Abstract
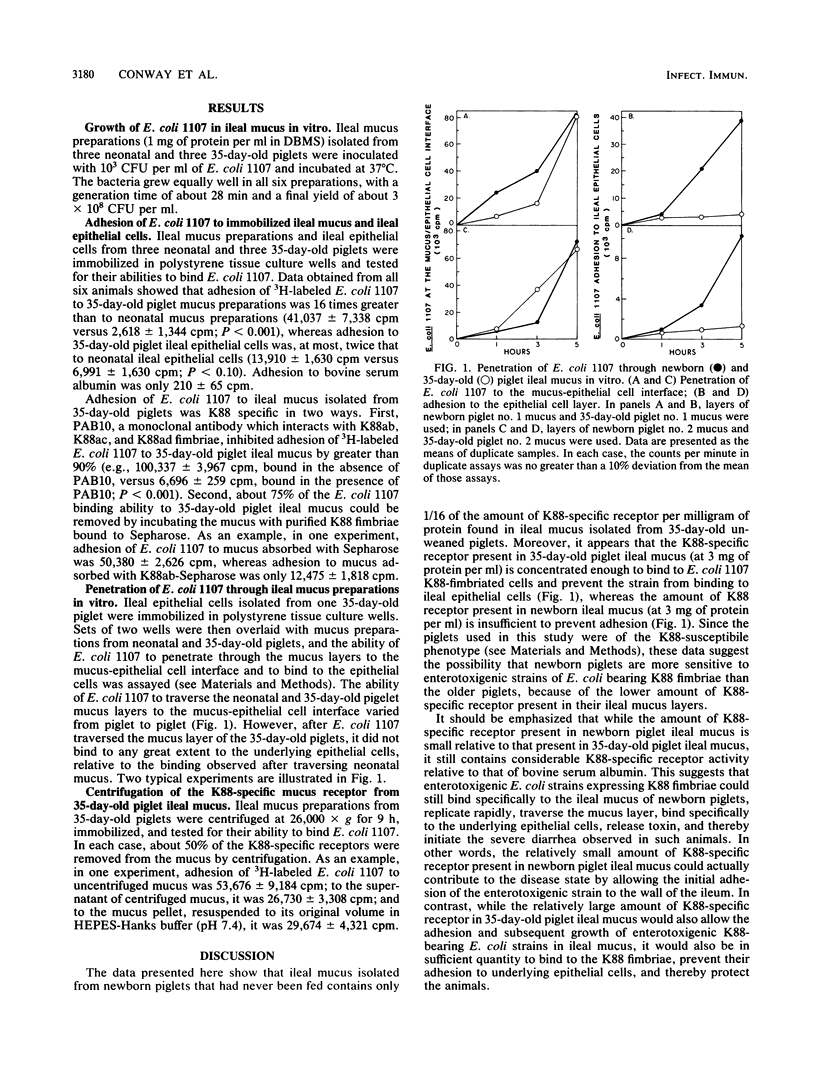
Ileal mucus and epithelial cells were isolated from newborn piglets that had never been fed and 35-day-old unweaned piglets. Both newborn and 35-day-old piglet mucus preparations supported growth of Escherichia coli Bd 1107/75 08, a K88-fimbriated porcine enterotoxigenic strain, equally well (i.e., generation times of 28 min were observed in both cases). Adhesion of E. coli Bd 1107/75 08 to 35-day-old piglet ileal epithelial cells was, at most, 2 times that of the same strain to newborn piglet ileal epithelial cells; however, adhesion of E. coli Bd 1107/75 08 to 35-day-old piglet ileal mucus was 16 times that of the same strain to newborn piglet ileal mucus. The receptor in 35-day-old piglet ileal mucus was K88 specific, since it could be removed by purified K88ab fimbriae. Furthermore, adhesion of E. coli Bd 1107/75 08 to 35-day-old piglet ileal mucus was blocked by PAB10, a K88ab-, K88ac-, K88ad-specific monoclonal antibody. Although E. coli Bd 1107/75 08 traversed both newborn and 35-day-old piglet ileal mucus about equally well in vitro and bound well to underlying ileal epithelial cells after passing through newborn ileal mucus, it did not bind to ileal epithelial cells after passing through 35-day-old piglet ileal mucus. The data are discussed with respect to the role that K88-specific receptors present in newborn and ileal mucus might play in the pathogenesis of porcine enterotoxigenic E. coli strains which bear K88 fimbriae.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertschinger H. U., Moon H. W., Whipp S. C. Association of Escherichia coli with the small intestinal epithelium. I. Comparison of enteropathogenic and nonenteropathogenic porcine strains in pigs. Infect Immun. 1972 Apr;5(4):595–605. doi: 10.1128/iai.5.4.595-605.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean E. A., Whipp S. C., Moon H. W. Age-specific colonization of porcine intestinal epithelium by 987P-piliated enterotoxigenic Escherichia coli. Infect Immun. 1989 Jan;57(1):82–87. doi: 10.1128/iai.57.1.82-87.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneke C. F., McGowan K., Thorne G. M., Gorbach S. L. Attachment of enterotoxigenic Escherichia coli to human intestinal cells. Infect Immun. 1983 Mar;39(3):1102–1106. doi: 10.1128/iai.39.3.1102-1106.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm B., Roberton A. M., Sherman P. M. Inhibition of attachment of Escherichia coli RDEC-1 to intestinal microvillus membranes by rabbit ileal mucus and mucin in vitro. Infect Immun. 1988 Sep;56(9):2437–2442. doi: 10.1128/iai.56.9.2437-2442.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner G. G. (1-14C)glucosamine incorporation by subcellular fractions of small intestinal mucosa. Identification by precursor labeling of three functionally distinct glycoprotein classes. J Biol Chem. 1970 Jul 25;245(14):3584–3592. [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Contribution of the K88 antigen of Escherichia coli to enteropathogenicity; protection against disease by neutralizing the adhesive properties of K88 antigen. Am J Clin Nutr. 1974 Dec;27(12):1441–1449. doi: 10.1093/ajcn/27.12.1441. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux D. C., McSweegan E. F., Williams T. J., Wadolkowski E. A., Cohen P. S. Identification and characterization of mouse small intestine mucosal receptors for Escherichia coli K-12(K88ab). Infect Immun. 1986 Apr;52(1):18–25. doi: 10.1128/iai.52.1.18-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouricout M. A., Julien R. A. Pilus-mediated binding of bovine enterotoxigenic Escherichia coli to calf small intestinal mucins. Infect Immun. 1987 May;55(5):1216–1223. doi: 10.1128/iai.55.5.1216-1223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouricout M., Petit J. M., Carias J. R., Julien R. Glycoprotein glycans that inhibit adhesion of Escherichia coli mediated by K99 fimbriae: treatment of experimental colibacillosis. Infect Immun. 1990 Jan;58(1):98–106. doi: 10.1128/iai.58.1.98-106.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevola J. J., Laux D. C., Cohen P. S. In vivo colonization of the mouse large intestine and in vitro penetration of intestinal mucus by an avirulent smooth strain of Salmonella typhimurium and its lipopolysaccharide-deficient mutant. Infect Immun. 1987 Dec;55(12):2884–2890. doi: 10.1128/iai.55.12.2884-2890.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C. S., Allen T. D. Ultrastructure of cell loss in intestinal mucosa. J Ultrastruct Res. 1977 Aug;60(2):272–277. doi: 10.1016/s0022-5320(77)80071-3. [DOI] [PubMed] [Google Scholar]
- QUASTLER H., SHERMAN F. G. Cell population kinetics in the intestinal epithelium of the mouse. Exp Cell Res. 1959 Jun;17(3):420–438. doi: 10.1016/0014-4827(59)90063-1. [DOI] [PubMed] [Google Scholar]
- Rutter J. M., Jones G. W., Brown G. T., Burrows M. R., Luther P. D. Antibacterial activity in colostrum and milk associated with protection of piglets against enteric disease caused by K88-positive Escherichia coli. Infect Immun. 1976 Mar;13(3):667–676. doi: 10.1128/iai.13.3.667-676.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter J. M., Jones G. W. Protection against enteric disease caused by Escherichia coli--a model for vaccination with a virulence determinant? Nature. 1973 Apr 20;242(5399):531–532. doi: 10.1038/242531a0. [DOI] [PubMed] [Google Scholar]
- Slomiany A., Yano S., Slomiany B. L., Glass G. B. Lipid composition of the gastric mucous barrier in the rat. J Biol Chem. 1978 Jun 10;253(11):3785–3791. [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Observations on the pathogenic properties of the K88, Hly and Ent plasmids of Escherichia coli with particular reference to porcine diarrhoea. J Med Microbiol. 1971 Nov;4(4):467–485. doi: 10.1099/00222615-4-4-467. [DOI] [PubMed] [Google Scholar]
- Stirm S., Orskov F., Orskov I., Birch-Andersen A. Episome-carried surface antigen K88 of Escherichia coli. 3. Morphology. J Bacteriol. 1967 Feb;93(2):740–748. doi: 10.1128/jb.93.2.740-748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti J. R., Salyers A. A., Bullard W. S., Wilkins D. Breakdown of mucin and plant polysaccharides in the human colon. Can J Biochem. 1977 Nov;55(11):1190–1196. doi: 10.1139/o77-178. [DOI] [PubMed] [Google Scholar]
- Wilson M. R., Hohmann A. W. Immunity to Escherichia coli in pigs: adhesion of enteropathogenic Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1974 Oct;10(4):776–782. doi: 10.1128/iai.10.4.776-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



