Abstract
Although Foxp3+ regulatory T (Treg) cells are thought to express autoreactive TCRs, it is not clear how individual TCRs influence Treg cell development, phenotype, and function in vivo. We have generated TCR transgenic mice (termed SFZ70 mice) using Tcra and Tcrb genes cloned from an autoreactive CD4+ T cell isolated from a Treg cell-deficient scurfy mouse. The SFZ70 TCR recognizes a cutaneous autoantigen and drives development of both conventional CD4+ Foxp3− T cells (Tconv) and Foxp3+ Treg cells. SFZ70 Treg cells display an activated phenotype evidenced by robust proliferation and expression of skin-homing molecules such as CD103 and P-selectin ligand. Analysis of Foxp3-deficient SFZ70 mice demonstrates Treg cells inhibit Tconv cell expression of tissue-homing receptors and their production of pro-inflammatory cytokines. Additionally, Treg cell suppression of SFZ70 Tconv cells can is overcome by non-specific activation of antigen-presenting cells. These results provide new insights into the differentiation and function of tissue-specific Treg cells in vivo and provide a tractable system for analyzing the molecular requirements of Treg cell-mediated tolerance toward a cutaneous autoantigen.
Introduction
Thymocytes expressing TCRs with high affinity for self-antigen are subject to a variety of fates. Many are eliminated via negative selection while agonistic selection diverts others into alternative lineages such as CD4+Foxp3+ regulatory T (Treg) cells and CD8αα intestinal epithelial (IEL) T cells (1). However, a fraction of autoreactive thymocytes are neither deleted nor redirected, but instead emerge from the thymus as conventional T (Tconv) cells with the potential to cause autoimmunity. As a result, T cell intrinsic and extrinsic peripheral tolerance mechanisms have evolved to control this population (2).
At barrier tissues such as the skin and intestines, the immune system must mount robust responses against invading pathogens while simultaneously maintaining self-tolerance. Indeed, several lines of evidence indicate that Treg cells are essential for maintaining proper immune homeostasis in the skin. Treg cell-deficient scurfy mice develop severe CD4+ T cell-mediated cutaneous inflammation and have autoantibodies directed against skin-specific antigens (3, 4). Additionally, human and mouse skin contain a high frequency of Treg cells, and the majority of human peripheral blood Treg cells express skin-homing receptors (5, 6). Finally, we have found that selectively blocking Treg cell migration to the skin impairs their ability to maintain cutaneous tolerance (5, 7). Conversely, in mice expressing the model antigen ovalbumin (OVA) under a skin-specific promoter, Treg cells do not play a critical role in the regulation of OVA-specific T cell migration to the skin (8). Thus, the extent to which, and mechanisms whereby Treg cells regulate skin-specific autoreactive T cells merits further investigation.
TCR-transgenic (Tg) mice recognizing endogenously expressed self-antigens have been valuable tools for studying T cell activation and tolerance in autoimmune disease (9–11). To further examine the development and behavior of autoreactive T cells in the skin, we generated a new line of TCR Tg mice, termed SFZ70 mice, using TCR chains cloned from a scurfy mouse–derived CD4+ T cell reactive towards a cutaneous self-antigen. These animals have allowed us to explore the behavior of skin antigen-specific Tconv and Treg cells in vivo and to further define the function of Treg cells in maintaining cutaneous tolerance.
Materials and Methods
Hybridoma generation and T cell activation assay
Generation and maintenance of β-galactosidase (lacZ)-inducible T cell hybridomas has been previously described (12). Briefly, CD4+ T cells isolated from the skin-draining LN of scurfy mice were fused with the lacZ-inducible fusion partner BWZ.36 to generate a lacZ-inducible T cell hybridoma panel. CD11c+ APCs were isolated using magnetic bead enrichment (Miltenyi) and cocultured overnight with LacZ-inducible T cell hybridomas. In some experiments the APCs were incubated with various amounts of anti-I-Ab mAb (Y3P) during co-culture as indicated. To assess T cell hybridoma activation, lysis buffer containing the lacZ substrate chlorophenolred-β-D-galactopyranoside (CPRG) was added to overnight cultures (0.15mM CPRG, .125% NP-40, and 9 mM MgCl2 in PBS). Results show lacZ activity as the absorbance of the CPRG cleavage product at 570 nm (with 630 nm as the reference wavelength).
Mice
C57BL/6J, Rag1−/− (B6.129S7-Rag1tm1Mom/J), OT-II (C57BL/6-Tg(TcraTcrb)425Cbn/J), and scurfy mice (B6.Cg-Foxp3sf/J) were obtained from The Jackson Laboratory. SFZ70 TCR Tg mice were generated by cloning cDNA encoding the rearranged TCR α and β variable regions (using the TRAV13D-1*01 and TRBV13-1*01 variable gene segments) into cassette vectors containing endogenous TCR regulatory elements (13). Linearized plasmids were then injected into the pronuclei of single-cell mouse embryos. The following PCR primers were used for identifying mice carrying the SFZ70 transgene: TCRα: (forward) GAGACCCGGGCAGCAACCACACAAGCACCA, (reverse) GAGAGCGGCCGCCCCAGCCAAAGCTGGGAATTT, expected product size: 800 bp; TCRβ: (forward) GAGACTCGAGTGGTCGCGAGATGGGCTCCA, (reverse) GAGAGACCGCGGAAATGCTCCCTCCCCTTCATT, expected product size: 600 bp. All animals were housed and bred under specific pathogen-free conditions in the Benaroya Research Institute animal facility, and the Benaroya Research Institute Institutional Animal Care and Use Committee approved all experiments.
Flow cytometry
For surface staining, cells were incubated at 4°C for 30 minutes in staining buffer (HBSS, 3% FBS, 0.09% NaN3) with the following monoclonal antibodies from eBioscience, Biolegend and BD Biosciences: anti-CD4 (GK1.5), anti-CD8 (53–6.7), anti-CD25 (PC61.5), anti-CD103 (2E7) and anti-CD44 (IM7). For P-selectin ligand staining, cells were incubated with P-selectin–human IgM fusion proteins, followed by biotinylated goat anti–human IgM (Jackson Immunoresearch) and streptavidin-phycoerythrin (eBioscience). For activated caspase staining, cells were incubated with FITC-tagged z- VAD-fmk (Promega). For intracellular staining of IFN-γ, IL-17, TNF-α, IL-4, and Foxp3, cells were surface stained as described above, permeabilized with Fix/Perm buffer (eBioscience), and stained for 30 minutes with the following antibodies purchased from eBioscience or BioLegend: anti-IFN-γ (XMG1), anti-IL-17 (TC11-18H10.1), anti-TNF-α (MP6-XT22), anti-IL-4 (11B11) and/or anti-Foxp3 (FKJ-16s) in BD Perm/Wash. Data were acquired on a LSR II (BD Biosciences) and analyzed using FlowJo software (TreeStar).
In vitro T cell stimulation
Prior to intracellular cytokine staining, cells from the skin-draining LN or spleen were depleted of red blood cells and stimulated in complete medium (RPMI plus 10% FCS, 4 mM L-glutamine, 1 mM sodium pyruvate, 50 units/mL penicillin and 50 μg/mL streptomycin) for 5 hours at 37°C with PMA (50 ng/mL) and ionomycin (1μg/mL) in the presence of monensin (10 μg/mL).
BrdU incorporation assay
BrdU (5-bromodeoxyuridine) was administered to mice in drinking water at a concentration of 0.8 mg/mL for 72 hours prior to analysis. Lymphocytes from treated mice were surface stained as described above, fixed for 20 minutes using BD Cytofix/Cytoperm, and then treated for 1 hour at 37°C with DNase I (Sigma #D4513, 300μg/mL). For analysis of BrdU incorporation, cells were stained intracellularly using antibodies directed against BrdU (MoBU-1) and Foxp3 in BD Perm/Wash 30 min.
Anti-CD40 treatment
Mice were injected intraperitoneally on days 0, 2, and 4 with 25 μg of anti-CD40 (FGK4.5; UCSF Monoclonal Laboratory Core) or 25 μg rat IgG (Sigma) in PBS. Mice were euthanized for analysis on day 6.
Tissue histology
Tissues were fixed using 10% neutral buffered formalin, paraffin embedded, and cut into 5 μm sections which were stained with hematoxylin and eosin.
Statistical analysis
Statistical significance was determined by one-way repeated-measures analysis of variance or two-tailed unpaired Student's t-test where appropriate as indicated in the figure legends.
Results
The SFZ70 TCR recognizes a cutaneous self-antigen
Foxp3-deficient scurfy mice suffer from severe CD4+ T cell–mediated skin inflammation, and therefore we reasoned they would provide an abundant source of CD4+ T cells specific for cutaneous antigens. We fused CD4+ T cells from the spleens and skin-draining lymph nodes (LN) of scurfy mice with the BWZ.36 partner to generate a panel of CD4+ T cell hybridomas expressing β-galactosidase under the control of NFAT regulatory elements (12). To identify clones reactive toward cutaneous self-antigens, each hybridoma was co-cultured with CD11c+ dendritic cells (DCs) isolated from either the skin-draining LNs or spleens of WT mice. T cell activation was then assessed by measuring cleavage of CPRG, a chromogenic β-galactosidase substrate. We identified several hybridoma clones that selectively recognized antigens presented by DCs from skin draining LN but not from the spleen. Of these, we selected one, termed scurfy-derived LacZ inducible hybridoma 70 (SFZ70) for further study based on its particularly robust, MHC class II-restricted response to skin-draining LN DCs (Fig. S1A). The SFZ70 hybridoma reacted strongly to DCs isolated from either the skin-draining LNs or directly from the skin, but minimally to DCs isolated from the spleen, mesenteric LN, thymus, intestine, liver, and lung (Fig. 1, top panels and data not shown). By contrast, DCs from all tissues were able to activate an ovalbumin-specific hybridoma (OTIIZ) when pulsed with OVA peptide 323–339, confirming their ability to present antigen. When analyzed separately, DCs from the inguinal, axillary, brachial, and superficial cervical skin-draining LNs activated the SFZ70 hybridoma to a similar extent (Fig. S1B).
Figure 1. Skin-specific activation of the SFZ70 hybridoma.
CD11c+ APCs were isolated from the indicated tissues of WT mice (top panels), WT and Ccr7−/− (middle panels) or conventional (CNV) and germ-free (GF) mice (bottom panels). APCs were then co-cultured with 1×105 SFZ70 hybridoma cells (left panels) or pulsed with OVA peptide and co-cultured with 1×105 OTIIZ hybridoma cells (right panels). After 16 hours, cells were lysed and the hybridoma response was assessed by measuring the absorbance (A570-A630) of the chromogenic product released following CPRG cleavage by β-gal. Results are representative of at least two independent experiments with APCs pooled from 2–8 mice per group.
The skin-draining LNs contain multiple subsets of DCs including LN resident DCs and several populations of migratory DCs. Migratory DCs transport cutaneous antigens to the draining LN via efferent lymphatics in a process dependent on the chemokine receptor CCR7 (14). DCs from the skin-draining LNs of CCR7-deficient mice failed to activate the SFZ70 hybridoma but were still capable of presenting antigen to the OTIIZ cells (Fig. 1, middle panels). Additionally, DCs from both germ-free and conventional mice were equally capable of activating the SFZ70 hybridoma, demonstrating that the SFZ70 TCR does not recognize an antigen derived from the cutaneous microflora (Fig. 1, bottom panels). Instead, these results demonstrate that the SFZ70 hybridoma recognizes a cutaneous self-antigen that is transported to the skin-draining LNs in a CCR7-dependent manner.
T cell development in SFZ70.Rag1−/− TCR transgenic mice
To study the development and behavior of T cells specific for an endogenously expressed cutaneous antigen in vivo, we generated SFZ70 TCR Tg mice which were subsequently backcrossed onto the C57BL/6 background. To prevent the confounding effects of endogenous TCR rearrangement, we further crossed the SFZ70 mice to Rag1−/−mice (SFZ70.Rag1−/−) and performed all subsequent experiments using these animals. The majority of SFZ70.Rag1−/− mice did not display any overt signs of autoimmune or inflammatory disease and appeared externally and histopathologically normal. However, < 2% developed caudal dermatitis reminiscent of that seen in Foxp3-deficient scurfy mice (Fig. S2).
Total thymocyte numbers, as well as the number of CD4/CD8 double positive (DP) and double negative (DN) cells in SFZ70.Rag1−/− mice were similar to those found in WT mice. However, we observed a 10-fold decrease in the number of CD4+CD8−thymocytes (Fig. 2A). Additionally, SFZ70.Rag1−/− mice contained a CD8SP T cell population not found in OVA-specific OT-II.Rag1−/− TCR Tg mice. CD4SP thymocytes expressing TCRs with higher affinity for self-antigen are thought to be preferentially diverted into the Treg cell lineage, and Treg cell development can only be recapitulated in TCR Tg mice that also express the TCR’s cognate antigen. In accordance with this model, we found a high frequency of Foxp3+ thymocytes within the SFZ70.Rag1−/−CD4SP subset, which on average was slightly larger than that found in WT mice, although this difference was not statistically significant (Fig. 2B). Moreover, although the frequency of the CD4SP Foxp3+ cells among CD4SP thymocytes varied substantially between mice, we consistently found ~100,000 CD4SP Foxp3+ thymocytes in SFZ70.Rag1−/− animals.
Figure 2. T cell development in SFZ70.Rag1−/− mice.
(A) Expression of CD4 and CD8 by thymocytes from SFZ70.Rag1−/−, OT-II.Rag1−/− or WT mice. Graphs show corresponding absolute numbers of DN, DP, and CD4SP thymocytes. (B) Expression of Foxp3 and CD25 by thymocytes isolated from SFZ70.Rag1−/−, OT-II.Rag1−/− or WT mice. Graphs show corresponding percentage and absolute number of CD4SP thymocytes expressing Foxp3. Data are representative of three independent experiments with 1–5 mice per group.
SFZ70.Rag1−/− mice contain activated peripheral Treg cells
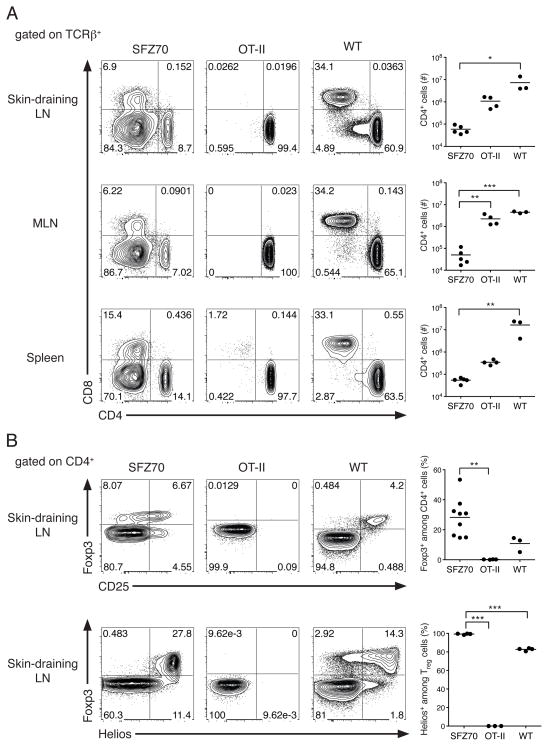
CD4+ T cells were present in the skin-draining LNs, mesenteric LNs, and spleen SFZ70.Rag1−/− mice (Fig. 3A). In addition, the level of surface TCRβ expressed by these cells was equivalent to that observed in CD4+ T cells from WT mice (data not shown), indicating that tolerance in these animals is not the simply the result of TCR downregulation. However, the peripheral lymphoid organs of SFZ70.Rag−/− mice were highly lymphopenic compared to those of OT-II.Rag1−/− or WT animals, containing ~10–100-fold fewer CD4+ T cells. Moreover, the majority of TCRβ+ SFZ70.Rag1−/− T cells in the periphery did not express CD4 or CD8, whereas TCRβ+ CD4/CD8 DN cells were rare in WT and OT-II.Rag1−/− mice. CD8αα+ IEL T cells are thought to originate from T cells expressing self-reactive TCRs, and the development of IEL and Treg cells has been genetically linked (15, 16). Consistent with this link, the intestinal epithelium of SFZ70.Rag1−/− mice contained a large population of CD8αα T cells (Fig. S3).
Figure 3. SFZ70.Rag1−/− mice contain peripheral CD4+ and CD8+ T cells and Helios+ Treg cells.
(A) Expression of CD4 and CD8 by TCRβ+ lymphocytes isolated from the skin-draining lymph nodes (LN), mesenteric lymph node (MLN) and spleens of SFZ70.Rag1−/−, OT-II.Rag1−/− or WT mice. Graphs show corresponding absolute numbers of CD4+ lymphocytes. (B) Expression of Foxp3 and CD25 (top) or Foxp3 and Helios (bottom) by CD4+ lymphocytes isolated from SFZ70.Rag1−/−, OT-II.Rag1−/− or WT mice. Statistical significance was determined using a one-way repeated-measures analysis of variance. A Tukey post-test was used to obtain the p-value for the bracketed pairwise comparisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Data are representative of two experiments with 3–9 mice per group.
Upon examination of the peripheral SFZ70.Rag1−/− Treg cell compartment, we found Foxp3+ expression by up to 50% of the CD4+ T cells within the spleen, mesenteric LN, and skin-draining LNs (Fig. 3B, top and data not shown). However, no Foxp3+ cells were present in either the DN or CD8+ T cell populations (data not shown). The disparity in the size of the Treg cell population in the periphery vs. the thymus of SFZ70.Rag1−/− mice suggests that peripheral Treg cells are either derived from Tconv cells that have upregulated Foxp3 or that the Treg cells possess a peripheral homeostatic proliferative/survival advantage over their Tconv cell counterparts. We examined expression of the Ikaros family transcription factor Helios, which is expressed by essentially all thymically-derived Treg cells, but not by Treg cells induced either in vivo in response to antigen feeding, or in vitro by activation in the presence of TGF-β (17). Interestingly, nearly all of the peripheral Treg cells in SFZ70.Rag1−/− mice were Helios+ indicating that they originated in the thymus, and not through peripheral conversion (Fig. 3B, bottom).
To determine if enhanced proliferation might account for the increased frequency of peripheral Treg cells and to directly compare Tconv and Treg cell proliferation in SFZ70.Rag1−/− mice, we measured incorporation of the thymidine analogue bromodeoxyuridine (BrdU) by T cells following a 72-hour treatment. As a control, we also examined BrdU incorporation in OT-II.Rag1−/− animals. Indeed, the percentage of BrdU+ Tconv cells was significantly greater in the autoreactive SFZ70.Rag1−/− mice than in OT-II.Rag1−/− animals (Fig. 4A). Furthermore, consistent with studies documenting the robust proliferation of Treg cells in vivo (18), BrdU labeling was observed in a larger proportion of the SFZ70.Rag1−/− Treg cells than their Tconv cell counterparts.
Figure 4. SFZ70.Rag1−/− T cells display an activated phenotype.
(A) Incorporation of BrdU by CD4+ lymphocytes from the skin-draining LN of SFZ70.Rag1−/−, OT-II.Rag1−/−or WT mice. Graph indicates the percent BrdU+ cells amongst Foxp3+ or Foxp3− cells from each group. (B) Expression of CD44, CD103, and P-selectin ligand (P-lig) by CD4+ lymphocytes isolated from the skin draining LN of SFZ70.Rag1−/−, OT-II.Rag1−/− or WT mice. Statistical significance was determined using a one-way repeated-measures analysis of variance; a Tukey post-test was used to obtain the p-value for the bracketed pairwise comparison. **, P < 0.01; ***, P < 0.001. Data are representative of three independent experiments with 3–5 mice per group.
Despite their robust proliferative response, SFZ70.Rag1−/− T cells do not accumulate, suggesting they may undergo increased apoptosis. To address this possibility, we stained T cells from the skin-draining LN of SFZ70.Rag1−/−, OT-II.Rag1−/−, and WT mice with FITC conjugated z-VAD-fmk, which specifically binds activated caspases and labels cells undergoing apoptosis (19). Indeed, CD4+ T cells from SFZ70.Rag1−/− mice show high levels of caspase activation compared to those from OT-II.Rag1−/− or WT animals, indicating that activation-induced apoptosis may help prevent the accumulation of autoreactive T cells and subsequent autoimmune disease in SFZ70.Rag1−/− mice (Fig. S4).
Both Tconv and Treg cells in SFZ70.Rag1−/− mice uniformly displayed a CD44hi effector/memory cell phenotype (Fig. 4B, top panels). Moreover, SFZ70.Rag1−/− Treg cells expressed skin homing receptors such as CD103 and P-selectin ligand (P-lig) (Fig. 4B, middle and bottom panels). Thus, in accordance with their specificity for a cutaneous autoantigen, activated Treg cells with a skin-homing phenotype comprise a large percentage of the peripheral CD4+ T cell compartment in SFZ70.Rag1−/− mice.
Treg cells are critical for maintenance of peripheral tolerance in SFZ70.Rag1−/− mice
Although the SFZ70 TCR recognizes a self-antigen, most SFZ70.Rag1−/− mice do not develop overt autoimmunity. To examine the role of Treg cells in preventing autoimmune disease in these animals, we bred SFZ70.Rag1−/− and Rag1−/−Foxp3sf animals to generate Treg cell-deficient SFZ70 (SFZ70.Rag1−/−Foxp3sf) mice. By 24 days of age, SFZ70.Rag1−/−Foxp3sf mice displayed a moribund phenotype, and weighed significantly less than their SFZ70.Rag1−/−Foxp3WT littermates (Fig. 5A). By contrast, OT-II.Rag1−/− mice that are unable to express functional Foxp3 do not develop any inflammatory disease and are indistinguishable from WT littermates (20). While there was no visible cutaneous inflammation in the SFZ70.Rag1−/−Foxp3sf mice, histological analysis revealed substantial lymphocytic infiltration of the skin and liver that was not observed in the SFZ70.Rag1−/−Foxp3WT littermate controls (Fig. 5B). Furthermore, despite their reduced weight and runted appearance, there was no evidence of inflammation in other tissues normally impacted in Foxp3-deficient mice such as the lungs and intestines (data not shown).
Figure 5. Treg cells are required for maintenance of peripheral tolerance in SFZ70.Rag1−/− mice.
(A) Weight of SFZ70.Rag1−/−Foxp3sf and SFZ70.Rag1−/−Foxp3WT mice at 24 days of age. (B) Photomicrographs of hematoxylin and eosin–stained sections of the skin (20×), and liver (20×) from SFZ70.Rag1−/−Foxp3sf and SFZ70.Rag1−/−Foxp3WT mice. (C) Expression of Ki-67, P-selectin ligand (P-lig), and CXCR3 by CD4+ T cells from the skin-draining LN of SFZ70.Rag1−/−Foxp3sf (solid black line) or SFZ70.Rag1−/− Foxp3WT (gray filled histogram) mice. (D) Production of IFN-γ, TNF-α, IL-4, and IL-17 by SFZ70.Rag1−/−Foxp3sf and SFZ70.Rag1−/−Foxp3WT CD4+Foxp3−CD44hi T cells from the skin-draining LN following in vitro PMA and ionomycin stimulation. ***, P < 0.001. Statistical significance was assessed using a two-tailed, unpaired Student’s t-test.
Treg cells can suppress autoimmunity by inhibiting the priming and activation of autoreactive T cells. However, in SFZ70.Rag1−/− mice the majority of Tconv cells were CD44hi and underwent robust proliferation (Fig. 4). Additionally, expression of the proliferation-associated protein Ki-67 was similar in cells from SFZ70.Rag1−/−Foxp3WT and SFZ70.Rag1−/−Foxp3sf mice (Fig. 5C, top panels). However, expression of P-lig and CXCR3, two molecules associated with T cell homing to inflamed tissues, were substantially increased in Tconv cells from the skin-draining LN and spleen of SFZ70.Rag1−/−Foxp3sf mice (Fig. 5C, middle and bottom panels). Furthermore, following ex vivo restimulation with PMA/ionomycin an increased percentage of SFZ70.Rag1−/− Foxp3sf CD4+ T cells produced pro-inflammatory cytokines such as IFN-γ, TNF-α, and IL-4 compared to those from SFZ70.Rag1−/−Foxp3WT littermate controls, whereas cytokine production by CD8+ and DN T cells was unchanged between the two groups (Fig. 5D, and data not shown). Thus, consistent with previous reports (8, 21, 22), Treg cells in SFZ70.Rag1−/− mice do not appear to inhibit the initial activation of Tconv cells, but instead function primarily by modulating Tconv cell migration and cytokine production.
Systemic DC maturation overcomes Treg cell-mediated suppression in SFZ70.Rag1−/− mice
The activation status of the antigen presenting DCs is a key factor in determining the outcome of T cell priming. Whereas immature DCs drive a tolerogenic T cell response, mature DCs express increased levels of costimulatory molecules and produce pro-inflammatory cytokines, which enable full T cell activation and effector differentiation (23). To examine the relationship between DC activation and Treg cell function, we treated SFZ70.Rag1−/− mice over a six-day period with either an agonistic anti-CD40 mAb that potently activates DCs, or control rat IgG. During the last 72 hours of anti-CD40 treatment, BrdU was administered to measure T cell proliferation. Indicative of robust DC activation, we found that proliferation of both Tconv and Treg cells was increased in the skin-draining LN and spleen of anti-CD40-treated SFZ70.Rag1−/− mice compared to controls (Fig. 6A). Furthermore, in response to ex vivo restimulation, there was a substantial increase in the percentage of Tconv cells producing the pro-inflammatory cytokines IFN-γ and IL-17 in the anti-CD40 treated mice (Fig. 6B). By contrast, anti-CD40-treatment did not augment proliferation or cytokine production by Tconv cells in non-autoreactive OT-II.Rag1−/− animals (data not shown). Thus, DC activation can overcome Treg cell-mediated suppression of Tconv cells, resulting in their increased proliferation and effector cytokine production.
Figure 6. Treg cell-mediated suppression in SFZ70.Rag1−/− mice is overcome by systemic DC activation.
On days 0, 2, and 4 SFZ70.Rag1−/− mice were injected intraperitoneally with 25 μg of anti-mouse CD40 or 25 μg rat IgG (control) in PBS. Mice were euthanized on day 6 and CD4+ T cells from the skin-draining LN and spleen were examined for (A) incorporation of BrdU and (B) production of IFN-γ and IL-17 following in vitro PMA and ionomycin stimulation.
Discussion
To examine the behavior of autoreactive T cells specific for an endogenously expressed cutaneous antigen, we generated SFZ70 TCR Tg mice using an autoreactive TCR isolated from a scurfy mouse. Notably, this TCR is compatible with the development of both CD4+Foxp3− Tconv cells and Foxp3+ Treg cells. Our analyses of these animals provide new insights into control of skin reactive T cells, and describe a new platform for elucidating the molecular and cellular mechanisms required for generation and maintenance of Treg cell-mediated immunological tolerance.
In mice containing a polyclonal TCR repertoire, Treg cell-mediated suppression of autoreactive Tconv cells is required throughout the animal’s lifespan for prevention of autoimmune disease (24). However, the specificity and behavior of the pathogenic T cells that cause disease in the absence of Treg cells has been poorly defined. Although long inferred, our results, together with a recent report demonstrating that Treg cell ablation in germ-free mice results in cutaneous inflammatory disease (25), provide direct evidence demonstrating that dysregulated CD4+ T cell responses in scurfy mice are aimed in part toward cutaneous autoantigens.
Despite the fact that it was recovered from a CD4+ T cell in the periphery of a scurfy mouse, the ability of the SFZ70 TCR to drive the development of CD4SP cells is greatly limited. Instead, a large fraction of TCR+ cells present in the thymus and periphery of SFZ70 mice express neither CD4 nor CD8. The development of these mature DN T cells may be due to the autoreactive nature of the SFZ70 TCR. Indeed, T cell coreceptor downregulation has been shown to lower overall TCR avidity for peptide:MHC complexes and has been previously described as a mechanism of tolerance (26). Alternatively, the large DN T cell population may reflect the altered timing of TCR expression during thymic development of SFZ70 T cells. Early expression of Tg TCR α and β chains under the control of endogenous transcriptional regulatory elements results in accelerated T cell development and the emergence of a large population of DN T cells in the thymus and periphery that phenotypically and functionally resemble γδ T cells (27). Similarly, development of CD8αα cells in TCR Tg mice has also been linked to premature TCRα chain expression during thymic development. However a detailed comparison of SFZ70 mice with other systems is complicated by differences in antigen expression and prevalence, MHC I vs. MHC II restriction, and TCR affinity.
In SFZ70 mice, the large proportion of DP thymocytes and relative absence of CDSP cells suggests deletion occurs at the DP to CD4SP transition, and this most likely reflects extensive negative selection of the autoreactive SFZ70 thymocytes. The timing and extent of negative selection in TCR Tg mice is dependent on factors including TCR affinity for peptide:MHC and the location and cell type in which antigen is presented in the thymus (28). Although thymic DCs can induce negative selection, these cells did not potently activate the SFZ70 hybridoma. Thus, thymic DCs may present the SFZ70 antigen at levels sufficient to induce negative selection in vivo, but below the detection limit of our hybridoma assay. For instance, low-level DC migration from the skin to thymus has been reported to contribute to negative selection and may play a role in shaping the SFZ70 thymocyte population (29). Additionally, presentation of the SFZ70 cognate antigen may be mediated in large part by medullary thymic epithelial cells (mTECs) capable of expressing and presenting tissue-specific antigens. Indeed, antigen presentation by mTECs can drive both negative selection and development of Foxp3+ Treg cells (30, 31). Crossing SFZ70 mice to animals deficient in various pathways of cellular migration and antigen presentation will help determine the relative contributions of DCs and mTECs for driving both selection and induction of Foxp3. Additionally, poor positive selection may also contribute to the paucity of SFZ70 CD4SP thymocytes, and we cannot exclude the possibility that the peptide:MHC complex mediating the thymic selection of SFZ70 T cells is distinct from the cutaneous antigen they respond to in the periphery.
In TCR Tg mice generated using TCRs isolated from Treg cells, only a very small percentage of CD4SP thymocytes were found to express Foxp3 (32, 33). However, mixed bone marrow chimeras revealed that these TCRs drove the development of Treg cells when their clonal frequency was limited to a small fraction of a polyclonal repertoire. These experiments demonstrated that a specific niche exists for a TCR of any given specificity that can only accommodate a limited number of Treg cells. In contrast to these mice, Treg cells comprise a substantial percentage of the thymic CD4SP population in SFZ70 mice at frequencies higher or comparable to that observed in WT mice. However, in agreement with a limited Treg cell clonal niche size, the absolute number of SFZ70 thymic Treg cells is consistently around 100,000 cells. Given the low absolute number of CD4SP thymocytes, intraclonal competition for Treg cell-inducing peptide:MHCII may be modest in SFZ70 mice, thereby accounting for the relatively large percentage of Treg cells among CD4SP thymocytes observed in these animals.
In the periphery, Treg cells undergo robust homeostatic proliferation that is thought to be dependent on their recognition of self-antigens presented by DCs on MHCII (34, 35). Additionally Treg cell homeostasis is controlled in part by IL-2 produced by CD44hi effector/memory Tconv cells (36). Therefore, the robust proliferation and peripheral expansion of Treg cells in SFZ70 mice may be driven by a combination of TCR autoantigen recognition and IL-2 produced by the relatively large population of CD44hi Tconv cells in these animals. Indeed, even when comparing cells of identical TCR specificity, Treg cells have a substantial proliferative/survival advantage over their Tconv counterparts. Moreover, the large percentage of Foxp3+ T cells in SFZ70 mice suggests that the scurfy T cell from which we isolated the SFZ70 TCR may have been a Treg cell unable to express functional Foxp3. Activated T cells from Foxp3-deficient mice express similar TCRs to those found in the Treg cells from WT mice (37). These ‘would-be’ Treg cells share many traits with bona fide Treg cells, but comprise a distinct peripheral T cell population that lacks functional suppressive activity and is substantially expanded in Foxp3-deficient animals (38, 39).
Treg cells are not phenotypically and functionally homogenous, but instead can be divided into distinct subsets based activation marker and homing receptor expression. CD44lo Treg cells share characteristics with naïve Tconv cells including a low level of homeostatic proliferation and a CD62LhighCD103− phenotype that facilitates their entry into lymphoid tissues. By contrast, CD44hi Treg cells resemble effector/memory Tconv cells and are characterized by low CD62L expression, increased expression of homing receptors such as CD103 and E/P-lig that target cells to non-lymphoid sites, and robust homeostatic proliferation (40–42). Although the molecular basis for this diversity is still poorly understood, the uniform effector/memory phenotype of SFZ70 Treg cells indicates that TCR specificity strongly influences Treg cell migration, homeostasis and function.
Effector/memory Treg cells display a high suppressive capability under inflammatory conditions, but the mechanisms and targets of Treg cell-mediated suppression remain largely unknown. Our finding that an increased percentage of Tconv cells express peripheral homing receptors and produce inflammatory cytokines in Treg cell-deficient SFZ70 mice argues that Treg cells function at least in part by limiting trafficking and cytokine production by activated autoreactive Tconv cells. Given the known ability of DCs to imprint T cells with specific patterns of cytokine and homing receptor expression (43), our data indicate that SFZ70 Treg cells may control Tconv cells indirectly by acting on DCs. However, strong activating stimuli can overcome Treg cell suppression of DCs (44), and we found that DC activation with an agonistic anti-CD40 mAb resulted in increased effector cytokine production by SFZ70 Tconv cells. Thus, even antigen-matched Treg cells are not sufficient to inhibit acquisition of effector function by autoreactive Tconv cells in the during periods of robust innate immune stimulation. This suggests that during the course of an infection with a strong innate stimulatory component, autoreactive Tconv cells may override Treg cell-mediated suppression, potentially contributing to the development of autoimmune disease. Indeed, dysregulation of DC activity can result in spontaneous autoimmunity even in the presence of Treg cells (45).
Treg cells have typically been studied as a bulk population composed of cells differing in TCR specificity, activation status, and origin. Thus, although Treg cells are thought to be largely self-reactive, how their specificity contributes to their unique phenotype, function and homeostasis has been difficult to examine experimentally. The SFZ70 mouse has allowed us to distinguish differences in proliferation, tissue-homing receptor expression, and effector function between Treg and Tconv cells expressing the same TCR. Given that the SFZ70 mice allow the study of Treg cells as a monospecific population, they are uniquely poised elucidate the underlying mechanisms involved in the generation and maintenance of Treg cell-mediated immune tolerance in the skin.
Supplementary Material
Acknowledgments
We would like to thank K. Klonowski (University of Georgia) for tissues from CCR7−/− mice, D. Artis (University of Pennsylvania) for tissues from germ-free mice, and A. Rudensky (Memorial Sloan Kettering Cancer Center) for Y3P monoclonal antibody.
Footnotes
This work was supported by grants AR055695, DK072295, and AI067750 to D.J.C. from the N.I.H. J.R.K. was supported in part by the Cancer Research Institute Predoctoral Emphasis Pathway in Tumor Immunology.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Cheroutre H, Mucida D, Lambolez F. The importance of being earnestly selfish. Nature immunology. 2009;10:1047–1049. doi: 10.1038/ni1009-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller DL. Mechanisms maintaining peripheral tolerance. Nature immunology. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 3.Blair PJ, Bultman SJ, Haas JC, Rouse BT, Wilkinson JE, Godfrey VL. CD4+CD8- T cells are the effector cells in disease pathogenesis in the scurfy (sf) mouse. J Immunol. 1994;153:3764–3774. [PubMed] [Google Scholar]
- 4.Huter EN, Natarajan K, Torgerson TR, Glass DD, Shevach EM. Autoantibodies in Scurfy mice and IPEX patients recognize keratin 14. J Invest Dermatol. 2010;130:1391–1399. doi: 10.1038/jid.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. The Journal of experimental medicine. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol. 2006;177:4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 7.Dudda JC, Perdue N, Bachtanian E, Campbell DJ. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. The Journal of experimental medicine. 2008;205:1559–1565. doi: 10.1084/jem.20072594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi T, Pincus LB, Wurbel MA, Rich BE, Kupper TS, Fuhlbrigge RC, Boes M. Maintenance of peripheral tolerance through controlled tissue homing of antigen-specific T cells in K14-mOVA mice. J Immunol. 2009;182:4665–4674. doi: 10.4049/jimmunol.0803628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 10.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 11.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 12.Sanderson S, Shastri N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int Immunol. 1994;6:369–376. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 13.Kouskoff V, Signorelli K, Benoist C, Mathis D. Cassette vectors directing expression of T cell receptor genes in transgenic mice. J Immunol Methods. 1995;180:273–280. doi: 10.1016/0022-1759(95)00002-r. [DOI] [PubMed] [Google Scholar]
- 14.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Forster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Leishman AJ, Gapin L, Capone M, Palmer E, MacDonald HR, Kronenberg M, Cheroutre H. Precursors of functional MHC class I- or class II-restricted CD8alphaalpha(+) T cells are positively selected in the thymus by agonist self-peptides. Immunity. 2002;16:355–364. doi: 10.1016/s1074-7613(02)00284-4. [DOI] [PubMed] [Google Scholar]
- 16.Holler PD, Yamagata T, Jiang W, Feuerer M, Benoist C, Mathis D. The same genomic region conditions clonal deletion and clonal deviation to the CD8alphaalpha and regulatory T cell lineages in NOD versus C57BL/6 mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7187–7192. doi: 10.1073/pnas.0701777104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. The Journal of experimental medicine. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayaraman S. Intracellular determination of activated caspases (IDAC) by flow cytometry using a pancaspase inhibitor labeled with FITC. Cytometry Part A : the journal of the International Society for Analytical Cytology. 2003;56:104–112. doi: 10.1002/cyto.a.10094. [DOI] [PubMed] [Google Scholar]
- 20.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 22.DiPaolo RJ, Glass DD, Bijwaard KE, Shevach EM. CD4+CD25+ T cells prevent the development of organ-specific autoimmune disease by inhibiting the differentiation of autoreactive effector T cells. J Immunol. 2005;175:7135–7142. doi: 10.4049/jimmunol.175.11.7135. [DOI] [PubMed] [Google Scholar]
- 23.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 24.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature immunology. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 25.Chinen T, Volchkov PY, Chervonsky AV, Rudensky AY. A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. The Journal of experimental medicine. 207:2323–2330. doi: 10.1084/jem.20101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Illes Z, Waldner H, Reddy J, Anderson AC, Sobel RA, Kuchroo VK. Modulation of CD4 co-receptor limits spontaneous autoimmunity when high-affinity transgenic TCR specific for self-antigen is expressed on a genetically resistant background. Int Immunol. 2007;19:1235–1248. doi: 10.1093/intimm/dxm094. [DOI] [PubMed] [Google Scholar]
- 27.Baldwin TA, Sandau MM, Jameson SC, Hogquist KA. The timing of TCR alpha expression critically influences T cell development and selection. The Journal of experimental medicine. 2005;202:111–121. doi: 10.1084/jem.20050359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 29.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nature immunology. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 30.Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nature immunology. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 31.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 32.Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nature immunology. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung MW, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. The Journal of experimental medicine. 2009;206:2121–2130. doi: 10.1084/jem.20091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimoda M, Mmanywa F, Joshi SK, Li T, Miyake K, Pihkala J, Abbas JA, Koni PA. Conditional ablation of MHC-II suggests an indirect role for MHC-II in regulatory CD4 T cell maintenance. J Immunol. 2006;176:6503–6511. doi: 10.4049/jimmunol.176.11.6503. [DOI] [PubMed] [Google Scholar]
- 35.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. The Journal of experimental medicine. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. The Journal of experimental medicine. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nature immunology. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 38.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 39.Lahl K, Mayer CT, Bopp T, Huehn J, Loddenkemper C, Eberl G, Wirnsberger G, Dornmair K, Geffers R, Schmitt E, Buer J, Sparwasser T. Nonfunctional regulatory T cells and defective control of Th2 cytokine production in natural scurfy mutant mice. J Immunol. 2009;183:5662–5672. doi: 10.4049/jimmunol.0803762. [DOI] [PubMed] [Google Scholar]
- 40.Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, Brunner M, Scheffold A, Hamann A. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25- regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13031–13036. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, Niesner U, de la Rosa M, Schmidt CA, Brauer R, Buer J, Scheffold A, Hamann A. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. The Journal of experimental medicine. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Min B, Thornton A, Caucheteux SM, Younes SA, Oh K, Hu-Li J, Paul WE. Gut flora antigens are not important in the maintenance of regulatory T cell heterogeneity and homeostasis. Eur J Immunol. 2007;37:1916–1923. doi: 10.1002/eji.200737236. [DOI] [PubMed] [Google Scholar]
- 43.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nature immunology. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serra P, Amrani A, Yamanouchi J, Han B, Thiessen S, Utsugi T, Verdaguer J, Santamaria P. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–889. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 45.Chervonsky AV. Influence of microbial environment on autoimmunity. Nature immunology. 11:28–35. doi: 10.1038/ni.1801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.