Abstract
Background
Varicella-zoster virus (VZV)-specific cell-mediated immunity is important for protection against VZV disease. We studied the relationship between VZV cell-mediated immunity and age after varicella or VZV vaccination in healthy and human immunodeficiency virus (HIV)–infected individuals.
Methods
VZV responder cell frequency (RCF) determinations from 752 healthy and 200 HIV-infected subjects were used to identify group-specific regression curves on age.
Results
In healthy individuals with past varicella, VZV RCF peaked at 34 years of age. Similarly, VZV-RCF after varicella vaccine increased with age in subjects aged <1 to 43 years. In subjects aged 61–90 years, VZV RCF after zoster vaccine decreased with age. HIV-infected children had lower VZV RCF estimates than HIV-infected adults. In both groups, VZV RCF results were low and constant over age. Varicella vaccination of HIV-infected children with CD4 levels ≥20% generated VZV RCF values higher than wild-type infection and comparable to vaccine-induced responses of healthy children.
Conclusions
In immunocompetent individuals with prior varicella, VZV RCF peaked in early adulthood. Administration of varicella vaccine to HIV-infected or uninfected individuals aged >5 years generated VZV RCF values similar to those of immunocompetent individuals with immunity induced by wild-type infection. A zoster vaccine increased the VZV RCF of elderly adults aged <75 years to values higher than peak values induced by wild-type infection.
Varicella-zoster virus (VZV)–specific cell-mediated immunity responses are essential for recovery from primary (varicella) or reactivation (herpes zoster) infection with VZV [1-4]. Patients who lack adequate VZV-specific cell-mediated immunity often have severe and prolonged infections with VZV, some of which are fatal [3-5]. Thus, VZV-specific cell-mediated immunity is a marker for protection against primary VZV infection, and the presence and magnitude of this response correlates with recovery from varicella and with the incidence and severity of reactivation as manifested by herpes zoster. VZV-specific cell-mediated immunity is also a component of primary responses to varicella vaccine administered to susceptible children and adults and has been used to evaluate candidate vaccines to prevent herpes zoster in immunocompromised and elderly individuals [6-9].
In this report, we present a regression model of VZV-specific memory CD4 responses as a function of age, from early childhood to advanced adulthood among healthy individuals with prior VZV wild-type infectionas a reference against which responses of other select groups of individuals are compared. Comparisons are made to elderly recipients of a zoster vaccine and to human immunodeficiency virus (HIV)–infected children and adults. We demonstrate that VZV-specific cell-mediated immunity is determined by the nature of primary immunization (natural infection vs VZV vaccination) and the age and immune status of the subject tested.
SUBJECTS AND METHODS
Subjects
We analyzed VZV-specific responder cell frequency (RCF) data obtained from 752 healthy and 200 HIV-infected individuals with a history of wild-type VZV infection and/or vaccination with the varicella or zoster vaccines and no history of herpes zoster. Data were generated from 1987 through 2005 for the following groups of subjects: healthy individuals with wild-type VZV infection (1987–2005); children and young adults who received varicella vaccine (1988–2005); elderly recipients of zoster vaccine (1999–2002); HIV-infected children and adults with wild-type VZV infection (1996–2003); and HIV-infected children who received varicella vaccine (1996–2003).
VZV-specific RCF
VZV-specific CD4 memory cells were enumerated by adding a limiting dilution step to a conventional lymphocyte proliferation assay [10]. The RCF assay used 24 replicate cultures of 6 serial dilutions of peripheral blood mononuclear cells (PBMCs) ranging from 100,000 to 3125 cells per well. These cells were stimulated with VZV or mock-infected control antigen for 8–10 days, were pulsed with 3H-thymidine for 6 h, and incorporated radioactivity was measured with a beta counter. The RCF was calculated as described by Henry et al [11]. Responder wells were defined as those in which counts per min exceeded the mean counts per min plus 3 standard deviations of the control cultures at the same cell concentration. The percentage of nonresponder wells was plotted on a log scale against the number of cells per well plotted on a linear scale, and the RCF was interpolated at the 37% nonresponder well frequency. Results were expressed as number of responder cells per 1 × 105 PBMCs. The analytical sensitivity of this assay is limited by the highest concentration of cells per well. For this analysis, the lower limit of detection of the assay was 1 responder/105 PBMCs. Values <1 responder/105 PBMCs were censored at 1.
The antigens used in the assays were prepared as described elsewhere [12] by the same laboratory, from the same VZV strain pool. Each new batch of antigen was compared with the previous one by lymphocyte proliferation assay with use of PBMCs from 3–5 donors, including high and low responders. The concentration of each preparation of newly prepared antigen was adjusted to stimulate a proliferative response equal to that of previously used preparations. An analysis described by Bland and Altman [13] of 202 RCF results was used to assess the concordance between RCF results obtained in early and late years of the study. In this analysis, the mean RCF difference of 0.09 (95% confidence interval, −0.09 to 0.27) and the plot of the difference between early and late years against the mean suggested good agreement between assay characteristics throughout the study.
Statistical analysis
VZV-specific RCF data were not normally distributed and were therefore transformed by base 10 logarithm for all analyses. To describe the fluctuation of VZV-specific RCF with age, we used a regression model of log10 RCF as a function of age, a continuous independent variable. A regression model with random intercepts and slopes for each subject was applied if subjects had multiple RCF observations over time [14]. For censored RCF data (below or above the limit of detection of the assay), the regression analysis was modified [15, 16]. The best model was evaluated by a forward step-wise procedure based on a likelihood ratio test. A separate regression analysis resulting in a population average curve of RCF was generated for each group of subjects, defined by vaccination status, and HIV status. In addition, a regression model of RCF from healthy subjects after wild-type VZV infection resulted in a reference population average curve for RCF. We then compared the vertical distance between the curves over observed age ranges from each group. For interpretability, we reported the results on the original scale with 2-sided confidence intervals. These analyses were performed using SAS, version 9.1 (SAS Institute), procedure NLMIXED.
RESULTS
VZV-specific RCF values after wild-type VZV infection of healthy individuals
RCF values, which indicate the frequency of VZV-specific CD4 memory cells, were obtained at a single time point from 540 subjects aged 3–90 years with a history of varicella but no history of herpes zoster. The time after varicella when RCF was measured varied from 10 days in one young adult, to 6 months to 5 years in children, to several decades in the majority of young and in all older adults. The following regression model of log10 RCF on age best described the data:
The model was used to construct a continuous reference curve of VZV RCF on age (Figure 1) and allowed us to estimate the RCF values that resulted from wild-type VZV infection of healthy individuals over a broad age interval. The highest frequency of VZV-specific CD4 memory cells occurred in midadulthood, with a mean of 5.2 responders/105 PBMCs (95% confidence interval, 4.1–6.6 responders/105 PBMCs) at 34 years of age.
Figure 1.
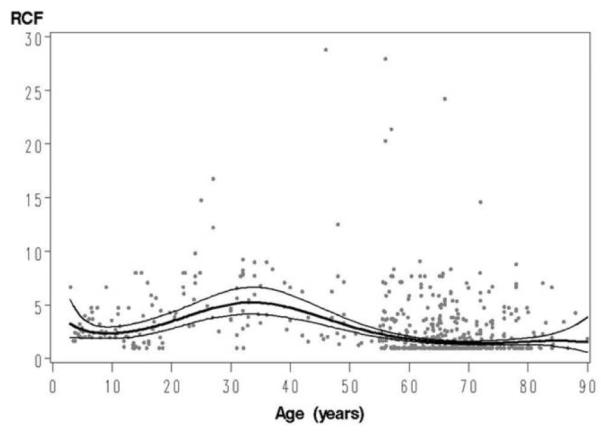
Reference curve of varicella-zoster virus (VZV) responder cell frequency (RCF) on age. Data were derived from 540 individuals with an antibody-confirmed history of wild-type varicella infection and without history of herpes zoster. Lines indicate the regression curve of VZV RCF on age and 95% confidence intervals.
VZV RCF responses to the varicella vaccine in healthy individuals
VZV RCF was measured at a single time point in 27 varicella vaccine recipients aged 1.5–43 years. Vaccination regimens consisted of 1 and 2 doses of vaccine in children and adults, respectively. RCF was measured after vaccination and compared with the unvaccinated reference group with wildtype infection shown in Figure 1. The time elapsed between vaccination and the measurement of RCF varied between 24 days and 7 years. A linear regression model of log10 RCF on age described best the responses to vaccine:
The age-specific comparison of the RCF values of the 27 vaccine recipients in the model with the reference curve of subjects with wild-type varicella (Figure 2) indicated that RCF values of vaccinees were significantly lower than those of the reference group before the age of 5 years but not thereafter. Because of the low number of vaccine recipients aged 11–43 years (n = 7), the results have to be interpreted with caution for persons aged >10 years.
Figure 2.
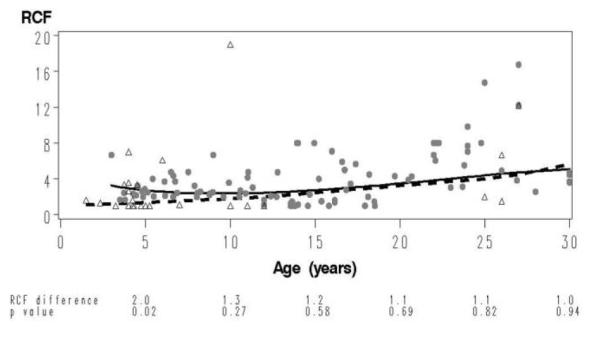
Comparison of the varicella-zoster virus (VZV) responder cell frequency (RCF) on age curve of healthy individuals vaccinated against varicella with the reference curve. The data of the vaccine recipients (open triangles), derived from 27 healthy children and adults aged 1.5–43 years, vaccinated with 1 and 2 doses, respectively, of the varicella vaccine, are superimposed on the data from the reference group (closed circles) for the same age interval. The dashed line depicts the VZV RCF as a function of age in vaccine recipients, and the continuous line depicts that in the reference group. At the bottom of the graph, we show VZV RCF differences between groups and corresponding P values calculated at 5-year intervals from 5 to 30 years of age.
VZV RCF responses to the zoster vaccine in elderly individuals
VZV RCF (n = 332) was measured in 185 varicella-experienced subjects aged 61–90 years, from 6 weeks to 2.3 years after administration of a zoster vaccine. A linear regression model of VZV RCF on age was the best fit for this group:
The age-specific comparison of the VZV RCF of the vaccinated group with the reference group, which acquired VZV immunity solely after varicella, showed significantly higher estimated mean RCF values in vaccinees aged 65–85 years (Table 1). The difference in estimated RCF between the vaccine recipients and the reference group decreased with increasing age.
Table 1.
Age-Specific Varicella-Zoster Virus Responder Cell Frequency (RCF) Responses to the Zoster Vaccine and to Distant Wild-Type Varicella
| RCF estimate, responder cells/105 PBMCs |
|||
|---|---|---|---|
| Age, years | Vaccinateda | Wild- type varicella | P |
| 65 | 30.9 | 1.6 | <.001 |
| 70 | 19.5 | 1.5 | <.001 |
| 75 | 12.3 | 1.5 | <.001 |
| 80 | 7.6 | 1.6 | <.001 |
| 85 | 4.8 | 1.7 | .003 |
NOTE. PBMC, peripheral blood mononuclear cell.
All vaccinated subjects had varicella-zoster wild-type infection before vaccination.
A comparison of the RCF values of the vaccinated elderly subjects with the RCF values of 34-year-old subjects who had only prior varicella (and had the highest VZV RCF estimates of subjects with prior varicella) showed that the 60–75-year-old recipients of zoster vaccine developed higher VZV RCF estimates than this younger unvaccinated group (RCF estimates, 50.1 and 5.2 responder cells/105 PBMCs, respectively; P < .01). However, the VZV RCF of vaccinees aged 80 years was not higher (RCF estimate, 7.6 responder cells/105 PBMCs; P = .11).
VZV RCF responses after wild-type varicella in HIV-infected children and adults
The VZV-specific RCF values from 88 HIV-infected children and adolescents (171 samples) aged 1–23 years were analyzed. Children had mean CD4 and log10 HIV RNA levels in plasma of 33% and 2.7 copies/mL, respectively [17, 18]. The RCF estimate for the group was <1 responder cell/105 PBMCs. In the final model, the RCF was not a function of age. The age-specific comparison between the RCF results of the HIV-infected children and adolescents with the healthy-subject reference group showed significant differences of ≥3.2 responder cells/105 PBMCs (P < .01) for all age groups (Figure 3).
Figure 3.
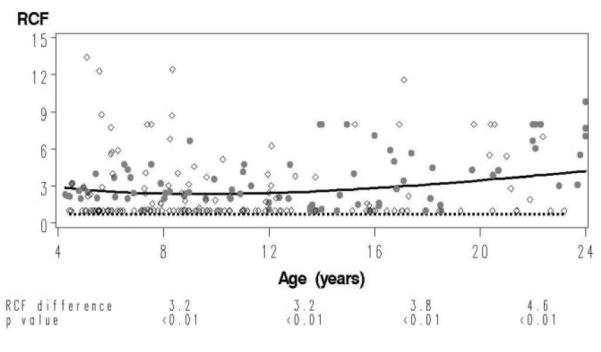
Comparison of varicella-zoster virus (VZV) responder cell frequency (RCF) as a function of age in human immunodeficiency virus (HIV)–infected children after chickenpox with the reference curve. The data derived from 88 HIV-infected children aged 1–23 years (open circles) with an antibody-confirmed history of varicella are superimposed on the data from the reference group (closed circles) for the same age interval. The dotted line depicts the VZV RCF as a function of age in HIV-infected children, and the continuous line depicts that in the reference group. At the bottom of the graph, we show VZV RCF differences between groups and corresponding P values calculated at 4-year intervals from 8 to 20 years of age.
VZV RCF was also measured in 64 HIV-infected adults distributed among the following 3 age groups: 21–35 years (n = 14), 36–50 (n = 32), and 51–65 years (n = 18) [19]. All received highly active antiretroviral therapy for ≥3 months, had CD4 counts >400 cells/μL, and had plasma HIV RNA levels <1000 copies/mL. The VZV RCF estimates for each group were <1, 1.5, and 1.4 responders/105 PBMCs, respectively. These values were compared with RCF estimates from the reference group at 28, 43, and 59 years of age (Table 2). The difference between HIV-infected subjects and the reference group was highly significant for age 28 and age 43 (P < .01) but not for age 59.
Table 2.
Varicella-Zoster Virus–Specific Responder Cell Frequency (RCF) after Wild-Type Varicella-Zoster Virus Infection in Human Immunodeficiency Virus (HIV)–Infected Adults, Compared with the Healthy Reference Group
| RCF estimate, responder cells/105 PBMCs |
|||
|---|---|---|---|
| Age, years | HIV-infected | Healthy | P |
| 28 | <1 | 4.8 | <.001 |
| 43 | 1.5 | 4.3 | <.001 |
| 59 | 1.4 | 1.9 | .13 |
NOTE. PBMC, peripheral blood mononuclear cell.
VZV-specific RCF responses of HIV-infected children to the varicella vaccine
VZV RCF responses (n = 100) to varicella vaccine in 48 HIV-infected children aged 2–10 years were analyzed. Before vaccination, children had median CD4 and log10 HIV RNA levels in plasma of 36% and 3.2 copies/mL, respectively [6, 7]. RCF was measured 8–104 weeks after they received 2 doses of varicella vaccine administered 12 weeks apart. The RCF estimate was 1.7 responder cells/105 PBMCs. In the final model, RCF was not a function of age. The RCF estimate of HIV-infected vaccine recipients was significantly higher than that of HIV-infected children with prior varicella (RCF difference, 2.2 responder cells/105 PBMCs [95% confidence interval, 1.2–4.2 responder cells/105 PBMCs]; P = .008). Furthermore, at any age, the RCF estimates of the vaccinated HIV-infected children were not significantly different from those of healthy children with wild-type VZV infection (Table 3).
Table 3.
Age-Specific Comparison of Responder Cell Frequency (RCF) Estimates in Healthy Children with Wild-Type Varicella-Zoster Virus (VZV) Infection Versus Human Immunodeficiency Virus (HIV)–Infected Children Who Received Varicella Vaccine
| RCF estimate, in responder cells/105 PBMCs |
|||
|---|---|---|---|
| Age, years | Healthy children with wild-type VZV infection |
HIV-infected children after 2 doses of VZV vaccine |
P |
| 3 | 3.2 | 1.7 | .07 |
| 4 | 3.0 | 1.7 | .09 |
| 5 | 2.7 | 1.7 | .12 |
| 6 | 2.5 | 1.7 | .15 |
| 7 | 2.5 | 1.7 | .18 |
| 8 | 2.3 | 1.7 | .21 |
| 9 | 2.3 | 1.7 | .22 |
| 10 | 2.3 | 1.7 | .22 |
NOTE. PBMC, peripheral blood mononuclear cell.
DISCUSSION
We developed a regression model of VZV-specific memory CD4 responses as a function of age, from early childhood to advanced adulthood, among healthy individuals who had wildtype VZV infection. This model allowed us to construct a reference curve of VZV RCF on age, against which we compared VZV RCF results of select groups of VZV-experienced hosts. Because we defined a continuous relationship between VZV RCF and age, the comparisons of the select groups with the reference group did not require exact age matching.
The reference model showed that VZV RCF was highest in young adults, despite the fact that most individuals contract VZV infection during childhood. This is contrast with other studies [20, 21], which failed to detect a bell shape relationship of VZV-specific cell-mediated immunity on age, perhaps because of limited sample size and use of semiquantitative or qualitative cell-mediated immunity measurements.
In our study, the majority of the VZV cell-mediated immunity data were generated prior to widespread administration of varicella vaccine, when wild-type VZV was circulating in the community. The curve of the VZV RCF as a function of age, with a peak at the age of 34 years, most likely reflects environmental boosting in response to exposure to varicella at home and in the community. Environmental boosting has been demonstrated by measuring responses in individuals after known exposures to varicella [22, 23] and has been suggested by modeling and case-control studies of large populations [24-26]. Universal varicella vaccination may remove environmental boosting and accelerate the loss of VZV-specific memory CD4 cells that normally occurs with age. This may increase the likelihood that herpes zoster will occur earlier in life for individuals with latent wild-type VZV infection [24-27].
After the age of 55 years, VZV-specific memory CD4 responses became undetectable in 30%–40% of subjects, contributing to the decrease of the VZV RCF estimates in the older groups. This age-specific decrease in VZV memory CD4 cells is the likely explanation for the increase in the incidence of herpes zoster with age. It is noteworthy that VZV RCF values began to decrease at ~35 years, although it is unlikely that environmental exposure ceased at that age.
An effective vaccine against herpes zoster was licensed in 2006 in the United States [28]. Our studies of the VZV RCF responses to a vaccine similar to the licensed zoster vaccine indicate that there is an inverse relationship between age and VZV RCF responses to the vaccine, which is in agreement with other studies that used the licensed zoster vaccine [8]. Although the VZV RCF estimate decreased with increasing age in older zoster vaccine recipients, it was significantly higher than the VZV RCF of individuals over the same age range in the reference group. It is noteworthy that elderly vaccinees aged ≤75 years had higher VZV RCF results than the young adults after wild-type infection, but this observation has to be interpreted with caution because the VZV RCF of elderly vaccinees was measured 6 months to 2 years after immunization, whereas the VZV RCF of young adults was measured after an unknown length of time from the last exposure to VZV. We previously showed that the RCF values observed in older individuals after vaccination are comparable to the RCF values after herpes zoster [29]. The boost in VZV-specific cell-mediated immunity conferred by the vaccine is probably important for preventing or aborting reactivation of latent VZV and is the presumed explanation for the efficacy of the zoster vaccine in preventing and attenuating herpes zoster in elderly people [8, 29].
In initially seronegative varicella vaccine recipients, the VZV RCF was positively correlated with age at the time of testing. Because the VZV RCF was measured at variable times after the administration of the vaccine, environmental boosting may have contributed to the growth curve of VZV RCF on age. This was also demonstrated with measurements of VZV-specific antibodies in vaccinees followed over a decade [30]. In these children, antibodies increased with the interval after vaccination. The comparison of VZV RCF of varicella vaccine recipients with the reference group showed lower VZV RCF in the vaccinees aged <5 years, compared with children of the same age who had wild-type VZV infection. This result is consistent with the observation that some children do not seroconvert after vaccination when seroconversion is measured with a highly specific VZV antibody test, FAMA [31]. Our observation is also consistent with the higher incidence of varicella break-through disease in vaccine recipients, compared with the extremely low incidence of secondary cases of varicella after wild-type VZV infection [30, 32]. To address the varicella break-through issue, the childhood vaccination schedule currently includes a second dose of varicella vaccine at 5 or 6 years of age [33].
The VZV RCF estimates were very low and did not vary with age in HIV-infected children and adolescents with previous wild-type VZV infection. In HIV-infected adults, the limitations of the available data did not support construction of a regression model of VZV RCF on age, but the differences in VZV RCF among 3 age categories (21–35, 36–50, and >50 years) were not appreciable. The flatness of the VZV RCF on age curve in HIV-infected individuals suggests a lack of environmental boosting, most likely because the underlying immunosuppression impedes significant postexposure boosts of cell-mediated immunity. The low VZV RCF values in HIV-infected individuals are consistent with the high incidence of herpes zoster in this population, which persists in the era of highly active antiretroviral therapy [34, 35]. The excellent VZV RCF responses to the varicella vaccine observed in HIV-infected children (see below) suggest that HIV-infected individuals with prior varicella may respond adequately to a zoster vaccine and become protected against herpes zoster.
We studied VZV RCF responses to a 2-dose varicella vaccine regimen in VZV-seronegative HIV-infected children with CD4 levels ≥20% receiving stable antiretroviral therapy. In contrast to normal hosts with wild-type infection or primary VZV vaccination, VZV RCF of HIV-infected vaccine recipients did not vary with age. Responses of HIV-infected children were uniformly assessed at the same intervals after vaccination, minimizing the potential interaction between age and environmental boost. However, it is likely that the underlying immunosuppression in these children may have also contributed to the obfuscation of the effect of age on immune responses, as it did for RCF responses after wild-type infection in HIV-infected children. Although there was a difference in the ethnic composition of HIV-infected children in this study (50% African American, 25% white, and 24% Hispanic), compared with the ethnic composition of the uninfected children in this study (5% African American, 60% white, and 20% Hispanic), this difference is unlikely to have affected the comparison of their responses to VZV, because within each group of HIV-infected or uninfected children, responses did not vary with ethnicity. VZV RCF estimates of HIV-infected vaccinees were higher than those of HIV-infected children with wild-type infection. This is most likely attributable to the fact that vaccination occurred at a time when the HIV infection was under relatively good control, with or without antiretroviral treatment, attested by CD4 levels ≥20% [6, 7]. In contrast, wild-type VZV infection may have occurred during or been followed by periods of profound immunosuppression, when children were unable to mount vigorous VZV RCF responses or lost specific T cell responses, respectively [5]. VZV RCF of HIV-infected children after vaccination or chickenpox did not correlate with CD4 percentage at the time of vaccination or blood sample collection. In vaccine recipients, the RCF correlated with the plasma HIV RNA level at the time of vaccination only, whereas after varicella, the RCF correlated with the plasma HIV RNA at the time of the blood sample collection. VZV RCF estimates of HIV-infected varicella vaccine recipients did not significantly differ from those of the reference group, supporting the protective effect of the vaccine in this population of immunocompromised children and adolescents.
The magnitude of VZV RCF varied with the nature of the primary immunizing event (wild-type infection or vaccine) and the age and immunocompetence of the host. The latter are important determinants of clinically apparent VZV reactivation in individuals with latent wild-type VZV infection. The effect of aging and immunocompetence on VZV reactivation in individuals whose latent virus is of vaccine origin is uncertain.
Acknowledgments
We thank Li Zhang, Julie Patterson-Bartlett, Laura Enomoto, Mary Cosyns, and Michelle Jones for technical support.
Financial support: Eunice Kennedy Shriver National Institute of Child Health and Human Development contracts N01-HD-33162 (to A.W.) and N01-HD-3-3345 (to M.J.L.), National Institute of Allergy and Infectious Diseases contract U01 AI068632, Merck Research Laboratories (to M.J.L.), and T32 CA-09337 (to A.A.L.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies.
Footnotes
Potential conflicts of interest: A.W., A.A.G., and M.J.L. received research funds or consultation fees from or are on the Speakers’ Bureau for Merck; M.J.L. claims intellectual property in Merck regarding the use of VZV vaccine to prevent herpes zoster; I.S.F.C., J.L.S., and R.V. are employees of Merck.
a Present affiliations: Department of Biostatistics, Harvard School of Public Health and Dana-Farber Cancer Institute, Boston, MA (A.A.L). National Institutes of Health, Bethesda, MD (A.R.H.).
References
- 1.Berger R, Florent G, Just M. Decrease of the lymphoproliferative response to varicella-zoster virus antigen in the aged. Infect Immun. 1981;32:24–27. doi: 10.1128/iai.32.1.24-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchbinder SP, Katz MH, Hessol NA, et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992;166:1153–1156. doi: 10.1093/infdis/166.5.1153. [DOI] [PubMed] [Google Scholar]
- 3.Buda K, Tubergen DG, Levin MJ. The frequency and consequences of varicella exposure and varicella infection in children receiving maintenance therapy for acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 1996;18:106–112. doi: 10.1097/00043426-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Furth SL, Sullivan EK, Neu AM, Tejani A, Fivush BA. Varicella in the first year after renal transplantation: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Pediatr Transplant. 1997;1:37–42. [PubMed] [Google Scholar]
- 5.Gershon AA, Mervish N, LaRussa P, et al. Varicella-zoster virus infection in children with underlying human immunodeficiency virus infection. J Infect Dis. 1997;176:1496–1500. doi: 10.1086/514147. [DOI] [PubMed] [Google Scholar]
- 6.Levin MJ, Gershon AA, Weinberg A, et al. Immunization of HIV-infected children with varicella vaccine. J Pediatr. 2001;139:305–310. doi: 10.1067/mpd.2001.115972. [DOI] [PubMed] [Google Scholar]
- 7.Levin MJ, Gershon AA, Weinberg A, Song LY, Fentin T, Nowak B. Administration of live varicella vaccine to HIV-infected children with current or past significant depression of CD4(+) T cells. J Infect Dis. 2006;194:247–255. doi: 10.1086/505149. [DOI] [PubMed] [Google Scholar]
- 8.Levin MJ, Oxman MN, Zhang JH, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberg A, Horslen SP, Kaufman SS, et al. Safety and immunogenicity of varicella-zoster virus vaccine in pediatric liver and intestine transplant recipients. Am J Transplant. 2006;6:565–568. doi: 10.1111/j.1600-6143.2005.01210.x. [DOI] [PubMed] [Google Scholar]
- 10.Hayward AR, Zerbe GO, Levin MJ. Clinical application of responder cell frequency estimates with four years of follow up. J Immunol Methods. 1994;170:27–36. doi: 10.1016/0022-1759(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 11.Henry C, Marbrook J, Vann DC, Kodlin DC, Wojsy C. Limiting dilution analysis. In: Mishell BB, Shigii SM, editors. Selected methods in cell mediated immunity. Freeman Press; San Francisco: 1980. pp. 138–152. [Google Scholar]
- 12.Zaia JA, Leary PL, Levin MJ. Specificity of the blastogenic response of human mononuclear cells to herpesvirus antigens. Infect Immun. 1978;20:646–651. doi: 10.1128/iai.20.3.646-651.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 14.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 15.Hughes JP. Mixed effects models with censored data with application to HIV RNA levels. Biometrics. 1999;55:625–629. doi: 10.1111/j.0006-341x.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 16.Thiébaut R, Jacqumin-Gadda H, Leport C, et al. Bivariate longitudinal model for the analysis of the evolution of HIV RNA and CD4 cell count in HIV infection taking into account left censoring of HIV RNA measures. J Biopharm Stat. 2003;13:271–282. doi: 10.1081/BIP-120019271. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg A, Wiznia AA, LaFleur BJ, Shah S, Levin MJ. Varicella-zoster virus-specific cell-mediated immunity in HIV-infected children receiving highly active antiretroviral therapy. J Infect Dis. 2004;190:267–270. doi: 10.1086/422011. [DOI] [PubMed] [Google Scholar]
- 18.Gershon A, Levin MJ, Weinberg A, et al. A phase I-II study of live attenuated varicella-zoster virus vaccine to boost immunity in human immunodeficiency virus-infected children with previous varicella. Pediatr Infect Dis. 2009;28(7):653–655. doi: 10.1097/INF.0b013e3181998f06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg A, Levin MJ, MacGregor RR. Safety and immunogenicity of a live attenuated varicella vaccine in VZV-seropositive HIV-infected adults. Human Vaccines. doi: 10.4161/hv.6.4.10654. (in press) [DOI] [PubMed] [Google Scholar]
- 20.Burke BL, Steele RW, Beard OW, Wood JS, Cain TD, Marmer DJ. Immune responses to varicella-zoster in the aged. Arch Intern Med. 1982;142:291–293. [PubMed] [Google Scholar]
- 21.Miller AE. Selective decline in cellular immune response to varicellazoster in the elderly. Neurology. 1980;30:582–587. doi: 10.1212/wnl.30.6.582. [DOI] [PubMed] [Google Scholar]
- 22.Arvin AM, Koropchak CM, Wittek AE. Immunologic evidence of reinfection with varicella-zoster virus. J Infect Dis. 1983;148:200–205. doi: 10.1093/infdis/148.2.200. [DOI] [PubMed] [Google Scholar]
- 23.Gershon AA, LaRussa P, Steinberg S, Mervish N, Lo SH, Meier P. Theprotective effect of immunologic boosting against zoster: an analysis in leukemic children who were vaccinated against chickenpox. J Infect Dis. 1996;173:450–453. doi: 10.1093/infdis/173.2.450. [DOI] [PubMed] [Google Scholar]
- 24.Brisson M, Gay NJ, Edmunds WJ, Andrews NJ. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine. 2002;20:2500–2507. doi: 10.1016/s0264-410x(02)00180-9. [DOI] [PubMed] [Google Scholar]
- 25.Chaves SS, Gargiullo P, Zhang JX, et al. Loss of vaccine-induced immunity to varicella over time. N Engl J Med. 2007;356:1121–1129. doi: 10.1056/NEJMoa064040. [DOI] [PubMed] [Google Scholar]
- 26.Thomas SL, Wheeler JG, Hall AJ. Contacts with varicella or with children and protection against herpes zoster in adults: a case-control study. Lancet. 2002;360:678–682. doi: 10.1016/S0140-6736(02)09837-9. [DOI] [PubMed] [Google Scholar]
- 27.Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28(11):954–959. doi: 10.1097/INF.0b013e3181a90b16. [DOI] [PubMed] [Google Scholar]
- 28.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg A, Zhang JH, Oxman MN, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200:1068–1077. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuter B, Matthews H, Shinefield H, et al. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004;23:132–137. doi: 10.1097/01.inf.0000109287.97518.67. [DOI] [PubMed] [Google Scholar]
- 31.Michalik DE, Steinberg SP, Larussa PS, et al. Primary vaccine failure after 1 dose of varicella vaccine in healthy children. J Infect Dis. 2008;197:944–949. doi: 10.1086/529043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shu L, Chan ISF, Matthews H, Heyse JF. Inverse relationship between six week postvaccination varicella antibody response to vaccine and likelihood of long term breakthrough infection. Pediatr Infect Dis J. 2002;21:337–342. doi: 10.1097/00006454-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Prevention of varicella: recommendations for use of varicella vaccines in children, including a recommendation for a routine 2-dose varicella immunization schedule. Pediatrics. 2007;120:221–231. doi: 10.1542/peds.2007-1089. [DOI] [PubMed] [Google Scholar]
- 34.Levin MJ, Anderson JP, Seage GR, 3rd, Williams PL. Short-term and long-term effects of highly active antiretroviral therapy on the incidence of herpes zoster in HIV-infected children. J Acquir Immune Defic Syndr. 2009;50:182–191. doi: 10.1097/QAI.0b013e31819550a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanhems P, Voisin L, Gayet-Ageron A, et al. The incidence of herpes zoster is less likely than other opportunistic infections to be reduced by highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2005;38:111–113. doi: 10.1097/00126334-200501010-00020. [DOI] [PubMed] [Google Scholar]


