (including early and advanced malignant stages) and thrombin protein expression on epithelium and stroma. These results renew interest in evaluating the role of specific coagulation proteases in cancer specific molecular pathways in prostate cancer as previously a lack of expression of coagulation pathway proteins in malignant prostatic tissue had been suggested. In an extensive evaluation of several coagulation factors in prostate tissue, no expression of F VII, FXa, factor XIII, protein S, and protein C was detected (17), while prothrombin–thrombin expression in prostatic tissue had not been reported. This led to generally concluding that prostate tumor cells lack expression of coagulation proteins unlike several other tumor types. Despite the early conclusions, recent studies have confirmed expression of several coagulation related proteins in prostate cancer including tissue factor as well as expression of thrombin receptor, PAR-1 (18). Strong expression of PAR-1 has been observed on malignant epithelium albeit, only in advanced stages of prostate cancer (4, 19, 20), while it is absent in benign prostate tissue with low to moderate expression observed on malignant epithelium in localized cancer stage (4, 19, 21). At a functional level direct activation of PAR-1 by thrombin enhances tumorigenesis by increased tumor cell motility and metastasis (22) and VEGF family proteins expression (23) as well as EGFR transactivation leading to cancer cell proliferation (24). Thrombin activation of PAR-1 in PC-3 cell lines results in NFκB signal transduction leading to increased tumor cell IL-6 and IL-8 production that serve as survival and proliferating factors (20). Finally, the observation that thrombin mediated PAR-1 activation interferes and abrogates docetaxel mediated cancer cell apoptosis through upregulation of BcL-xL protein expression (20) in advanced prostate cancer has profound therapeutic potential, as direct thrombin inhibition during docetaxel chemotherapy could enhance docetaxel cytotoxicty. Such a clinical strategy of using direct thrombin inhibitors as chemosensitizers of docetaxel may result in achieving enhanced response rates and/or survival of patients on docetaxel chemotherapy. These observations make it clinically relevant to evaluate the expression of not just PAR-1 in prostate cancer but also identify a functional role of the thrombin-PAR-1 axis in prostate cancer progression in advanced stages in order to target coagulation pathophysiology using novel and emerging agents such as direct thrombin inhibitors for enhancing therapeutic gain.
In summary, we report a novel finding of thrombin expression in prostate tissue not previously reported and hypothesize the implications of an active thrombin-PAR axis in advanced prostate cancer stage in therapy. The rich reservoir of thrombin in prostate cancer via PAR activation may promote tumorigenesis and possibly abrogate the effects of chemotherapies in advanced stages that should be identified in future mechanistic studies. Thrombin expression may specifically provide a potential therapeutic target in advanced prostate cancer for chemosensitization of docetaxel chemotherapy and needs to be further explored. If successful, such an intervention could serve a dual purpose of not only preventing the morbidity of venous thrombosis in prostate cancer patients, but also resulting in enhancing the effects of cancer treatments and prolonging survival when administered in combination with docetaxel chemotherapy.
ACKNOWLEDGMENTS
The study was supported by Departmental funds from Medicine and Urology, University of Rochester, NCI grant no. CA091956 (Prostate SPORE Awarded to Mayo Clinic), and Funding source, CA123199 (WAR).
The authors are grateful to Professor Charles Francis, MD, Director, Coagulation Lab, University of Rochester, NY, for editorial comments and suggestion with manuscript preparation.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article. There are no financial disclosures from any author.
REFERENCES
- 1.Zacharski LR, Memoli VA, Morain WD, Schlaeppi JM, Rousseau SM. Cellular localization of enzymatically active thrombin in intact human tissues by hirudin binding. Thromb Haemost. 1995;73(5):793–797. [PubMed] [Google Scholar]
- 2.Guttridge DC, Lau A, Tran L, Cunningham DD. Thrombin causes a marked delay in skeletal myogenesis that correlates with the delayed expression of myogenin and p21CIP1/WAF1. J Biol Chem. 1997;272(39):24117–24120. doi: 10.1074/jbc.272.39.24117. [DOI] [PubMed] [Google Scholar]
- 3.Derian CK, Eckardt AJ. Thrombin receptor-dependent prostaglandin E2 synthesis in hamster fibroblasts: synergistic interactions with interleukin-1beta. Exp Cell Res. 1997;232(1):1–7. doi: 10.1006/excr.1997.3483. [DOI] [PubMed] [Google Scholar]
- 4.Kaushal V, Kohli M, Dennis RA, Siegel ER, Chiles WW, Mukunyadzi P. Thrombin receptor expression is upregulated in prostate cancer. Prostate. 2005;66(3):273–282. doi: 10.1002/pros.20326. [DOI] [PubMed] [Google Scholar]
- 5.Kaushal V, Mukunyadzi P, Siegel ER, Dennis RA, Johnson DE, Kohli M. Expression of tissue factor in prostate cancer correlates with malignant phenotype. Appl Immunohistochem Mol Morphol. 2008;16(1):1–6. doi: 10.1097/01.pai.0000213157.94804.fc. [DOI] [PubMed] [Google Scholar]
- 6.Kaushal V, Mukunyadzi P, Dennis RA, Siegel ER, Johnson DE, Kohli M. Stage-specific characterization of the vascular endothelial growth factor axis in prostate cancer: expression of lymphangiogenic markers is associated with advanced-stage disease. Clin Cancer Res. 2005;11(2 Pt 1):584–593. [PubMed] [Google Scholar]
- 7.Arai T, Miklossy J, Klegeris A, Guo JP, McGeer PL. Thrombin and prothrombin are expressed by neurons and glial cells and accumulate in neurofibrillary tangles in Alzheimer disease brain. J Neuropathol Exp Neurol. 2006;65(1):19–25. doi: 10.1097/01.jnen.0000196133.74087.cb. [DOI] [PubMed] [Google Scholar]
- 8.Yao JL, Ryan CK, Francis CW, Kohli M, Taubman MB, Khorana AA. Tissue factor and VEGF expression in prostate carcinoma: a tissue microarray study. Cancer Invest. 2009;27(4):430–434. doi: 10.1080/07357900802527247. [DOI] [PubMed] [Google Scholar]
- 9.Boccaccio C, Sabatino G, Medico E, Girolami F, Follenzi A, Reato G, et al. The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature. 2005;434(7031):396–400. doi: 10.1038/nature03357. [DOI] [PubMed] [Google Scholar]
- 10.Xie WZ, Leibl M, Clark MR, Dohrmann P, Kunze T, Gieseler F. Activation of the coagulation system in cancerogenesis and metastasation. Biomed Pharmacother. 2005;59(3):70–75. doi: 10.1016/j.biopha.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Miller GJ, Bauer KA, Howarth DJ, Cooper JA, Humphries SE, Rosenberg RD. Increased incidence of neoplasia of the digestive tract in men with persistent activation of the coagulant pathway. J Thromb Haemost. 2004;2(12):2107–2114. doi: 10.1111/j.1538-7836.2004.01011.x. [DOI] [PubMed] [Google Scholar]
- 12.Levitan N, Dowlati A, Remick SC, Tahsildar HI, Sivinski LD, Beyth R, Rimm AA. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 1999;78(5):285–291. doi: 10.1097/00005792-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 14.Kakkar AK, Levine MN, Kadziola Z, Lemoine NR, Low V, Patel HK, Rustin G, Thomas M, Quigley M, Williamson RC. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS) J Clin Oncol. 2004;22(10):1944–1948. doi: 10.1200/JCO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Lee AY, Rickles FR, Julian JA, Gent M, Baker RI, Bowden C, Kakkar AK, Prins M, Levine MN. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol. 2005;23(10):2123–2129. doi: 10.1200/JCO.2005.03.133. [DOI] [PubMed] [Google Scholar]
- 16.Zacharski LR, Wojtukiewicz MZ, Costantini V, Ornstein DL, Memoli VA. Pathways of coagulation/fibrinolysis activation in malignancy. Semin Thromb Hemost. 1992;18(1):104–116. doi: 10.1055/s-2007-1002415. [DOI] [PubMed] [Google Scholar]
- 17.Wojtukiewicz MZ, Zacharski LR, Memoli VA, Kisiel W, Kudryk BJ, Moritz TE, Rousseau SM, Stump DC. Fibrin formation on vessel walls in hyperplastic and malignant prostate tissue. Cancer. 1991;67(5):1377–1383. doi: 10.1002/1097-0142(19910301)67:5<1377::aid-cncr2820670517>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Kaushal V, Kohli M, Dennis RA, Siegel ER, Chiles WW, Mukunyadzi P. Thrombin receptor expression is upregulated in prostate cancer. Prostate. 2006;66(3):273–282. doi: 10.1002/pros.20326. [DOI] [PubMed] [Google Scholar]
- 19.Chay CH, Cooper CR, Gendernalik JD, Dhanasekaran SM, Chinnaiyan AM, Rubin MA, Schmaier AH, Pienta KJ. A functional thrombin receptor (PAR1) is expressed on bone-derived prostate cancer cell lines. Urology. 2002;60(5):760–765. doi: 10.1016/s0090-4295(02)01969-6. [DOI] [PubMed] [Google Scholar]
- 20.Tantivejkul K, Loberg RD, Mawocha SC, Day LL, John LS, Pienta BA, Rubin MA, Pienta KJ. PAR1-mediated NFkappaB activation promotes survival of prostate cancer cells through a Bcl-xL-dependent mechanism. J Cell Biochem. 2005;96(3):641–652. doi: 10.1002/jcb.20533. [DOI] [PubMed] [Google Scholar]
- 21.Cooper CR, Chay CH, Gendernalik JD, Lee HL, Bhatia J, Taichman RS, McCauley LK, Keller ET, Pienta KJ. Stromal factors involved in prostate carcinoma metastasis to bone. Cancer. 2003;97(3 Suppl):739–747. doi: 10.1002/cncr.11181. [DOI] [PubMed] [Google Scholar]
- 22.Shi X, Gangadharan B, Brass LF, Ruf W, Mueller BM. Protease-activated receptors (PAR1 and PAR2) contribute to tumor cell motility and metastasis. Mol Cancer Res. 2004;2(7):395–402. [PubMed] [Google Scholar]
- 23.Yin YJ, Salah Z, Grisaru-Granovsky S, Cohen I, Even-Ram SC, Maoz M, Uziely B, Peretz T, Bar-Shavit R. Human protease-activated receptor 1 expression in malignant epithelia: a role in invasiveness. Arterioscler Thromb Vasc Biol. 2003;23(6):940–944. doi: 10.1161/01.ATV.0000066878.27340.22. [DOI] [PubMed] [Google Scholar]
- 24.Darmoul D, Gratio V, Devaud H, Laburthe M. Protease-activated receptor 2 in colon cancer: trypsin-induced MAPK phosphorylation and cell proliferation are mediated by epidermal growth factor receptor transactivation. J Biol Chem. 2004;279(20):20927–20934. doi: 10.1074/jbc.M401430200. [DOI] [PubMed] [Google Scholar]