Abstract
Subjective tinnitus, the phantom ringing or buzzing sensation that occurs in the absence of sound, affects 12–14% of adults; in some cases the tinnitus is so severe or disabling that patients seek medical treatment. However, although the economic and emotional impact of tinnitus is large, there are currently no FDA-approved drugs to treat this condition. Clinical trials are now underway to evaluate the efficacy of N-methyl-d-aspartate (NMDA) and dopamine D2 antagonists, selective serotonin reuptake inhibitors (SSRIs), γ-aminobutyric acid (GABA) agonists and zinc dietary supplements. Previous off-label clinical studies, while not definitive, suggest that patients with severe depression may experience improvement in their tinnitus after treatment with antidepressants such as nortriptyline or sertraline. A small subpopulation of patients with what has been described as “typewriter tinnitus” have been shown to gain significant relief from the anticonvulsant carbamazepine. Preliminary studies with misoprostol, a synthetic prostaglandin E1 analogue, and sulpiride, a dopamine D2 antagonist, have shown promise. Animal behavioral studies suggest that GABA transaminase inhibitors and potassium channel modulators can suppress tinnitus. Additionally, improvements in tinnitus have also been noted in patients taking melatonin for significant sleep disturbances. Like other complex neurological disorders, one drug is unlikely to resolve tinnitus in all patients; therapies targeting specific subgroups are likely to yield the greatest success.
INTRODUCTION
Tinnitus, from the Latin word tinnire, refers to a condition in which a patient experiences a ringing, buzzing or hissing auditory sensation in the absence of an external sound. Tinnitus is broadly classified as being either objective or subjective. The objective form, which is rare, refers to a condition in which a real sound is generated by an internal biological activity, such as vascular turbulence or pulsations or spasm of the muscles in the middle ear, Eustachian tube or soft palate. Subjective tinnitus, the most common type and the subject of this review, refers to a phantom auditory sensation for which no objective sound can be identified.
Individuals who experience severe and disabling tinnitus often seek medical treatment from an otologist, neurologist or psychiatrist with the hope of finding a drug or surgical treatment that can completely switch off their tinnitus and bring back silence. Disappointment and disbelief set in when patients are told by their physician that they must learn to live with it or try nonmedicinal approaches involving some form of sound therapy and counseling. While sound therapy and counseling have been found to reduce the severity of tinnitus, often by reducing the emotional or psychological impact, many patients would prefer a “magic pill” that completely abolishes their tinnitus in a matter of hours or days. This review highlights some of the recent pharmacological studies that have been carried out with a variety of agents in the treatment of tinnitus.
PREVALENCE, CHARACTERISTICS AND IMPACT OF TINNITUS
Prevalence
Most individuals at one time or another experience transient tinnitus lasting seconds or minutes; however, others experience long-lasting tinnitus that can persist for a lifetime. The prevalence of tinnitus among those over 65 years of age ranges from 12% to 15% (1–6). Approximately < 1% of the population suffers from severe, debilitating tinnitus that negatively impacts sleep, concentration, work and quality of life, and which requires medical treatment or counseling. Tinnitus occurs roughly equally in females and males (7, 8). As with most disorders, the prevalence of tinnitus increases with age and peaks at around 60–70 years (7).
In 2005, it was estimated that the potential market in the U.S. for tinnitus treatment was approximately $10 billion (9). The Veterans Administration Benefits Report ranked tinnitus as the second most prevalent service-related disability. Among those who began receiving benefits in 2006, tinnitus was ranked first among service-related disabilities at 9.7% of the total (http://www.vba.va.gov/bln/21/). In 2005, the annual compensation for tinnitus-related disability was $418 million. The economic impact of this disabling disorder, combined with the growing number of noise-exposed combat personnel and the swelling ranks of elderly individuals with tinnitus (10, 11), has generated renewed interest in finding therapeutic compounds that can suppress or manage tinnitus.
Perceptual characteristics of tinnitus
A little over half of tinnitus patients perceive the phantom sound as coming from inside the ear (tinnitus aurium), suggesting that tinnitus might originate in the inner ear. Others report that the phantom sound is located inside the head (tinnitus cerebri), raising the possibility of a central origin (12, 13), while a few patients perceive the phantom sound as coming from outside the head. Approximately 60% of patients experience bilateral tinnitus, while the remainder hear the sound in just one ear (14).
Patients often undergo detailed audiometric and psychoacoustic evaluation to assess the status of the auditory pathway. The pure- tone audiogram, which identifies the frequencies where hearing is impaired, is an important procedure, as tinnitus is generally associated with hearing loss. In younger individuals hearing loss is most often associated with noise exposure, while in older individuals it is often associated with age-related hearing loss or presbycusis. The prevalence of tinnitus steadily increases with the degree of age-related hearing loss (7). Despite the frequent association between hearing loss and tinnitus, many individuals with hearing loss do not experience tinnitus. Conversely, some individuals with normal hearing experience tinnitus. The definition of “normal” hearing, however, can be misleading, because most hearing tests are only performed at octave intervals from 125 to 8000 Hz. It is conceivable that these “normal” listeners may actually have undetected hearing loss at interoctave intervals, or hearing loss between 8000 and 20,000 Hz, where hearing is seldom evaluated, or other auditory disorders that are not detected by an audiogram. Clinical trials that assess the efficacy of drugs used to treat tinnitus often use psychoacoustic tests to determine if the loudness, pitch or other qualities of the tinnitus change as a result of treatment. In some individuals, the perceptual qualities of tinnitus may fluctuate from morning to evening or from day to day, while in others the tinnitus remains stable over time (15). If the patient’s tinnitus fluctuates, it is important to have several baseline measurements prior to the start of treatment.
The pitch and spectral qualities of tinnitus vary from one individual to the next, but the most common descriptors used are those of ringing, buzzing, hissing or whistling (16). Because the auditory system is organized tonotopically such that different frequencies activate different anatomical locations in the auditory pathway (similar to a piano keyboard), the pitch and spectral qualities of the tinnitus may provide clues regarding the neurons that trigger the phantom sensation. The pitch or spectral qualities of tinnitus are often located in the region of maximum hearing loss or at the boundary between normal and abnormal hearing (17, 18). These results suggest that the neurons responsible for the perception of tinnitus may be located at the edge or boundary of maximum hearing loss (19).
Loudness, a perceptual phenomenon, is most closely related to sound intensity, a physical measure assessed in units of decibels (dB). Loudness measurements of tinnitus are often obtained with a 10-point visual analog scale, with a score of 10 being very loud and a score of 1 being soft. When patients are asked to rate the subjective loudness of their tinnitus, the average loudness rating is ~6.3, suggesting that the phantom sound is moderately loud (20). Other approaches estimate tinnitus loudness by having the subject adjust the level of an external sound so that it matches the perceived loudness of their tinnitus. In most cases, the tinnitus is matched to an external tone less than 10 dB above the threshold of hearing (< 10 dB sensation level, or 10 dB SL). These low dB SL values may suggest that the tinnitus is not very loud; however, such results may be misleading because dB SL is a measure referenced to the individual’s threshold of hearing at that frequency. If a patient has a 40-dB hearing loss and matches the loudness of the tinnitus to 10 dB SL, the sound level would be 50 dB HL, i.e., 50 dB above the normal threshold for hearing at that frequency. Additionally, when a patient suffers from hearing loss, they often experience loudness recruitment, i.e., the loudness of a sound increases rapidly once the sound level exceeds the patient’s threshold.
Masking, the ability of one loud sound to cover up a less intense sound, has been used as a treatment for tinnitus, allowing the patient to voluntarily escape from the tinnitus (21, 22). The minimum masking intensity provides an objective method of assessing the loudness of tinnitus. Masking can be expressed in dB SPL (sound pressure level), an acoustic measure, or in terms of dB SL, the sound level relative to the subject’s threshold. The minimum masking level can be used to assess the severity of tinnitus (23) and to evaluate the ability of a drug or devices to ameliorate tinnitus severity (24–27). In many cases, tinnitus can be masked at relatively low intensities; in others, high intensities are required, and in a few cases, the tinnitus is unmaskable or made worse by external sounds (16, 28, 29). Masking also provides insights into the neural origins of tinnitus. When a real tone is presented to one ear, it is easily masked by sounds presented to the same ear, but difficult to mask by sounds presented to the opposite ear (30). In contrast, when the phantom sound of tinnitus is perceived in one ear, it can usually be masked by a sound presented to the same ear or the opposite ear (31, 32). One interpretation of these finding is that the neural generator for tinnitus resides in the central auditory system where neural responses from the two ears can interact with one another, rather than in the inner ear where the neural responses are largely independent. Another striking difference between a real sound and tinnitus is that the masker sometimes becomes less effective in covering up the tinnitus the longer it is presented, whereas masking of external sounds remains relatively constant over time (33, 34).
Emotional and psychological responses to tinnitus
A patient’s emotional or psychological response to tinnitus can vary from one individual to the next, even among patients whose tinnitus is similar. Consequently, it is important to determine how a drug affects a person’s social or emotional response to tinnitus, as well as its perceptual qualities. Frequently used questionnaires for assessing a patient’s reaction to tinnitus are the Tinnitus Handicap Inventory, Tinnitus Handicap Questionnaire and Tinnitus Reaction Questionnaire (35–38). These questionnaires focus on issues related to the degree of handicap, sleep, social interactions, emotion, concentration, depression and annoyance of tinnitus. Drugs used to treat tinnitus should either reduce the tinnitus perception or the emotional response, and ideally both.
Tinnitus classification
Subjective tinnitus is associated with many different diseases and disorders, and it sometimes emerges as a side effect of medications used to treat other diseases (39). Attempts have been made to classify different forms of tinnitus based on the site of lesion, the patient’s description, the clinician’s observations, the perceptual characteristics, causation, neurophysiological characteristics, response to treatment and various combinations of these factors (12, 40–43). While none of the classification schemes is widely accepted, there is evidence that certain types of tinnitus may respond well to a particular type of drug therapy (44–47).
DRUG THERAPY FOR TINNITUS: PHARMACOLOGICAL STUDIES
Although tinnitus is a significant health and economic problem, there are no FDA-approved drugs to treat tinnitus (9) and few drugs reliably suppress or eliminate chronic tinnitus in the majority of patients (48–50). The lack of drug therapies is due in part to a limited understanding of the biological basis of tinnitus, the lack of an accepted tinnitus nosology, the heterogeneity of the tinnitus population, the wide range of medical conditions that appear to cause tinnitus and the huge cost associated with developing drugs to specifically treat tinnitus. Consequently, drugs developed for other medical conditions have generally been evaluated to determine whether they can relieve tinnitus. While several double-blind, placebo-controlled, crossover studies have been carried out in tinnitus patients, many reports suffer from the lack of proper experimental controls and small sample sizes. However, advances have been made in the development of animal models in which to test compounds that can suppress noise- or drug-induced tinnitus (51–56). The following sections review many of the drugs used to suppress tinnitus, with a focus primarily on drugs administered systemically rather than locally (57, 58), as oral dosing is most likely to gain widespread acceptance because of convenience, ease of titration and scheduling.
Acamprosate
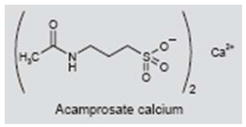
Acamprosate (Campral®) is approved for the treatment of alcoholism in the U.S. and Europe. It presumably blocks excitatory glutamatergic N-methyl-d-aspartate (NMDA) receptors (59–61) while enhancing γ-aminobutyric acid (GABA)-mediated nerve inhibition (62–64). However, some studies indicate that acamprosate enhances NMDA function, while others indicate that the compound enhances NMDA-induced excitability at low concentrations and suppresses it at high concentrations (62–65). Other reports suggest that acamprosate has no effect on GABA-mediated currents (66), and some reports suggest that acamprosate inhibits the binding of taurine to taurine receptors (67).
One paper has been published on the use of acamprosate to treat tinnitus patients, most of whom had mild to profound noise-induced hearing loss (68, 69). The rationale for treatment assumes that tinnitus arises from excess glutamatergic activity through NMDA receptors and/or hyperactivity resulting from the loss of GABA-mediated inhibition (70–75). In this double-blind study, patients received placebo or acamprosate (333 mg t.i.d.) and rated the loudness and annoyance of their tinnitus before and at monthly intervals of treatment. Acamprosate had no beneficial effects after 30 days of treatment, a modest benefit at 60 days and a significant effect at 90 days. Approximately 87% of the subjects in the acamprosate group showed some improvement, including three subjects in whom tinnitus disappeared, compared to 44% in the placebo group. A larger clinical trial is currently under way to further assess the encouraging results from this preliminary study (http://clinicaltrials.gov/ct2/show/NCT00596531).
Caroverine
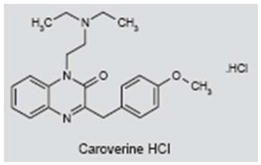
Caroverine (Spasmium-R®) is used as a spasmolytic drug and acts as an antagonist of calcium and non-NMDA and NMDA glutamate receptors (76–80). Because of a limited uptake with oral administration, caroverine is administered intravenously or locally (58, 81). It has been proposed that cochlear synaptic tinnitus arises from a synaptic disturbance of NMDA or non-NMDA receptors on the afferent dendrites of the spiral ganglion neurons (70, 82–84). To test this hypothesis, patients with putative cochlear synaptic tinnitus were enrolled in a single-blind study in which subjects were randomly assigned to a placebo group (i.v. saline) or a caroverine group (a maximum dose of 160 mg i.v.; less if tinnitus reduced or worsened). Tinnitus loudness (5-point scale) and tinnitus matching (intensity and frequency) were measured before and after treatment and at 1 week post-treatment. Significant responders were those that showed a reduction in loudness of 1 point or more and a 50% reduction in intensity (−3 dB). Immediately post-treatment, 63% of the caroverine group and 0% of the placebo group showed a significant response. At 1 week post-treatment, 43% in the caroverine group had a significant positive response compared to 3% in the placebo group. The low positive response rate in the placebo group was unusual, because placebo response rates are typically around 40% (85). While these initial results were encouraging, a subsequent study following the same protocol found no positive effect of caroverine treatment (86).
Memantine
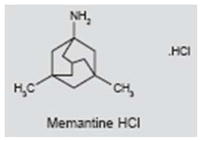
Memantine (Namenda®) is currently used in the treatment of Alzheimer’s disease (AD) and has shown positive effects in depression (87, 88). It acts as a voltage-dependent antagonist of NMDA receptors and reduces excitotoxicity by preventing prolonged influx of calcium (89, 90). However, memantine is also known to block serotonin (5-HT) and nicotinic acetylcholine receptors (91, 92). Excitotoxicity mediated by NMDA receptors has been proposed as a mechanism for cochlear tinnitus (70, 82–84). High doses of salicylate, the active ingredient in aspirin, reliably induce tinnitus and augment currents through NMDA receptors on cochlear spiral ganglion neurons (93–95). NMDA antagonists applied locally to the inner ear blocked behavioral evidence of salicylate-induced tinnitus (83). In another behavioral experiment, cochlear application of the selective NMDA antagonist ifenprodil in the first 4 days following noise exposure significantly reduced the probability of developing noise-induced tinnitus (84). In contrast to the positive results seen with cochlear application of NMDA antagonists, systemic memantine administration failed to completely suppress salicylate-induced tinnitus in a behavioral model of tinnitus (51). Likewise, in a prospective, randomized, double-blind, crossover clinical study using the Tinnitus Handicap Inventory to assess efficacy, 90-day treatment with memantine was no more effective than placebo. Moreover, memantine caused side effects in 9% of patients (96).
AM-101
AM-101 is a noncompetitive NMDA antagonist that is being evaluated as a treatment for tinnitus. Based on positive results in animal studies, a double-blind, randomized, placebo-controlled phase IIb trial of intratympanic delivery of AM-101 is currently being carried out. The study involves patients with acute tinnitus (< 3 months) arising from noise trauma or sudden hearing loss who have not responded to glucocorticoid treatment (http://www.aurismedical.com/p/therapies/am_101.php). Patients with acute tinnitus are being enrolled in a clinical trial with AM-101 (http://clinicaltrials.gov/ct2/show/NCT00860808).
Neramexane
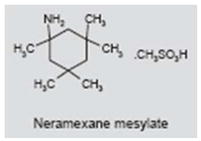
Neramexane, a drug similar to memantine, is being evaluated for AD, drug dependence, depression and pain. Like memantine, neramexane acts as a noncompetitive, voltage-dependent NMDA antagonist. It also blocks α9 and α10 nicotinic cholinergic receptors which are expressed on inner hair cells in the inner ear (97, 98). Developer Merz & Co GmbH is currently conducting a multicenter clinical trial to determine the efficacy of neramexane for treating tinnitus (http://clinicaltrials.gov/ct2/show/NCT00772980).
Gacyclidine
Gacyclidine is another NMDA antagonist under evaluation for the treatment of tinnitus. In animal behavioral studies of salicylate-induced tinnitus, gacyclidine suppressed tinnitus-like behavior when applied bilaterally to the cochlea (83). Nine days of intracochlear perfusion of gacyclidine did not have any adverse effects on the guinea pig auditory evoked responses, suggesting that this treatment may be relatively safe. In preliminary studies in unilateral deaf humans perfusion of gacyclidine on the round window membrane for several days resulted in the temporary relief of tinnitus (99). While the results in the published abstract are encouraging, further work is needed to determine the generality of the findings and if gacyclidine has any long-term benefit.
Alprazolam
Alprazolam (Xanax®) is a short-acting triazolobenzodiazepine used to treat anxiety, panic attacks and depression (100–103). Alprazolam binds to the benzodiazepine site of the GABAA receptor, where it acts as a GABA agonist by increasing the permeability of chloride ions, leading to hyperpolarization and decreased excitability (104, 105). Complications associated with alprazolam include drug dependency and difficulty of discontinuing use. In a prospective, double-blind, placebo-controlled study, alprazolam was administered to patients with tinnitus (26); the dose was increased until it caused side effects or had an effect on tinnitus. Alprazolam reduced tinnitus loudness, measured with a tinnitus synthesizer and visual analog scale, in 76% of subjects, whereas only 5% showed a reduction in tinnitus loudness in the control group. Although the positive effects of alprazolam observed in this study are encouraging, the study design has been criticized because of the small sample size, drug dosing method, failure to assess emotional effect and the need for replication (49, 106).
Diazepam
The benzodiazepine diazepam (Valium®) is used to treat anxiety, insomnia, epilepsy, and muscle spasms. It binds to a specific subunit on the GABAA receptor (107) and is a positive allosteric modulator of GABA that increases hyperpolarization and decreases neuronal excitability. However, diazepam can also bind to voltage-gated sodium channels and reduce excitability by slowing sodium channel inactivation (108). Diazepam was evaluated in a double-blind, triple crossover trial involving 21 tinnitus patients (109). The drug had no effect on tinnitus loudness, a result which is surprising considering that its mechanisms of action are similar to those of alprazolam. One possible explanation for the discrepancy is that the dose of alprazolam, but not diazepam, was adjusted for each patient to maximize its effects on tinnitus (26). Complications associated with diazepam include drug dependency and difficulty of discontinuing use (110, 111).
Clonazepam
Clonazepam (Klonopin®) is a benzodiazepine derivative used as a muscle relaxant, anxiolytic and anticonvulsant. Like other benzodiazepines, it binds to specific subunits of the GABA receptor, where it acts as an agonist, leading to hyperpolarization and decreased excitability (107). In a retrospective study of medical records from over 3,000 patients taking clonazepam (0.5–1 mg/day for 60–180 days) for vestibular or cochleovestibular disorders, 32% reported an improvement in their tinnitus (112). The lack of a control group makes it difficult to evaluate the significance of these findings. In a prospective, randomized, single-blind clinical trial involving 10 patients per group, clonazepam significantly reduced tinnitus loudness and annoyance (visual analog scale) relative to the control group (113). Because of the small sample size, lack of double-blind and a crossover design, additional studies are needed to evaluate the efficacy of clonazepam (114). Like other benzodiazepines, drug dependency and difficulty discontinuing use are complicating factors associated with its use in the treatment of tinnitus.
Vigabatrin and tiagabine
Vigabatrin (Sabril®) and tiagabine (Gabitril®), two drugs that act on different aspects of GABAergic neurotransmission, have been studied in an animal model of noise-induced tinnitus. Vigabatrin is used as an anticonvulsant and to treat infantile spasms. It irreversibly inhibits GABA transaminase (GABA-T), the enzyme that catabolizes GABA, thereby increasing GABA levels (115–117). Vigabatrin also induces tonic release of GABA by causing the GABA transporter to operate in reverse (118). Tiagabine is used to treat seizures and panic disorders (119–121) and acts by inhibiting the uptake of GABA via the GAT-1 transporter, thereby increasing the availability of GABA at its receptor (122, 123).
It has been proposed that tinnitus arises from loss of inhibition in the CNS as a result of cochlear deafferentation caused by noise, aging or ototoxic drugs (72, 124–126). To test this hypothesis, noise-exposed rats with behavioral evidence of tinnitus were treated with vigabatrin or tiagabine. Tiagabine did not suppress noise-induced tinnitus; however, vigabatrin suppressed noise-induced tinnitus, and the tinnitus reappeared when treatment was discontinued (73). We are unaware of any clinical trials in which vigabatrin has been used to treat tinnitus; however, given the positive animal data, vigabatrin is a potential drug candidate for a clinical study in tinnitus. However, it is known that the drug can cause irreversible visual disturbances, limiting it use in humans (127).
Lidocaine
Lidocaine (Xylocaine®) is generally used as a local anesthetic or to treat cardiac arrhythmias (128). It is believed to bind to fast voltage-gated sodium channels, reducing the magnitude of the sodium current during depolarization (129–131). However, the mode of action of lidocaine is more complex, as it is known to affect calcium-, potassium-, and glycine-induced chloride currents at micromolar concentrations (132, 133). In 1935, lidocaine was inadvertently found to suppress tinnitus following nasal administration (134). Since that time, many clinical studies have shown that intravenous lidocaine suppresses tinnitus in a subpopulation of subjects. High positive response rates (~70%) have been reported in some studies, while others have reported lower response rates (~40%), as well as a large percentage of subjects in whom tinnitus became worse (~30%) (135–139). Relatively few patients show large reductions in tinnitus loudness; in those that do, the positive effects tend to be short-lasting (140). The sites of action of intravenous lidocaine are not well understood, but there is evidence that it affects both the cochlea and CNS (139, 141, 142). In one human brain imaging study in which lidocaine either increased or decreased the loudness of tinnitus, the changes in loudness were associated with altered neural activity in the right auditory association cortex (139). Tocainide, an analogue of lidocaine that can be taken orally, was evaluated as a potential long-term therapy for tinnitus. While preliminary results were encouraging, several randomized, controlled studies found that tocainide had little benefit for tinnitus (143–147).
Potassium channel modulators
Tinnitus is thought to arise from neural hyperactivity. As potassium ion channels play an important role in regulating the resting potential and spontaneous and evoked neural activity, potassium channel modulators may represent potential therapeutic targets for tinnitus therapy. Potassium channel modulators have also attracted attention as potential therapeutic targets for pain, epilepsy, anxiety and other hyperexcitability disorders (148, 149). In a preliminary report utilizing a rat behavioral model, the potassium channel modulators flindokalner (BMS-204352; MaxiPost™), and its (R)-enantiomer (R-MaxiPost™) reduced behavioral evidence of salicylate-induced tinnitus in a dose-dependent manner (150). Both enantiomers are KCa1.1 (BK) positive modulators and Kv7.1 negative modulators (149, 151). MaxiPost and its (R)-enantiomer modulate Kv7.2-Kv7.5 ion channels positively and negatively, respectively. These results suggest that potassium channel modulators may represent new therapeutic candidates for tinnitus management.
Carbamazepine
Carbamazepine (Tegretol®) is an anticonvulsant and mood stabilizer used to treat a variety of clinical disorders, including epilepsy, bipolar disorder, schizophrenia, pain and trigeminal neuralgia (152–156). The drug binds to voltage-gated sodium channels and stabilizes the sodium inactivation state, thereby reducing neural firing (157–159). Its mode of action, however, may be more complex than this, as some reports indicate that carbamazepine enhances outward, voltage-dependent potassium currents, inhibits L-type calcium channels and enhances the release of 5-HT (160–162). The results for carbamazepine in the treatment of tinnitus have been mixed. Several randomized clinical trials reported no beneficial effect of carbamazepine on tinnitus (163–165). However, the doses in these studies tended to be low (200 mg/day), and in one study only a single dose was given, which may explain the lack of effect (165). Other reports have shown that, among those patients who responded positively to intravenous lidocaine, 56% had a good or excellent response to carbamazepine (typically administered at 600–1000 mg daily), whereas those that responded poorly to lidocaine also responded poorly to carbamazepine (166). Patients most likely to respond positively to lidocaine were those with cochlear hearing loss. A more recent report found that among positive lidocaine responders, 50% responded positively to carbamazepine (ascending doses of 50–600 mg), carbamazepine had no effect in 29% of lidocaine responders, 15% withdrew because of side effects, and tinnitus worsened in 6% of patients (137). These results suggest that carbamazepine may provide tinnitus relief in roughly half the patients that respond positively to lidocaine.
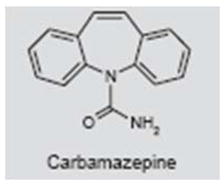
A rare group of patients who derive significant benefit from carbamazepine are those who have intermittent “typewriter tinnitus”, which is described as sounding like a typewriter, popcorn or ear clicking (44, 45). Radiological analysis showed evidence of vascular compression of the auditory nerve on the same side as the clicking. This suggests that there may be a tinnitus subgroup that can be classified on the basis of tinnitus perceptual characteristics, radiological features and response to drug treatment.
Finally, an animal study evaluated the ability of carbamazepine to suppress salicylate (aspirin)-induced tinnitus in rats. Tinnitus was assessed with a behavioral conditioned, lick-suppression paradigm. Salicylate-induced tinnitus was significantly reduced by 15 mg/kg of carbamazepine, but not by lower or higher doses (167). The neural mechanisms by which carbamazepine suppresses salicylate-induced tinnitus are unclear (83, 126, 167–170).
Sodium valproate/valproic acid
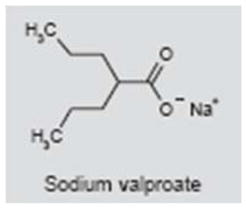
Sodium valproate (Depakene®) is used in the treatment of seizures, bipolar disorders, mood disorders and depression. Valproic acid has a broad spectrum of action that includes inhibition of GABA-T, inhibition of histone and blockade of voltage-gated sodium channels and T-type calcium channels. One case study found that sodium valproate (200 mg b.i.d.) was effective in suppressing tinnitus (171). However, another case study reported that sodium valproate induced tinnitus, and that the tinnitus gradually disappeared when treatment was discontinued (172). Because sodium valproate is sometimes used clinically to treat patients with severe tinnitus (173), well-controlled clinical trials are needed to assess its efficacy.
Gabapentin
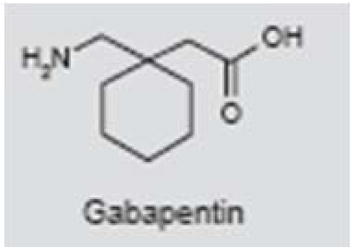
Gabapentin (Neurontin®) is widely used in the treatment of seizures, neuropathic pain and migraine (174–177). Because of its structural similarity to GABA, gabapentin was thought to bind to GABA receptors (178); however, its mechanisms of action are not fully understood. Gabapentin does not act directly on GABA, glycine or glutamatergic receptors, or voltage-gated sodium or calcium channels (179). The drug enhances the stimulated-release of GABA (180), increases GABA levels in patients (181) and, with chronic application, inhibits calcium currents by disrupting calcium channel trafficking (182).
An early case report and several clinical studies suggest that gabapentin may suppress tinnitus (183, 184). In addition, an animal study indicated that gabapentin reversibly suppressed behavioral evidence of noise-induced tinnitus in rats (185). Despite these positive findings, several large, randomized clinical trials found that gabapentin was not significantly different from placebo in treating tinnitus, with the possible exception that it reduced tinnitus annoyance in a subgroup of patients with noise-induced tinnitus (186–189). The overall trend that has emerged from randomized clinical trials is that gabapentin may be of limited value in treating tinnitus.
Trimipramine
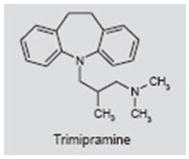
Trimipramine (Stangyl®) is classified as a tricyclic antidepressant; however, its mode of action differs from other tricyclic antidepressants in that it is a weak to moderate inhibitor of norepinephrine and 5-HT. However, trimipramine also blocks some dopamine and 5-HT receptors and alters the concentration of dopamine and 5-HT (190, 191). Trimipramine was evaluated in a double-blind, placebo-controlled, crossover study (192). Of the 19 subjects completing the study, 47% reported improvement and 37% claimed worsening of their tinnitus with trimipramine, whereas 42% reported improvement and 21% reported worsening with placebo. These results suggest that trimipramine has little or no benefit in the treatment of tinnitus.
Nortriptyline
Nortriptyline (Aventyl®) is tricyclic antidepressant that is also used to treat chronic fatigue, migraine and chronic pain (193–196). Its primary mode of action is to inhibit the reuptake of norepinephrine and, to a lesser extent, 5-HT. Nortriptyline also blocks muscarinic and 5-HT receptors. In a small, single-blind, placebo-washout study involving patients with severe tinnitus and major depression, nortriptyline significantly reduced depression and tinnitus loudness (10-dB reduction) (197). In a double-blind, placebo-controlled, follow-up study involving subjects with severe tinnitus and severe depression or depressive symptoms, nortriptyline significantly reduced depression scores, tinnitus disability scores and tinnitus loudness (6.4-dB reduction) relative to placebo (198). There was a significant correlation between the reduction in tinnitus disability scores and depression scores (199). These results suggest that nortriptyline is effective in reducing tinnitus loudness and severity in severely depressed tinnitus patients, but has less benefit in nondepressed individuals.
Paroxetine
Paroxetine (Paxil®) is an antidepressant that is also used to treat post-traumatic distress disorder, obsessive-compulsive disorder, anxiety and panic disorder (200–202). Paroxetine is a potent and highly selective serotonin reuptake inhibitor (SSRI) and shows weak binding to muscarinic acetylcholine receptors (203, 204). In a double-blind, placebo-controlled study involving chronic tinnitus patients, few of whom suffered from depression, the paroxetine group showed little difference from placebo on tinnitus loudness matching, Tinnitus Handicap Questionnaire scores and other measures; however the paroxetine group showed a significant improvement in tinnitus aggravation compared with the control group (205). Although SSRIs are widely used to treat tinnitus (206), the authors suggested that antidepressants should not be used to treat nondepressed tinnitus patients. However, in a case study, paroxetine significantly reduced tinnitus and improved mood in a patient with severe depression, anxiety and tinnitus (206).
Trazodone
Trazodone (Desyrel®) is an antidepressant with a dual mode of action: it is an SSRI and also selectively blocks postsynaptic 5-HT2A and 5-HT2C receptors. In addition to its use as an antidepressant, trazodone is also used to treat insomnia. It main side effects are drowsiness and hypotension. Trazodone was evaluated in a prospective, placebo-controlled, double-blind trial involving 83 subjects - approximately half each in the placebo and treatment groups. Subjects were treated with trazodone for 60 days at 50 mg/day. Patients in both the placebo and trazodone arms showed significant improvement in tinnitus loudness, discomfort and quality of life; however, the differences between the placebo and trazodone groups were not statistically significant. Thus, trazodone was not shown to be effective in treating tinnitus using this particular regimen and in this patient population.
Vestipitant/paroxetine combination therapy
Vestipitant and the combination of vestipitant and paroxetine are currently undergoing a phase II clinical trial for the treatment of tinnitus (http://clinicaltrials.gov/ct2/show/NCT00394056); information on the clinical efficacy of these drugs is currently unavailable. Vestipitant is a novel antagonist of the tachykinin NK1 receptor that binds substance P. Substance P acts as a neurotransmitter and neuromodulator. NK1 receptor antagonists suppress pain (207, 208). Neurokinin receptors are present in the inner ear and therefore represent a potential therapeutic target for tinnitus (209, 210). Paroxetine is an SSRI used to treat depression, obsessive-compulsive disorder and anxiety (211).
Sertraline
Sertraline (Zoloft®) is used to treat major depression, anxiety, obsessive-compulsive behavior and panic disorder (212–214). It mainly acts as an SSRI by suppressing the serotonin transporter (215); however, it also inhibits the dopamine transporter and the σ1 receptor (216). In a randomized, double-blind, placebo-controlled study in patients without severe hearing loss but with depression, anxiety and a high risk for developing severe tinnitus, sertraline was shown to be significantly more effective than placebo in reducing tinnitus loudness (12 dB versus 4 dB) and tinnitus severity (4.7 versus 2.7), but not tinnitus annoyance. Collectively, the results suggest that tinnitus patients with depression and anxiety may gain some benefit from antidepressant treatments (217).
Misoprostol
Misoprostol (Cytotec®) is a synthetic prostaglandin E1 (PGE1) analogue (218) that is primarily used to prevent gastric ulcers induced by non-steroidal anti-inflammatory drugs such as aspirin and to induce labor (219–221). It also inhibits the release of inflammatory cytokines (222). In a small, placebo-controlled, semi-crossover study, tinnitus was improved (reduced severity, improved sleep and concentration) in 33% of subjects during misoprostol treatment (escalating to 800 μg/day), whereas no subjects improved in the placebo group (223). In a subsequent double-blind, placebo-controlled study, the proportion of subjects showing a > 15-dB reduction in tinnitus loudness was significantly greater in the misoprostol group (800 μg/day) than in the placebo group. Surprisingly, misoprostol did not show significant benefit on other tinnitus measures. In a third randomized, controlled study involving tinnitus patients with hypertension and/or diabetes, the decrease in tinnitus loudness was significantly greater in the misoprostol group (46% of patients) than the placebo group (14% of patients); surprisingly, the subjective measures of tinnitus were not different (224). Overall, the studies suggest that misoprostol may provide some benefit to tinnitus patients with minimal risk, but larger studies are needed to confirm these trends before misoprostol can be considered of significant benefit for tinnitus.
Atorvastatin
The statin atorvastatin (Lipitor®) is used extensively to lower blood cholesterol and prevent strokes (225, 226). The drug inhibits HMG-CoA reductase, which suppresses the production of mevalonate, in turn reducing the synthesis of cholesterol (227). Atorvastatin also improves circulation and reduces inflammation and oxidative stress (228, 229). In a 13-month, randomized, double-blind, placebo-controlled study involving elderly patients with elevated cholesterol, atorvastatin failed to slow the progression of age-related hearing loss and did not significantly reduce tinnitus, although there was a trend toward a beneficial effect that did not reach significance (230).
Nimodipine
The L-type calcium channel blocker nimodipine (Nomotop®) was originally used for the treatment of high blood pressure, but is now primarily used in the treatment of subarachnoid hemorrhage (231, 232, 233). Nimodipine crosses the blood-brain barrier, dilates cerebral blood vessels and improves cerebral blood flow. The first open-label clinical trial found that nimodipine had positive effects on tinnitus in many patients, but the lack of a placebo control made it difficult to gauge its efficacy (234). Subsequent behavioral studies in rats showed that nimodipine significantly reduced tinnitus-like behavior caused by high doses of quinine or sodium salicylate (54, 235, 236). However, a second open-label clinical trial found significant improvement in the subject rating of tinnitus in only 5 of 31 (16%) patients; 17 patients (55%) showed no change and 6 patients (19%) showed marginal to major worsening of their tinnitus (237). Because there were few positive responders, it was concluded that a large-scale, randomized, placebo-controlled study was not warranted.
Furosemide
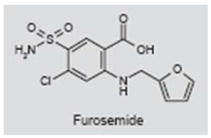
Furosemide (Lasix®) is a loop-inhibiting diuretic used to treat congestive heart failure and edema (238). It inhibits the Na-K-2Cl cotransporter that transports sodium, potassium and chloride ions into and out of cells (239). The Na-K-2Cl cotransporter is expressed in the inner ear, as well as in the brain (240, 241). Furosemide also blocks GABAA receptors (242). The drug has been proposed as a treatment for tinnitus of cochlear origin because it strongly suppresses the endolymphatic potential and other cochlear responses (243). It has been reported that ~50% of patients note a reduction in tinnitus symptoms following intravenous furosemide treatment; these positive responders were hypothesized to have cochlear tinnitus, whereas those who did not respond were assumed to have central tinnitus. In contrast, patients with presumably central tinnitus (i.e., tinnitus emanating from a surgically ablated ear) did not show a reduction in their tinnitus (244). Furosemide has also been found to suppress tinnitus in ~40% of patients with Meniere’s disease (245). However, high doses of furosemide can also induce temporary hearing loss and tinnitus (246–248). Thus, the data on the use of furosemide to treat tinnitus and conclusions regarding its central versus cochlear mode of action are difficult to interpret, especially when considering that Na-K-2Cl transporters are present in both the ear and brain.
Amino-oxyacetic acid
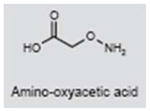
Amino-oxyacetic acid (AOAA) is an anticonvulsant that potently inhibits GABA-T, leading to the buildup of GABA in the brain, thereby strengthening its inhibitory effects (249, 250). AOAA also reduces the endocochlear potential in the cochlea (251). Because of its cochlear effects, AOAA was evaluated as a treatment for tinnitus presumably of cochlear origin (252). AOAA reduced tinnitus severity in ~20% of patients; however, > 70% of patients experienced severe side effects, thereby ruling out the clinical utility of this compound (253).
Scopolamine
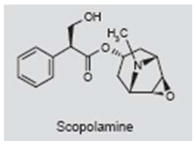
Scopolamine is used to treat motion sickness, nausea and intestinal cramps (254, 255). It acts as a competitive antagonist of M1 muscarinic acetylcholine receptors, which are widely distributed in the brain and along the auditory pathway (256–259). A literature search failed to turn up any clinical report in which scopolamine has been used to treat tinnitus. One animal study, however, suggested that scopolamine might be effective in suppressing salicylate-induced tinnitus (93, 94). The rationale for using scopolamine is based on the fact that high doses of salicylate significantly increase the expression of the activity and plasticity-related proteins c-fos and arg3.1 in the auditory cortex and amygdala; these proteins were considered putative markers of tinnitus-related neural activity (260). The amygdala sends axons to the nucleus basalis, which in turn sends cholinergic fibers to the auditory cortex, and scopolamine was proposed as a treatment for salicylate-induced tinnitus as it suppresses the expression of c-fos and arg3.1 in the auditory cortex. However, when behavioral measures of tinnitus were obtained from rats treated with salicylate or salicylate plus scopolamine, scopolamine failed to suppress salicylate-induced tinnitus (261). On the basis of these results, it seems unlikely that scopolamine will be effective in treating tinnitus.
Cyclandelate
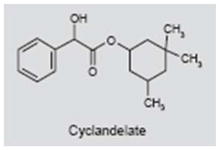
Cyclandelate is a vasodilator that is believed to act by blocking the influx of calcium into cells (262, 263). It is mainly used to treat peripheral vascular disorders, but is sometimes used to treat cerebrovascular disorders (264). Because some reports suggest that tinnitus arises from vascular insufficiency affecting the labyrinthine or brain (265, 266), cyclandelate was investigated as a treatment for tinnitus. In an open-label, nonrandomized, multicenter clinical trial in patients with tinnitus, vertigo and visual disturbances, 90 days of treatment with cyclandelate reportedly reduced the severity and frequency of these symptoms, with minimal side effects (267). However, in a subsequent placebo-controlled, double-blind study, cyclandelate did not significantly change audiometric measures of tinnitus loudness and pitch. The number of positive tinnitus responders was small and not significantly different from the placebo group, and many patients reported side effects (268). Thus, there is no clear evidence that vasodilators such as cyclandelate improve tinnitus symptoms.
Sulpiride
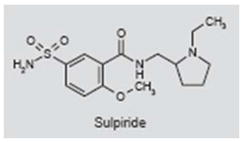
Sulpiride (Meresa®) is an antipsychotic drug that selectively blocks dopamine D2 receptors (269, 270). Several studies have explored the use of sulpiride to treat tinnitus based on models and data suggesting that dopaminergic pathways in limbic, prefrontal and temporal areas of the cortex contribute to tinnitus severity (271–274). In one double-blind, placebo-controlled study, sulpiride significantly reduced subjective ratings of tinnitus and tinnitus visual analog scale scores; the reductions with sulpiride were not significantly greater than those produced by placebo. However, the combination of sulpiride plus melatonin, which interacts with dopamine receptors, reduced tinnitus visual analog scale scores significantly more than placebo (275–277). In a single-blind, placebo-controlled study, sulpiride plus hydroxyzine, an antihistamine and anxiolytic, was significantly more effective in reducing tinnitus visual analog scale and tinnitus perception scores than placebo or sulpiride alone (278). These preliminary studies suggest that D2 antagonists such as sulpiride may help to suppress tinnitus symptoms, but further work is needed to confirm or refute these findings.
Vardenafil
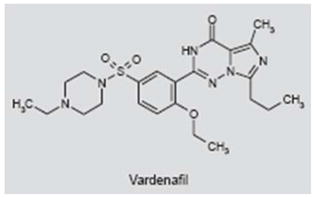
The phosphodiesterase type 5 (PDE5) inhibitor vardenafil (Levitra®) prevents the degradation of cyclic GMP found in the vascular smooth muscles of the penis and lungs (279). Vardenafil is primarily used to treat male impotence and sometimes pulmonary hypertension (280). While vardenafil is generally well tolerated, common side effects include headache, flushing, nasal congestion and dyspepsia (281). In a recent clinical report, the rationale for evaluating the effects of vardenafil on tinnitus was based on informal patient reports claiming that their tinnitus improved significantly when taking vardenafil (282). The efficacy of vardenafil was evaluated in a randomized, double-blind, placebo-controlled study with 21 patients receiving placebo or vardenafil (10 mg b.i.d. for 12 weeks). The major outcome variables relevant to tinnitus (Tinnitus Questionnaire, tinnitus loudness or pitch and other audiometric measure) failed to reveal any clinical or statistically significant improvement. Therefore, vardenafil does not appear to be a suitable treatment for tinnitus.
Ginkgo biloba
Extracts derived from the ancient Chinese Ginkgo biloba tree yield EGb-761, the active component, which contains bioactive flavonoids, terpenes and vasoactive compounds. Because of its vasodilating and antioxidant properties, Ginkgo has been used to treat a wide range of disorders, including tinnitus (283). Several studies have reported that Ginkgo alleviates tinnitus symptoms, particularly in patients with short-duration symptoms (265, 284). In addition, one behavioral study with rats found that EGb-761 reduced the behavioral manifestations of salicylate-induced tinnitus (285). However, a growing body of evidence from large, well-controlled, double-blind, placebo-controlled clinical studies clearly indicates that Ginkgo is no more effective in alleviating tinnitus symptoms than placebo (286–291).
Melatonin
Melatonin, a naturally occurring circulating hormone produced in the pineal gland and other tissues, binds to melatonin receptors and plays an important role in regulating circadian rhythms (292, 293). Melatonin is also a potent antioxidant that protects mitochondrial and nuclear DNA (294, 295) and has been found to protect against noise- and drug-induced hearing loss (296, 297). Because sleep disturbances represent a major complaint and complicating factor in tinnitus, melatonin was evaluated as a treatment for tinnitus in three studies. In the first double-blind, placebo-controlled, crossover study, scores of tinnitus severity measured with the Tinnitus Handicap Inventory improved by approximately the same degree with melatonin and placebo. There was a trend toward improved sleep scores with melatonin, but the effect was not statistically significant (298). A subsequent open-label study found statistically significant improvements on the Tinnitus Handicap Inventory and Pittsburgh Sleep Quality Index with melatonin treatment; however, it is difficult to evaluate the significance of these findings because of the lack of a placebo control group and moderate effect size (299). A more recent, randomized, double-blind, placebo-controlled study found that melatonin reduced subjective ratings of tinnitus and tinnitus loudness more than placebo; these improvements were substantially larger if melatonin was combined with the antipsychotic sulpiride, a selective dopamine D2 antagonist (275).
Zinc supplements
Zinc, an essential trace metal that plays an important role in numerous biological functions, is widely expressed in the auditory pathway, including the dorsal cochlear nucleus, a structure posited to be a major tinnitus generator (300–303). Zinc has been proposed as a treatment for tinnitus, particularly in patients with hypozincemia (304–307). While positive results have been reported in some patients (308), most well-controlled, double-blind, placebo-controlled studies have found little or no improvement in tinnitus with zinc therapy, except in a few patients with low zinc serum levels (290, 309, 310). A clinical trial to evaluate the efficacy of a dietary zinc supplement for treating tinnitus is under way (http://clinicaltrials.gov/ct2/show/NCT00683644).
SUMMARY AND OUTLOOK
The neural mechanisms that give rise to tinnitus are likely to be just as complex and multifaceted as other neurological disorders, such as epilepsy, pain or depression. The lack of a clinically relevant, objective and valid framework for classifying and distinguishing patients who present with tinnitus symptoms represents a major challenge for clinicians. Efforts are currently under way to develop more clinically relevant schemes for classifying tinnitus patients based on etiology, somatic, audiometric, perceptual and psychological features, as well as brain imaging findings (41, 272, 311–316). Advances in classifying tinnitus patients are likely to lead to improved success rates with drugs targeted to specific subpopulations.
For many years, the standard of care for dealing with tinnitus patients has been, “You need to learn to live with it.” Over the past two decades, a small cadre of clinicians has significantly advanced the standard of care by providing patients with sound therapy, counseling and education (24, 317–319). While many tinnitus patients demonstrate slow, steady improvement with these methods, most patients would prefer a drug therapy that would rapidly lead to improvement and complete suppression of their tinnitus. Aside from a few exceptional cases, most currently available drugs fail to completely suppress tinnitus, although some drugs have been reported to provide moderate relief of symptoms in a subset of patients. Careful clinical observations, along with data from clinical trials, have provided useful clues for deciding on a rational course of drug therapy for select patients. A rare subgroup of patients who describe their tinnitus as sounding like a typewriter is likely to gain significant tinnitus relief from carbamazepine (44, 45). For other patients, carbamazepine is likely to be no better than placebo. When a shotgun approach is used to treat all tinnitus patients with antidepressants, the overall success rates have proved disappointing (205, 206). However, if tinnitus patients with severe depression are treated with antidepressants, tinnitus symptoms often show noticeable improvement (197, 206, 217). Administering a depression inventory to all patients seeking treatment would help identify those patients likely to obtain tinnitus relief from antidepressant therapy (199, 320). Not surprisingly, patients with roaring tinnitus often find it difficult to fall asleep. Sleep deprivation adds to the fatigue, emotional burden and stress associated with tinnitus. Melatonin, an over-the-counter sleep aid with antioxidant properties and minimal side effects, may prove helpful in reducing tinnitus symptoms in patients with significant sleep disturbances. Preliminary studies combining melatonin with the dopamine D2 antagonist and antipsychotic sulpiride have yielded promising results, but further work is need to confirm these findings (275–277).
Because little is known about the underlying neural mechanisms that cause tinnitus, the clinical approaches to implementing drug therapies for treating tinnitus have been largely hit or miss. The development of behavioral measures of tinnitus in animals, combined with physiological, biochemical, molecular and imaging techniques are likely to provide important insights on the underlying causes of tinnitus (51, 54, 73, 321). Behavioral methods for detecting tinnitus in animals will allow new or existing drugs to be screened to determine if they can suppress tinnitus. The studies carried out to date suggest that GABA-T inhibitors and potassium channel modulators are potential drug candidates for tinnitus (73, 150).
The potential market for an FDA-approved drug to treat tinnitus is quite large; however, the acceptance by the medical community of new or old drugs to treat tinnitus ultimately rests on significant positive findings from well-controlled, multicenter clinical trials. Several existing drugs have been reported to provide significant relief from tinnitus; however, many of these studies suffer from small sample size, lack of replication, unusual dosing methods or weak experimental design, thereby hindering the acceptance of these drugs by the medical community (26, 106, 223, 224). Looking to the future, patients and clinicians may finally receive clear-cut answers from clinical trials currently under way to evaluate the efficacy of neramexane, vestipitant alone and in combination with paroxetine, acamprosate, AM-101 and dietary zinc supplements. Information gleaned from ongoing and future clinical studies may have the potential to revolutionize the treatment of tinnitus.
Footnotes
DISCLOSURE
The authors are supported in part by grants from NIH (R01DC009219, R01DC00909101), American Tinnitus Association, Tinnitus Research Initiative and RNID. No conflicts of interest exist in the publication of this article.
References
- 1.Demeester K, van Wieringen A, Hendrickx JJ, et al. Prevalence of tinnitus and audiometric shape. BENT. 2007;3(Suppl 7):37. [PubMed] [Google Scholar]
- 2.Hannaford PC, Simpson JA, Bisset AF, Davis A, McKerrow W, Mills R. The prevalence of ear, nose and throat problems in the community: Results from a national cross-sectional postal survey in Scotland. Fam Pract. 2005;22(3):227. doi: 10.1093/fampra/cmi004. [DOI] [PubMed] [Google Scholar]
- 3.Coles RR. Epidemiology of tinnitus: (1) Prevalence. J Laryngol Otol. 1984;9(Suppl):7. doi: 10.1017/s1755146300090041. [DOI] [PubMed] [Google Scholar]
- 4.McCombe A, Baguley D, Coles R, McKenna L, McKinney C, Windle-Taylor P. Guidelines for the grading of tinnitus severity: The results of a working group commissioned by the British Association of Otolaryngologists, Head and Neck Surgeons, 1999. Clin Otolaryngol. 2001;26(5):388. doi: 10.1046/j.1365-2273.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- 5.Medical Research Council’s Institute of Hearing Research. Epidemiology of tinnitus. In: Hazell JWP, editor. Tinnitus. Churchill Livingstone; Edinburgh: 1987. pp. 46–70. [Google Scholar]
- 6.Hoffmann HJ, Reed GW. Epidemiology of tinnitus. In: Snow JB, editor. Tinnitus: Theory and Management. B.C. Decker; Hamilton: 2004. pp. 16–41. [Google Scholar]
- 7.Davis A, Fafaie E. Epidemiology of tinnitus. In: Tyler R, editor. Tinnitus Handbook. Singular Publishing Group; San Diego: 2000. pp. 1–23. [Google Scholar]
- 8.Axelsson A, Ringdahl A. Tinnitus - A study of its prevalence and characteristics. Br J Audiol. 1989;23(1):53–62. doi: 10.3109/03005368909077819. [DOI] [PubMed] [Google Scholar]
- 9.Vio MM, Holme RH. Hearing loss and tinnitus: 250 Million people and a US$10 billion potential market. Drug Discov Today. 2005;10(19):1263. doi: 10.1016/S1359-6446(05)03594-4. [DOI] [PubMed] [Google Scholar]
- 10.Cave KM, Cornish EM, Chandler DW. Blast injury of the ear: Clinical update from the global war on terror. Mil Med. 2007;172(7):726. doi: 10.7205/milmed.172.7.726. [DOI] [PubMed] [Google Scholar]
- 11.Nicolas-Puel C, Faulconbridge RL, Guitton M, Puel JL, Mondain M, Uziel A. Characteristics of tinnitus and etiology of associated hearing loss: A study of 123 patients. Int Tinnitus J. 2002;8(1):37–44. [PubMed] [Google Scholar]
- 12.Douek E. Classification of tinnitus. In: Evered D, Lawrenson G, editors. Tinnitus Ciba Foundation Symposium 85. Pitman: Bath; 1981. pp. 4–15. [DOI] [PubMed] [Google Scholar]
- 13.Moller AR. Tinnitus: Presence and future. Prog Brain Res. 2007;166:3. doi: 10.1016/S0079-6123(07)66001-4. [DOI] [PubMed] [Google Scholar]
- 14.Meikle MB, Griest S. Asymmetry in tinnitus perception. In: Aran JM, Dauman R, editors. Tinnitus 91: Proceedings of the Fourth International Tinnitus Seminar. Kugler Publications; Amsterdam: 1992. pp. 231–7. [Google Scholar]
- 15.Hallberg LR, Erlandsson SI. Tinnitus characteristics in tinnitus complainers and noncomplainers. Br J Audiol. 1993;27(1):19–27. doi: 10.3109/03005369309077885. [DOI] [PubMed] [Google Scholar]
- 16.Tyler RS. The psychoacoustical measurement of tinnitus. In: Tyler RS, editor. Tinnitus Handbook. Singular Publishing; San Diego: 2000. pp. 149–79. [Google Scholar]
- 17.Loeb M, Smith R. Relation of induced tinnitus to physical characteristics of the inducing stimuli. J Acoust Soc Am. 1967;42(2):453–5. doi: 10.1121/1.1910600. [DOI] [PubMed] [Google Scholar]
- 18.Norena A, Micheyl C, Chery-Croze S, Collet L. Psychoacoustic characterization of the tinnitus spectrum: Implications for the underlying mechanisms of tinnitus. Audiol Neuro-Otol. 2002;7(6):358. doi: 10.1159/000066156. [DOI] [PubMed] [Google Scholar]
- 19.Kaltenbach JA. Neurophysiologic mechanisms of tinnitus. J Am Acad Audiol. 2000;11(3):125–37. [PubMed] [Google Scholar]
- 20.Stouffer JL, Tyler RS. Characterization of tinnitus by tinnitus patients. J Speech Hear Res. 1990;55(3):439–53. doi: 10.1044/jshd.5503.439. [DOI] [PubMed] [Google Scholar]
- 21.Wegel RL, Lane CE. The auditory masking of one pure tone by another and its probable relation to the dynamics of the inner ear. Physical Rev. 1924;23(2):266–85. [Google Scholar]
- 22.Vernon J, Meikle M. Tinnitus, CIBA Foundation Symposium 85. Pittman: Bath; 1981. Tinnitus masking: Unresolved problems; pp. 239–62. [DOI] [PubMed] [Google Scholar]
- 23.Henry JA, Rheinsburg B, Owens KK, Ellingson RM. Acta Oto-Laryngol. Suppl 556. 2006. New instrumentation for automated tinnitus psychoacoustic assessment; p. 34. [DOI] [PubMed] [Google Scholar]
- 24.Davis PB, Paki B, Hanley PJ. Neuromonics Tinnitus Treatment: Third clinical trial. Ear Hear. 2007;28(2):242. doi: 10.1097/AUD.0b013e3180312619. [DOI] [PubMed] [Google Scholar]
- 25.Teggi R, Bellini C, Piccioni LO, Palonta F, Bussi M. Transmeatal low-level laser therapy for chronic tinnitus with cochlear dysfunction. Audiol Neuro-Otol. 2008;14(2):115. doi: 10.1159/000161235. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RM, Brummett R, Schleuning A. Use of alprazolam for relief of tinnitus. A double-blind study. Arch Otolaryngol Head Neck Surg. 1993;119(8):842. doi: 10.1001/archotol.1993.01880200042006. [DOI] [PubMed] [Google Scholar]
- 27.Westerberg BD, et al. A double-blind placebo-controlled trial of baclofen in the treatment of tinnitus. Am J Otol. 1996;17(6):896. [PubMed] [Google Scholar]
- 28.Penner MJ, Zhang T. Masking patterns for partially masked tinnitus. Int Tinnitus J. 1996;2:105–9. [PubMed] [Google Scholar]
- 29.Feldmann H. Homolateral and contralateral masking of tinnitus by noise-bands and by pure tones. Audiology. 1971;10(3):138. doi: 10.3109/00206097109072551. [DOI] [PubMed] [Google Scholar]
- 30.Zwislocki JJ, Buining E, Glantz J. Frequency distribution of central masking. J Acoust Soc Am. 1968;43(6):1267–71. doi: 10.1121/1.1910978. [DOI] [PubMed] [Google Scholar]
- 31.Tyler RS, Babin R, Niebuhr D. Some observations on the masking and postmasking effects on tinnitus. J Laryngol Otol. 1984;9(Suppl):150. [Google Scholar]
- 32.Penner MJ. Masking of tinnitus and central masking. J Speech Hear Res. 1987;30(2):147–52. doi: 10.1044/jshr.3002.147. [DOI] [PubMed] [Google Scholar]
- 33.Penner MJ, Brauth S, Hood L. The temporal course of the masking of tinnitus as a basis for inferring its origin. J Speech Hear Res. 1981;24(2):257–61. doi: 10.1044/jshr.2402.257. [DOI] [PubMed] [Google Scholar]
- 34.Penner MJ, Bilger RC. Adaptation and the masking of tinnitus. J Speech Hear Res. 1989;32(2):339–46. doi: 10.1044/jshr.3202.339. [DOI] [PubMed] [Google Scholar]
- 35.Wilson PH, Henry J, Bowen M, Haralambous G. Tinnitus reaction questionnaire: Psychometric properties of a measure of distress associated with tinnitus. J Speech Hear Res. 1991;34(1):197–201. [PubMed] [Google Scholar]
- 36.Kuk FK, Tyler RS, Russell D, Jordan H. The psychometric properties of a tinnitus handicap questionnaire. Ear Hear. 1990;11(6):434. doi: 10.1097/00003446-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- 38.Baguley DM, Andersson G. Factor analysis of the Tinnitus Handicap Inventory. Am J Audiol. 2003;12(1):31–4. doi: 10.1044/1059-0889(2003/007). [DOI] [PubMed] [Google Scholar]
- 39.Seligmann H, Podoshin L, Ben-David J, Fradis M, Goldsher M. Drug-induced tinnitus and other hearing disorders. Drug Saf. 1996;14(3):198. doi: 10.2165/00002018-199614030-00006. [DOI] [PubMed] [Google Scholar]
- 40.Briner W. A behavioral nosology for tinnitus. Psychol Rep. 1995;77(1):27–34. doi: 10.2466/pr0.1995.77.1.27. [DOI] [PubMed] [Google Scholar]
- 41.Tyler R, Coelho C, Tao P, Ji H, Noble W, Gehringer A, Gogel S. Identifying tinnitus subgroups with cluster analysis. Am J Audiol. 2008;17(2):S176–84. doi: 10.1044/1059-0889(2008/07-0044). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldstein B, Shulman A. Tinnitus classification: Medical audiologic assessment. J Laryngol Otol. 1981;(4 Suppl):33. [PubMed] [Google Scholar]
- 43.Norena A, Cransac H, Chery-Croze S. Towards an objectification by classification of tinnitus. Clin Neurophysiol. 1999;110(4):666. doi: 10.1016/s1388-2457(98)00034-0. [DOI] [PubMed] [Google Scholar]
- 44.Levine RA. Typewriter tinnitus: A carbamazepine-responsive syndrome related to auditory nerve vascular compression. ORL - J Otorhinolaryngol Relat Spec. 2006;68(1):43. doi: 10.1159/000090490. [DOI] [PubMed] [Google Scholar]
- 45.Mardini MK. Ear-clicking “tinnitus” responding to carbamazepine. New Engl J Med. 1987;317(24):1542. doi: 10.1056/nejm198712103172418. [DOI] [PubMed] [Google Scholar]
- 46.Rahko T, Hakkinen V. Carbamazepine in the treatment of objective myoclonus tinnitus. J Laryngol Otol. 1979;93(2):123. doi: 10.1017/s0022215100086849. [DOI] [PubMed] [Google Scholar]
- 47.Shea JJ, Harell M. Management of tinnitus aurium with lidocaine and carbamazepine. Laryngoscope. 1978;88(9, Pt. 1):1477. doi: 10.1002/lary.1978.88.9.1477. [DOI] [PubMed] [Google Scholar]
- 48.Dobie RA. A review of randomized clinical trials in tinnitus. Laryngoscope. 1999;109(8):1202. doi: 10.1097/00005537-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Dobie RA. Clinical trials and drug therapy for tinnitus. In: Snow JBJ, editor. Tinnitus: Theory and Management. BC Decker, Inc; Hamilton: 2004. pp. 266–77. [Google Scholar]
- 50.Robinson S, Viirre ES, Stein MB. Antidepressant therapy for tinnitus. In: Snow JBJ, editor. Tinnitus: Theory and Management. BC Decker, Inc; Hamilton: 2004. pp. 278–93. [Google Scholar]
- 51.Lobarinas E, Yang G, Sun W, et al. Salicylate- and quinine-induced tinnitus and effects of memantine. Acta Oto-Laryngol. 2006;(Suppl 556):13. doi: 10.1080/03655230600895408. [DOI] [PubMed] [Google Scholar]
- 52.Lobarinas E, Sun W, Cushing R, Salvi R. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC) Hear Res. 2004;190(1–2):109. doi: 10.1016/S0378-5955(04)00019-X. [DOI] [PubMed] [Google Scholar]
- 53.Jastreboff PJ, Brennan JF, Sasaki CT. An animal model for tinnitus. Laryngoscope. 1988;98(3):280. doi: 10.1288/00005537-198803000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Jastreboff PJ, Brennan JF, Sasaki CT. Quinine-induced tinnitus in rats. Arch Otolaryngol Head Neck Surg. 1991;117(10):1162. doi: 10.1001/archotol.1991.01870220110020. [DOI] [PubMed] [Google Scholar]
- 55.Grigor RR, Spitz PW, Furst DE. Salicylate toxicity in elderly patients with rheumatoid arthritis. J Rheumatol. 1987;14(1):60. [PubMed] [Google Scholar]
- 56.Bateman DN, Dyson EH. Quinine toxicity. Adverse Drug React Acute Poisoning Rev. 1986;5(4):215. [PubMed] [Google Scholar]
- 57.Dodson KM, Sismanis A. Intratympanic perfusion for the treatment of tinnitus. Otolaryngol Clin North Am. 2004;37(5):991. doi: 10.1016/j.otc.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Schwab B, Lenarz T, Heermann R. Use of the round window micro cath for inner ear therapy - Results of a placebo-controlled, prospective study on chronic tinnitus. Laryngo-Rhino-Otol. 2004;83(3):164. doi: 10.1055/s-2004-814278. [DOI] [PubMed] [Google Scholar]
- 59.Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- 60.Williams SH. Medications for treating alcohol dependence. Am Fam Physician (1970) 2005;72(9):1775. [PubMed] [Google Scholar]
- 61.Mason BJ, Crean R. Acamprosate in the treatment of alcohol dependence: Clinical and economic considerations. Expert Rev Neurother. 2007;7(11):1465. doi: 10.1586/14737175.7.11.1465. [DOI] [PubMed] [Google Scholar]
- 62.Pierrefiche O, Daoust M, Naassila M. Biphasic effect of acamprosate on NMDA but not on GABAA receptors in spontaneous rhythmic activity from the isolated neonatal rat respiratory network. Neuropharmacology. 2004;47(1):35. doi: 10.1016/j.neuropharm.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Madamba SG, Schweitzer P, Zieglgansberger W, Siggins GR. Acamprosate (calcium acetylhomotaurinate) enhances the N-methyl-D-aspartate component of excitatory neurotransmission in rat hippocampal CA1 neurons in vitro. Alcohol Clin Exp Res. 1996;20(4):651. doi: 10.1111/j.1530-0277.1996.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 64.Berton F, Francesconi WG, Madamba SG, Zieglgansberger W, Siggins GR. Acamprosate enhances N-methyl-D-apartate receptor-mediated neurotransmission but inhibits presynaptic GABA(B) receptors in nucleus accumbens neurons. Alcohol Clin Exp Res. 1998;22(1):183. [PubMed] [Google Scholar]
- 65.Naassila M, Hammoumi S, Legrand E, Durbin P, Daoust M. Mechanism of action of acamprosate. Part I Characterization of spermidine-sensitive acamprosate binding site in rat brain. Alcohol Clin Exp Res. 1998;22(4):802. [PubMed] [Google Scholar]
- 66.Zeise ML, et al. Acamprosate (calciumacetylhomotaurinate) decreases postsynaptic potentials in the rat neocortex - Possible involvement of excitatory amino acid receptors. Eur J Pharmacol. 1993;231(1):47. doi: 10.1016/0014-2999(93)90682-8. [DOI] [PubMed] [Google Scholar]
- 67.Wu JY, Jin H, Schloss JV, Faiman MD, Ningaraj NS, Foos T, Chen W. Neurotoxic effect of acamprosate, n-acetyl-homotaurine, in cultured neurons. J Biomed Sci. 2001;8(1):96. doi: 10.1007/BF02255977. [DOI] [PubMed] [Google Scholar]
- 68.Azevedo AA, Figueiredo RR. Tinnitus treatment with acamprosate: Double-blind study. Braz J Otorhinolaryngol. 2005;71(5):618–23. doi: 10.1016/S1808-8694(15)31266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azevedo AA, Figueiredo RR. Treatment of tinnitus with acamprosate. Prog Brain Res. 2007;166:273. doi: 10.1016/S0079-6123(07)66025-7. [DOI] [PubMed] [Google Scholar]
- 70.Oestreicher E, Arnold W, Ehrenberger K, Felix D. Memantine suppresses the glutamatergic neurotransmission of mammalian inner hair cells. ORL - J Otorhinolaryngol Relat Spec. 1998;60(1):18. doi: 10.1159/000027556. [DOI] [PubMed] [Google Scholar]
- 71.Guitton MJ, Pujol R, Puel JL. m-Chlorophenylpiperazine exacerbates perception of salicylate-induced tinnitus in rats. Eur J Neurosci. 2005;22(10):2675. doi: 10.1111/j.1460-9568.2005.04436.x. [DOI] [PubMed] [Google Scholar]
- 72.Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM. GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res. 2000;147(1–2):251. doi: 10.1016/s0378-5955(00)00135-0. [DOI] [PubMed] [Google Scholar]
- 73.Brozoski TJ, Spires TJ, Bauer CA. Vigabatrin, a GABA transaminase inhibitor, reversibly eliminates tinnitus in an animal model. J Assoc Res Otolaryngol. 2007;8(1):105–18. doi: 10.1007/s10162-006-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daftary A, Shulman A, Strashun AM, Gottschalk C, Zoghbi SS, Seibyl JP. Benzodiazepine receptor distribution in severe intractable tinnitus. Int Tinnitus J. 2004;10(1):17–23. [PubMed] [Google Scholar]
- 75.Suneja SK, Potashner SJ, Benson CG. Plastic changes in glycine and GABA release and uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation. Exp Neurol. 1998;151(2):273. doi: 10.1006/exnr.1998.6812. [DOI] [PubMed] [Google Scholar]
- 76.Kudo Y, Shibata S. Effects of caroverine and diltiazem on synaptic responses, L-glutamate-induced depolarization and potassium efflux in the frog spinal cord. Br J Pharmacol. 1984;83(3):813. doi: 10.1111/j.1476-5381.1984.tb16237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koppi S, Eberhardt G, Haller R, Koenig P. Calcium-channel-blocking agent in the treatment of acute alcohol withdrawal - Caroverine versus meprobamate in a randomized double-blind study. Neuropsychobiology. 1987;17(1–2):49. doi: 10.1159/000118340. [DOI] [PubMed] [Google Scholar]
- 78.Saletu B, Gruenberger J, Anderer P, Linzmayer L, Koenig P. Acute central effects of the calcium channel blocker and antiglutamatergic drug caroverine. Double-blind, placebo-controlled, EEG mapping and psychometric studies after intravenous and oral administration. Arzneim-Forsch Drug Res. 1995;45(3):217. [PubMed] [Google Scholar]
- 79.Ehrenberger K, Felix D. Receptor pharmacological models for inner ear therapies with emphasis on glutamate receptors: A survey. Acta Oto-Laryngol. 1995;115(2):236. doi: 10.3109/00016489509139299. [DOI] [PubMed] [Google Scholar]
- 80.Ehrenberger K, Felix D. Caroverine depresses the activity of cochlear glutamate receptors in guinea pigs: In vivo model for drug-induced neuroprotection? Neuropharmacology. 1992;31(12):1259. doi: 10.1016/0028-3908(92)90054-s. [DOI] [PubMed] [Google Scholar]
- 81.Chen Z, Duan M, Lee H, Ruan R, Ulfendahl M. Pharmacokinetics of caroverine in the inner ear and its effects on cochlear function after systemic and local administrations in Guinea pigs. Audiol Neuro-Otol. 2003;8(1):49. doi: 10.1159/000067893. [DOI] [PubMed] [Google Scholar]
- 82.Denk DM, Heinzl H, Franz P, Ehrenberger K. Caroverine in tinnitus treatment. A placebo-controlled blind study. Acta Oto-Laryngol. 1997;117(6):825. doi: 10.3109/00016489709114208. [DOI] [PubMed] [Google Scholar]
- 83.Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL. Salicylate induces tinnitus through activation of cochlear NMDA receptors. J Neurosci. 2003;23(9):3944. doi: 10.1523/JNEUROSCI.23-09-03944.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guitton MJ, Dudai Y. Blockade of cochlear NMDA receptors prevents long-term tinnitus during a brief consolidation window after acoustic trauma. Neural Plast (Online) 2007;2007:80904. doi: 10.1155/2007/80904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duckert LG, Rees TS. Placebo effect in tinnitus management. Otolaryngol Head Neck Surg. 1984;92(6):697. doi: 10.1177/019459988409200618. [DOI] [PubMed] [Google Scholar]
- 86.Domeisen H, Hotz MA, Haeusler R. Caroverine in tinnitus treatment. Acta Oto-Laryngol. 1998;118(4):606. doi: 10.1080/00016489850154801. [DOI] [PubMed] [Google Scholar]
- 87.Peskind ER, Potkin SG, Pomara N, Ott BR, Graham SM, Olin JT, McDonald S. Memantine treatment in mild to moderate Alzheimer disease: A 24-week randomized, controlled trial. Am J Geriatr Psychiatry. 2006;14(8):704. doi: 10.1097/01.JGP.0000224350.82719.83. [DOI] [PubMed] [Google Scholar]
- 88.Muhonen LH, Loennqvist J, Juva K, Alho H. Double-blind, randomized comparison of memantine and escitalopram for the treatment of major depressive disorder comorbid with alcohol dependence. J Clin Psychiatry. 2008;69(3):392. doi: 10.4088/jcp.v69n0308. [DOI] [PubMed] [Google Scholar]
- 89.Rogawski MA, Wenk GL. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer’s disease. CNS Drug Rev. 2003;9(3):275. doi: 10.1111/j.1527-3458.2003.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deshpande SS, Smith CD, Filbert MG. Assessment of primary neuronal culture as a model for soman-induced neurotoxicity and effectiveness of memantine as a neuroprotective drug. Arch Toxicol. 1995;69(6):384. doi: 10.1007/s002040050188. [DOI] [PubMed] [Google Scholar]
- 91.Rammes G, Rupprecht R, Ferrari U, Zieglgaensberger W, Parsons CG. The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT3 receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neurosci Lett. 2001;306(1–2):81. doi: 10.1016/s0304-3940(01)01872-9. [DOI] [PubMed] [Google Scholar]
- 92.Oliver D, et al. Memantine inhibits efferent cholinergic transmission in the cochlea by blocking nicotinic acetylcholine receptors of outer hair cells. Mol Pharmacol. 2001;60(1):183. doi: 10.1124/mol.60.1.183. [DOI] [PubMed] [Google Scholar]
- 93.Jastreboff PJ, Brennan JF, Coleman JK, Sasaki CT. Phantom auditory sensation in rats: An animal model for tinnitus. Behav Neurosci. 1988;102(6):811. doi: 10.1037//0735-7044.102.6.811. [DOI] [PubMed] [Google Scholar]
- 94.Myers EN, Bernstein JM. Salicylate ototoxicity; a clinical and experimental study. Arch Otolaryngol Head Neck Surg. 1965;82(5):483. doi: 10.1001/archotol.1965.00760010485006. [DOI] [PubMed] [Google Scholar]
- 95.Peng BG, et al. Aspirin selectively augmented N-methyl-D-aspartate types of glutamate responses in cultured spiral ganglion neurons of mice. Neurosci Lett. 2003;343(1):21. doi: 10.1016/s0304-3940(03)00296-9. [DOI] [PubMed] [Google Scholar]
- 96.Figueiredo RR, Langguth B, Mello de Oliveira P, Aparecida de Azevedo A. Tinnitus treatment with memantine. Otolaryngol Head Neck Surg. 2008;138(4):492. doi: 10.1016/j.otohns.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 97.Gilling K, Jatzke C, Wollenburg C, Vanejevs M, Kauss V, Jirgensons A, Parsons CG. A novel class of amino-alkylcyclohexanes as uncompetitive, fast, voltage-dependent, N-methyl-D-aspartate (NMDA) receptor antagonists - In vitro characterization. J Neural Transm. 2007;114(12):1529. doi: 10.1007/s00702-007-0792-7. [DOI] [PubMed] [Google Scholar]
- 98.Plazas PV, Savino J, Kracun S, Gomez-Casati ME, Katz E, Parsons CG, Millar NS, Elgoyhen AB. Inhibition of the alpha9alpha10 nicotinic cholinergic receptor by neramexane, an open channel blocker of N-methyl-D-aspartate receptors. Eur J Pharmacol. 2007;566(1–3):11. doi: 10.1016/j.ejphar.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 99.Wenzel GI, Lim HH, Staver T, Lobl T, Schloss J, Schwab B, Lenarz T. Effects of gacyclidine extracochlear perfusion on tinnitus in humans and intracochlear perfusion on ABR thresholds in Guinea pigs. [Google Scholar]
- 100.Srisurapanont M, Boonyanaruthee V. Alprazolam and standard antidepressants in the treatment of depression: A meta-analysis of the antidepressant effect. J Med Assoc Thail. 1997;80(3):183. [PubMed] [Google Scholar]
- 101.Kravitz HM, Fawcett J, Newman AJ. Alprazolam and depression: A review of risks and benefits. J Clin Psychiatry. 1993;54(Suppl):78. [PubMed] [Google Scholar]
- 102.Freeman AM, 3rd, Fleece L, Folks DG, et al. Alprazolam treatment of postcoronary bypass anxiety and depression. J Clin Psychopharmacol. 1986;6(1):39. [PubMed] [Google Scholar]
- 103.Dunner DL, Ishiki D, Avery DH, Wilson LG, Hyde TS. Effect of alprazolam and diazepam on anxiety and panic attacks in panic disorder: A controlled study. J Clin Psychiatry. 1986;47(9):458. [PubMed] [Google Scholar]
- 104.Obata T, Yamamura HI. Modulation of GABA-stimulated chloride influx into membrane vesicles from rat cerebral cortex by triazolobenzodiazepines. Life Sci. 1988;42(6):659. doi: 10.1016/0024-3205(88)90457-2. [DOI] [PubMed] [Google Scholar]
- 105.Lopez F, Miller LG, Greenblatt DJ, Schatzki A, Lumpkin M, Shader RI. Chronic low-dose alprazolam augments gamma-aminobutyric acid(A) receptor function. J Clin Psychopharmacol. 1992;12(2):119. [PubMed] [Google Scholar]
- 106.Huynh L, Fields S. Alprazolam for tinnitus. Ann Pharmacother. 1995;29(3):311. doi: 10.1177/106002809502900313. [DOI] [PubMed] [Google Scholar]
- 107.Sieghart W. Pharmacology of benzodiazepine receptors: An update. J Psychiatry Neurosci. 1994;19(1):24. [PMC free article] [PubMed] [Google Scholar]
- 108.McLean MJ, Macdonald RL. Benzodiazepines, but not beta carbolines, limit high frequency repetitive firing of action potentials of spinal cord neurons in cell culture. J Pharmacol Exp Ther. 1988;244(2):789. [PubMed] [Google Scholar]
- 109.Kay NJ. Oral chemotherapy in tinnitus. Br J Audiol. 1981;15(2):123–4. doi: 10.3109/03005368109081425. [DOI] [PubMed] [Google Scholar]
- 110.Busto U, Fornazzari L, Naranjo CA. Protracted tinnitus after discontinuation of long-term therapeutic use of benzodiazepines. J Clin Psychopharmacol. 1988;8(5):359. [PubMed] [Google Scholar]
- 111.Busto U, Sellers EM, Naranjo CA, Cappell H, Sanchez-Craig M, Sykora K. Withdrawal reaction after long-term therapeutic use of benzodiazepines. New Engl J Med. 1986;315(14):854. doi: 10.1056/NEJM198610023151403. [DOI] [PubMed] [Google Scholar]
- 112.Gananca MM, Caovilla HH, Gananca FF, Gananca CF, Munhoz MS, da Silva ML, Serafini F. Clonazepam in the pharmacological treatment of vertigo and tinnitus. Int Tinnitus J. 2002;8(1):50–3. [PubMed] [Google Scholar]
- 113.Bahmad FM, Jr, Venosa AR, Oliveira CA. Benzodiazepines and GABAergics in treating severe disabling tinnitus of predominantly cochlear origin. Int Tinnitus J. 2006;12(2):140–4. [PubMed] [Google Scholar]
- 114.Murai K, Tyler RS, Harker LA, Stouffer JL. Review of pharmacologic treatment of tinnitus. Am J Otol. 1992;13(5):454. [PubMed] [Google Scholar]
- 115.Mattson RH, Petroff OAC, Rothman D, Behar K. Vigabatrin: Effect on brain GABA levels measured by nuclear magnetic resonance spectroscopy. Acta Neurol Scand. 1995;92(Suppl 162):27. doi: 10.1111/j.1600-0404.1995.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 116.Richens A. Pharmacology and clinical pharmacology of vigabatrin. J Child Neurol. 1991;(Suppl 2):S7. [PubMed] [Google Scholar]
- 117.Czuczwar SJ, Patsalos PN. The new generation of GABA enhancers. Potential in the treatment of epilepsy. CNS Drugs. 2001;15(5):339. doi: 10.2165/00023210-200115050-00001. [DOI] [PubMed] [Google Scholar]
- 118.Wu YM, et al. Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J Neurophysiol. 2003;89(4):2021. doi: 10.1152/jn.00856.2002. [DOI] [PubMed] [Google Scholar]
- 119.Smith SE, Parvez NS, Chapman AG, Meldrum BS. The gamma-aminobutyric acid uptake inhibitor, tiagabine, is anticonvulsant in two animal models of reflex epilepsy. Eur J Pharmacol. 1995;273(3):259. doi: 10.1016/0014-2999(94)00696-5. [DOI] [PubMed] [Google Scholar]
- 120.Adkins JC, Noble S. Tiagabine. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the management of epilepsy. Drugs. 1998;55(3):437. doi: 10.2165/00003495-199855030-00013. [DOI] [PubMed] [Google Scholar]
- 121.Zwanzger P, Rupprecht R. Selective GABAergic treatment for panic? Investigations in experimental panic induction and panic disorder. J Psychiatry Neurosci. 2005;30(3):167. [PMC free article] [PubMed] [Google Scholar]
- 122.Gram L. Tiagabine: A novel drug with a GABAergic mechanism of action. Epilepsia. 1994;35(Suppl 5):S85. doi: 10.1111/j.1528-1157.1994.tb05977.x. [DOI] [PubMed] [Google Scholar]
- 123.Chen L, Yung WH. Effects of the GABA-uptake inhibitor tiagabine in rat globus pallidus. Exp Brain Res. 2003;152(2):263. doi: 10.1007/s00221-003-1549-7. [DOI] [PubMed] [Google Scholar]
- 124.Potashner SJ, Suneja SK, Benson CG. Regulation of D-aspartate release and uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation. Exp Neurol. 1997;148(1):222. doi: 10.1006/exnr.1997.6641. [DOI] [PubMed] [Google Scholar]
- 125.Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211(Pt. 11):1781. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bauer CA, Brozoski TJ, Holder TM, Caspary DM. Effects of chronic salicylate on GABAergic activity in rat inferior colliculus. Hear Res. 2000;147(1–2):175. doi: 10.1016/s0378-5955(00)00130-1. [DOI] [PubMed] [Google Scholar]
- 127.Besag FM. Behavioural effects of the newer antiepileptic drugs: An update. Expert Opin Drug Saf. 2004;3(1):1. doi: 10.1517/14740338.3.1.1. [DOI] [PubMed] [Google Scholar]
- 128.Schwartz ML, Meyer MB, Covino BG, Narang RM, Sethi V, Schwartz AJ, Kamp P. Antiarrhythmic effectiveness of intramuscular lidocaine: Influence of different injection sites. J Clin Pharmacol. 1974;14(2):77. doi: 10.1002/j.1552-4604.1974.tb02293.x. [DOI] [PubMed] [Google Scholar]
- 129.Clarkson CW, Follmer CH, Ten Eick RE, Hondeghem LM, Yeh JZ. Evidence for two components of sodium channel block by lidocaine in isolated cardiac myocytes. Circ Res. 1988;63(5):869. doi: 10.1161/01.res.63.5.869. [DOI] [PubMed] [Google Scholar]
- 130.Courtney KR. Mechanism of frequency-dependent inhibition of sodium currents in frog myelinated nerve by the lidocaine derivative GEA. J Pharmacol Exp Ther. 1975;195(2):225. [PubMed] [Google Scholar]
- 131.Lenkowski PW, Shah BS, Dinn AE, Lee K, Patel MK. Lidocaine block of neonatal Nav1.3 is differentially modulated by co-expression of beta1 and beta3 subunits Eur J Pharmacol 2003, 467(1–3): 23 3 is differentially modulated by co-expression of beta1 and beta3 subunits. Eur J Pharmacol. 2003;467(1–3):23. doi: 10.1016/s0014-2999(03)01595-4. [DOI] [PubMed] [Google Scholar]
- 132.Josephson IR. Lidocaine blocks Na, Ca and K currents of chick ventricular myocytes. J Mol Cell Cardiol. 1988;20(7):593. doi: 10.1016/s0022-2828(88)80117-2. [DOI] [PubMed] [Google Scholar]
- 133.Yu M, Chen L. Modulation of major voltage- and ligand-gated ion channels in cultured neurons of the rat inferior colliculus by lidocaine. Acta Pharmacol Sin. 2008;29(12):1409. doi: 10.1111/j.1745-7254.2008.00893.x. [DOI] [PubMed] [Google Scholar]
- 134.Barany R. Die Beinflussung des Ohrensausens durch intravenös injizierte Lokalanaestetica. Acta Oto-Laryngol. 1935;23:201. doi: 10.1080/00016489.2018.1438148. [DOI] [PubMed] [Google Scholar]
- 135.Melding PS, Goodey RJ, Thorne PR. The use of intravenous lignocaine in the diagnosis and treatment of tinnitus. J Laryngol Otol. 1978;92(2):115. doi: 10.1017/s002221510008511x. [DOI] [PubMed] [Google Scholar]
- 136.Martin FW, Colman BH. Tinnitus: A double-blind crossover controlled trial to evaluate the use of lignocaine. Clin Otolaryngol. 1980;5(1):3. doi: 10.1111/j.1365-2273.1980.tb01622.x. [DOI] [PubMed] [Google Scholar]
- 137.Sanchez TG, Balbani AP, Bittar RS, Bento RF, Camara J. Lidocaine test in patients with tinnitus: Rationale of accomplishment and relation to the treatment with carbamazepine. Auris Nasus Larynx. 1999;26(4):411. doi: 10.1016/s0385-8146(99)00020-6. [DOI] [PubMed] [Google Scholar]
- 138.Duckert LG, Rees TS. Treatment of tinnitus with intravenous lidocaine: A double-blind randomized trial. Otolaryngol Head Neck Surg. 1983;91(5):550. doi: 10.1177/019459988309100514. [DOI] [PubMed] [Google Scholar]
- 139.Reyes SA, Salvi RJ, Burkard RF, Coad ML, Wack DS, Galantowicz PJ, Lockwood AH. Brain imaging of the effects of lidocaine on tinnitus. Hear Res. 2002;171(1–2):43. doi: 10.1016/s0378-5955(02)00346-5. [DOI] [PubMed] [Google Scholar]
- 140.Kallio H, Niskanen ML, Havia M, Neuvonen PJ, Rosenberg PH, Kentala E. I. V ropivacaine compared with lidocaine for the treatment of tinnitus. Br J Anaesth. 2008;101(2):261. doi: 10.1093/bja/aen137. [DOI] [PubMed] [Google Scholar]
- 141.Haginomori S, Makimoto K, Araki M, Kawakami M, Takahashi H. Effect of lidocaine injection of EOAE in patients with tinnitus. Acta Oto-Laryngol. 1995;115(4):488. doi: 10.3109/00016489509139353. [DOI] [PubMed] [Google Scholar]
- 142.Baguley DM, Jones S, Wilkins I, Axon PR, Moffat DA. The inhibitory effect of intravenous lidocaine infusion on tinnitus after translabyrinthine removal of vestibular schwannoma: A double-blind, placebo-controlled, crossover study. Otol Neurotol. 2005;26(2):169. doi: 10.1097/00129492-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 143.Cathcart JM. Assessment of the value of tocainide hydrochloride in the treatment of tinnitus. J Laryngol Otol. 1982;96(11):981. doi: 10.1017/s0022215100093397. [DOI] [PubMed] [Google Scholar]
- 144.Hulshof JH, Vermeij P. The value of tocainide in the treatment of tinnitus. A double-blind controlled study. Arch Oto-Rhino-Laryngol. 1985;241(3):279–83. doi: 10.1007/BF00453701. [DOI] [PubMed] [Google Scholar]
- 145.Emmett JR, Shea JJ. Treatment of tinnitus with tocainide hydrochloride. Otolaryngol Head Neck Surg. 1980;88(4):442. [PubMed] [Google Scholar]
- 146.Blayney AW, Phillips MS, Guy AM, Colman BH. A sequential double blind cross-over trial of tocainide hydrochloride in tinnitus. Clin Otolaryngol. 1985;10(2):97. doi: 10.1111/j.1365-2273.1985.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 147.Lenarz T. Treatment of tinnitus with lidocaine and tocainide. Scand Audiol Suppl. 1986;26:49–51. [PubMed] [Google Scholar]
- 148.Gribkoff VK. The therapeutic potential of neuronal KCNQ channel modulators. Expert Opin Ther Targets. 2003;7(6):737. doi: 10.1517/14728222.7.6.737. [DOI] [PubMed] [Google Scholar]
- 149.Korsgaard MP, Hartz BP, Brown WD, Ahring PK, Strobaek D, Mirza NR. Anxiolytic effects of Maxipost (BMS-204352) and retigabine via activation of neuronal KV7 channels. J Pharmacol Exp Ther. 2005;314(1):282. doi: 10.1124/jpet.105.083923. [DOI] [PubMed] [Google Scholar]
- 150.Lobarinas E, Dalby-Brown W, Stolzberg D, Mirza N, Salvi R. Effects of the BK agonists BMS-204352 and the enantiomeric compound (“R-enantiomer”) on transient, salicylate induced tinnitus in rats. [DOI] [PubMed] [Google Scholar]
- 151.Munro G, Erichsen HK, Mirza NR. Pharmacological comparison of anticonvulsant drugs in animal models of persistent pain and anxiety. Neuropharmacology. 2007;53(5):609. doi: 10.1016/j.neuropharm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 152.Israel M, Beaudry P. Carbamazepine in psychiatry: A review. Can J Psychiatry. 1988;33(7):577. doi: 10.1177/070674378803300701. [DOI] [PubMed] [Google Scholar]
- 153.Hirschfeld RM, Kasper S. A review of the evidence for carbamazepine and oxcarbazepine in the treatment of bipolar disorder. Int J Neuropsychopharmacol. 2004;7(4):507. doi: 10.1017/S1461145704004651. [DOI] [PubMed] [Google Scholar]
- 154.Cereghino JJ, Brock JT, Van Meter JC, Penry JK, Smith LD, White BG. The efficacy of carbamazepine combinations in epilepsy. Clin Pharmacol Ther. 1975;18(6):733. doi: 10.1002/cpt1975186733. [DOI] [PubMed] [Google Scholar]
- 155.Taylor JC, Brauer S, Espir ML. Long-term treatment of trigeminal neuralgia with carbamazepine. Postgrad Med J. 1981;57(663):16. doi: 10.1136/pgmj.57.663.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Herrera JM, Sramek JJ, Costa JF. Efficacy of adjunctive carbamazepine in the treatment of chronic schizophrenia. Drug Intel Clin Pharm. 1987;21(4):355. doi: 10.1177/106002808702100411. [DOI] [PubMed] [Google Scholar]
- 157.Willow M, Gonoi T, Catterall WA. Voltage clamp analysis of the inhibitory actions of diphenylhydantoin and carbamazepine on voltage-sensitive sodium channels in neuroblastoma cells. Mol Pharmacol. 1985;27(5):549. [PubMed] [Google Scholar]
- 158.Kuo CC, Chen RS, Lu L, Chen RC. Carbamazepine inhibition of neuronal Na+ currents: quantitative distinction from phenytoin and possible therapeutic implications. Mol Pharmacol. 1997;51(6):1077. doi: 10.1124/mol.51.6.1077. [DOI] [PubMed] [Google Scholar]
- 159.Cardenas CA, Cardenas CG, de Armendi AJ, Scroggs RS. Carbamazepine interacts with a slow inactivation state of NaV1.8-like sodium channels Neurosci Lett 2006, 408(2): 129 8-like sodium channels. Neurosci Lett. 2006;408(2):129. doi: 10.1016/j.neulet.2006.08.070. [DOI] [PubMed] [Google Scholar]
- 160.Dailey JW, Reith ME, Steidley KR, Milbrandt JC, Jobe PC. Carbamazepine-induced release of serotonin from rat hippocampus in vitro. Epilepsia. 1998;39(10):1054. doi: 10.1111/j.1528-1157.1998.tb01290.x. [DOI] [PubMed] [Google Scholar]
- 161.Zona C, Tancredi V, Palma E, Pirrone GC, Avoli M. Potassium currents in rat cortical neurons in culture are enhanced by the antiepileptic drug carbamazepine. Can J Physiol Pharmacol. 1990;68(4):545. doi: 10.1139/y90-079. [DOI] [PubMed] [Google Scholar]
- 162.Ambrosio AF, et al. Carbamazepine inhibits L-type Ca2+ channels in cultured rat hippocampal neurons stimulated with glutamate receptor agonists. Neuropharmacology. 1999;38(9):1349. doi: 10.1016/s0028-3908(99)00058-1. [DOI] [PubMed] [Google Scholar]
- 163.Donaldson I. Tegretol: A double blind trial in tinnitus. J Laryngol Otol. 1981;95(9):947. doi: 10.1017/s0022215100091659. [DOI] [PubMed] [Google Scholar]
- 164.Hulshof JH, Vermeij P. The value of carbamazepine in the treatment of tinnitus. ORL - J Otorhinolaryngol Relat Spec. 1985;47(5):262. doi: 10.1159/000275781. [DOI] [PubMed] [Google Scholar]
- 165.Marks NJ, Onisiphorou C, Trounce JR. The effect of single doses of amylobarbitone sodium and carbamazepine in tinnitus. J Laryngol Otol. 1981;95(9):941. doi: 10.1017/s0022215100091647. [DOI] [PubMed] [Google Scholar]
- 166.Melding PS, Goodey RJ. The treatment of tinnitus with oral anticonvulsants. J Laryngol Otol. 1979;93(2):111. doi: 10.1017/s0022215100086837. [DOI] [PubMed] [Google Scholar]
- 167.Zheng Y, Hooton K, Smith PF, Darlington CL. Carbamazepine reduces the behavioural manifestations of tinnitus following salicylate treatment in rats. Acta Oto-Laryngol. 2008;128(1):48. doi: 10.1080/00016480701361939. [DOI] [PubMed] [Google Scholar]
- 168.Liu J, Li X, Wang L, Dong Y, Han H, Liu G. Effects of salicylate on serotoninergic activities in rat inferior colliculus and auditory cortex. Hear Res. 2003;175(1–2):45. doi: 10.1016/s0378-5955(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 169.Liu Y, Zhang H, Li X, Wang Y, Lu H, Qi X, Ma C, Liu J. Inhibition of voltage-gated channel currents in rat auditory cortex neurons by salicylate. Neuropharmacology. 2007;53(7):870. doi: 10.1016/j.neuropharm.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 170.Wang HT, Luo B, Zhou KQ, Xu TL, Chen L. Sodium salicylate reduces inhibitory postsynaptic currents in neurons of rat auditory cortex. Hear Res. 2006;215(1–2):77. doi: 10.1016/j.heares.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 171.Menkes DB, Larson PM. Sodium valproate for tinnitus. J Neurol Neurosurg Psychiatry. 1998;65(5):803. doi: 10.1136/jnnp.65.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Reeves RR, Mustain DW, Pendarvis JE. Valproate-induced tinnitus misinterpreted as psychotic symptoms. South Med J. 2000;93(10):1030. [PubMed] [Google Scholar]
- 173.Goodey R. Drug treatment for tinnitus. Prog Brain Res. 2007;166:237. doi: 10.1016/S0079-6123(07)66022-1. [DOI] [PubMed] [Google Scholar]
- 174.Sivenius J, Kalviainen R, Ylinen A, Riekkinen P. Double-blind study of gabapentin in the treatment of partial seizures. Epilepsia. 1991;32(4):539. doi: 10.1111/j.1528-1157.1991.tb04689.x. [DOI] [PubMed] [Google Scholar]
- 175.Levendoglu F, et al. Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. Spine. 2004;29(7):743. doi: 10.1097/01.brs.0000112068.16108.3a. [DOI] [PubMed] [Google Scholar]
- 176.Smith DG, Ehde DM, Hanley MA, et al. Efficacy of gabapentin in treating chronic phantom limb and residual limb pain. J Rehabil Res Dev. 2005;42(5):645. doi: 10.1682/jrrd.2005.05.0082. [DOI] [PubMed] [Google Scholar]
- 177.Di Trapani G, Mei D, Marra C, Mazza S, Capuano A. Gabapentin in the prophylaxis of migraine: A double-blind randomized placebo-controlled study. Clin Ter (Rome) 2000;151(3):145. [PubMed] [Google Scholar]
- 178.Goa KL, Sorkin EM. Gabapentin. A review of its pharmacological properties and clinical potential in epilepsy. Drugs. 1993;46(3):409. doi: 10.2165/00003495-199346030-00007. [DOI] [PubMed] [Google Scholar]
- 179.Rock DM, Kelly KM, Macdonald RL. Gabapentin actions on ligand- and voltage-gated responses in cultured rodent neurons. Epilepsy Res. 1993;16(2):89. doi: 10.1016/0920-1211(93)90023-z. [DOI] [PubMed] [Google Scholar]
- 180.Honmou O, Kocsis JD, Richerson GB. Gabapentin potentiates the conductance increase induced by nipecotic acid in CA1 pyramidal neurons in vitro. Epilepsy Res. 1995;20(3):193. doi: 10.1016/0920-1211(94)00076-9. [DOI] [PubMed] [Google Scholar]
- 181.Petroff OA, Rothman DL, Behar KL, Lamoureux D, Mattson RH. The effect of gabapentin on brain gamma-aminobutyric acid in patients with epilepsy. Ann Neurol. 1996;39(1):95. doi: 10.1002/ana.410390114. [DOI] [PubMed] [Google Scholar]
- 182.Hendrich J, Van Minh AT, Heblich F, et al. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci USA. 2008;105(9):3628. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zapp JJ. Gabapentin for the treatment of tinnitus: A case report. Ear Nose Throat J. 2001;80(2):114. [PubMed] [Google Scholar]
- 184.Shulman A. Gabapentin and tinnitus relief. Int Tinnitus J. 2008;14(1):1–5. [PubMed] [Google Scholar]
- 185.Bauer CA, Brozoski TJ. Assessing tinnitus and prospective tinnitus therapeutics using a psychophysical animal model. J Assoc Res Otolaryngol. 2001;2(1):54–64. doi: 10.1007/s101620010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Witsell DL, Hannley MT, Stinnet S, Tucci DL. Treatment of tinnitus with gabapentin: A pilot study. Otol Neurotol. 2007;28(1):11. doi: 10.1097/01.mao.0000235967.53474.93. [DOI] [PubMed] [Google Scholar]
- 187.Bakhshaee M, Ghasemi M, Azarpazhooh M, Khadivi E, Rezaei S, Shakeri M, Tale M. Gabapentin effectiveness on the sensation of subjective idiopathic tinnitus: A pilot study. Eur Arch Oto-Rhino-Laryngol. 2008;265(5):525. doi: 10.1007/s00405-007-0504-9. [DOI] [PubMed] [Google Scholar]
- 188.Piccirillo JF, Finnell J, Vlahiotis A, Chole RA, Spitznagel E., Jr Relief of idiopathic subjective tinnitus: Is gabapentin effective? Arch Otolaryngol Head Neck Surg. 2007;133(4):390. doi: 10.1001/archotol.133.4.390. [DOI] [PubMed] [Google Scholar]
- 189.Bauer CA, Brozoski TJ. Effect of gabapentin on the sensation and impact of tinnitus. Laryngoscope. 2006;116(5):675. doi: 10.1097/01.MLG.0000216812.65206.CD. [DOI] [PubMed] [Google Scholar]
- 190.Gross G, Xin X, Gastpar M. Trimipramine: Pharmacological reevaluation and comparison with clozapine. Neuropharmacology. 1991;30(11):1159. doi: 10.1016/0028-3908(91)90160-d. [DOI] [PubMed] [Google Scholar]
- 191.Juorio AV, Li XM, Boulton AA. The effects of chronic trimipramine treatment on biogenic amine metabolism and on dopamine D2, 5-HT2 and tryptamine binding sites in rat brain. Gen Pharmacol. 1990;21(5):759. doi: 10.1016/0306-3623(90)91030-u. [DOI] [PubMed] [Google Scholar]
- 192.Mihail RC, Crowley JM, Walden BE, Fishburne J, Reinwall JE, Zajtchuk JT. The tricyclic trimipramine in the treatment of subjective tinnitus. Ann Otol Rhinol Laryngol. 1988;97(2 Pt 1):120. doi: 10.1177/000348948809700204. [DOI] [PubMed] [Google Scholar]
- 193.Gracious B, Wisner KL. Nortriptyline in chronic fatigue syndrome: A double blind, placebo-controlled single case study. Biol Psychiatry. 1991;30(4):405. doi: 10.1016/0006-3223(91)90297-y. [DOI] [PubMed] [Google Scholar]
- 194.Punay NC, Couch JR. Antidepressants in the treatment of migraine headache. Curr Pain Headache Rep. 2003;7(1):51. doi: 10.1007/s11916-003-0010-8. [DOI] [PubMed] [Google Scholar]
- 195.Atkinson JH, et al. A placebo-controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain. 1998;76(3):287. doi: 10.1016/S0304-3959(98)00064-5. [DOI] [PubMed] [Google Scholar]
- 196.Anderson J, Lambert NG, Pigott PV. An evaluation of fluphenazine with nortriptyline in anxiety and depression. Practitioner. 1972;208(246):511–7. [PubMed] [Google Scholar]
- 197.Sullivan MD, Dobie RA, Sakai CS, Katon WJ. Treatment of depressed tinnitus patients with nortriptyline. Ann Otol Rhinol Laryngol. 1989;98(11):867. doi: 10.1177/000348948909801107. [DOI] [PubMed] [Google Scholar]
- 198.Sullivan M, Katon W, Russo J, Dobie R, Sakai C. A randomized trial of nortriptyline for severe chronic tinnitus. Effects on depression, disability, and tinnitus symptoms. Arch Intern Med. 1993;153(19):2251. [PubMed] [Google Scholar]
- 199.Katon W, Sullivan M, Russo J, Dobie R, Sakai C. Depressive symptoms and measures of disability: A prospective study. J Affect Disord. 1993;27(4):245. doi: 10.1016/0165-0327(93)90048-o. [DOI] [PubMed] [Google Scholar]
- 200.Stein DJ, Davidson J, Seedat S, Beebe K. Paroxetine in the treatment of post-traumatic stress disorder: Pooled analysis of placebo-controlled studies. Expert Opin Pharmacother. 2003;4(10):1829. doi: 10.1517/14656566.4.10.1829. [DOI] [PubMed] [Google Scholar]
- 201.Oehrberg S, Christiansen PE, Behnke K, et al. Paroxetine in the treatment of panic disorder. A randomised, double-blind, placebo-controlled study. Br J Psychiatry. 1995;167(3):374. doi: 10.1192/bjp.167.3.374. [DOI] [PubMed] [Google Scholar]
- 202.Denys D, et al. A double blind comparison of venlafaxine and paroxetine in obsessive-compulsive disorder. J Clin Psychopharmacol. 2003;23(6):568. doi: 10.1097/01.jcp.0000095342.32154.54. [DOI] [PubMed] [Google Scholar]
- 203.Thomas DR, Nelson DR, Johnson AM. Biochemical effects of the antidepressant paroxetine, a specific 5-hydroxytryptamine uptake inhibitor. Psychopharmacology (Berlin) 1987;93(2):193. doi: 10.1007/BF00179933. [DOI] [PubMed] [Google Scholar]
- 204.Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs) Int Clin Psychopharmacol. 1994;9(Suppl 1):19. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- 205.Robinson SK, Viirre ES, Bailey KA, Gerke MA, Harris JP, Stein MB. Randomized placebo-controlled trial of a selective serotonin reuptake inhibitor in the treatment of nondepressed tinnitus subjects. Psychosom Med. 2005;67(6):981. doi: 10.1097/01.psy.0000188479.04891.74. [DOI] [PubMed] [Google Scholar]
- 206.Parnes SM. Current concepts in the clinical management of patients with tinnitus. Eur Arch Oto-Rhino-Laryngol. 1997;254(9–10):406. doi: 10.1007/BF02439968. [DOI] [PubMed] [Google Scholar]
- 207.Kingery WS, Davies MF, Clark JD. A substance P receptor (NK1) antagonist can reverse vascular and nociceptive abnormalities in a rat model of complex regional pain syndrome type II. Pain. 2003;104(1–2):75. doi: 10.1016/s0304-3959(02)00467-0. [DOI] [PubMed] [Google Scholar]
- 208.Dionne RA, Max MB, Gordon SM, Parada S, Sang C, Gracely RH, Sethna NF, MacLean DB. The substance P receptor antagonist CP-99,994 reduces acute postoperative pain. Clin Pharmacol Ther. 1998;64(5):562. doi: 10.1016/S0009-9236(98)90140-0. [DOI] [PubMed] [Google Scholar]
- 209.Vass Z, Nuttall AL, Coleman JK, Miller JM. Capsaicin-induced release of substance P increases cochlear blood flow in the guinea pig. Hear Res. 1995;89(1–2):86. doi: 10.1016/0378-5955(95)00127-4. [DOI] [PubMed] [Google Scholar]
- 210.Sun W, Ding DL, Wang P, Sun J, Jin X, Salvi RJ. Substance P inhibits potassium and calcium currents in inner ear spiral ganglion neurons. Brain Res. 2004;1012(1–2):82. doi: 10.1016/j.brainres.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 211.Holliday SM, Plosker GL. Paroxetine. A review of its pharmacology, therapeutic use in depression and therapeutic potential in diabetic neuropathy. Drugs Aging. 1993;3(3):278. doi: 10.2165/00002512-199303030-00008. [DOI] [PubMed] [Google Scholar]
- 212.Kronig MH, Apter J, Asnis G, Bystritsky A, Curtis G, Ferguson J, Landbloom R, Munjack D, Riesenberg R, Robinson D, RoyByrne P, Phillips K, DuPont IJ. Placebo-controlled, multicenter study of sertraline treatment for obsessive-compulsive disorder. J Clin Psychopharmacol. 1999;19(2):172. doi: 10.1097/00004714-199904000-00013. [DOI] [PubMed] [Google Scholar]
- 213.Hirschfeld RM. Sertraline in the treatment of anxiety disorders. Depress Anxiety. 2000;11(4):139. doi: 10.1002/1520-6394(2000)11:4<139::AID-DA1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 214.Hesslinger B, Van de Loo A, Klecha D, Harter M, Schmidt-Schweda S. Depression and panic disorder after heart transplantation - Treatment with sertraline. Pharmacopsychiatry. 2002;35(1):31. doi: 10.1055/s-2002-19835. [DOI] [PubMed] [Google Scholar]
- 215.Guthrie SK. Sertraline: A new specific serotonin reuptake blocker. DICP. 1991;25(9):952. doi: 10.1177/106002809102500910. [DOI] [PubMed] [Google Scholar]
- 216.Schmidt A, Lebel L, Koe BK, Seeger T, Heym J. Sertraline potently displaces (+)-[3H]3-PPP binding to sigma sites in rat brain. Eur J Pharmacol. 1989;165(2–3):335. doi: 10.1016/0014-2999(89)90734-6. [DOI] [PubMed] [Google Scholar]
- 217.Robinson S. Antidepressants for treatment of tinnitus. Prog Brain Res. 2007;166:263. doi: 10.1016/S0079-6123(07)66024-5. [DOI] [PubMed] [Google Scholar]
- 218.Miller MJ, Sadowska-Krowicka H, Jeng AY, et al. Substance P levels in experimental ileitis in guinea pigs: effects of misoprostol. Am J Physiol. 1993;265(2 Pt 1):G321. doi: 10.1152/ajpgi.1993.265.2.G321. [DOI] [PubMed] [Google Scholar]
- 219.Gullikson GW, Anglin CP, Kessler LK, Smeach S, Bauer RF, Dajani EZ. Misoprostol attenuates aspirin-induced changes in potential difference and associated damage in canine gastric mucosa. Clin Invest Med. 1987;10(3):145. [PubMed] [Google Scholar]
- 220.Roth SH. Misoprostol in the prevention of NSAID-induced gastric ulcer: A multicenter, double-blind, placebo-controlled trial. J Rheumatol. 1990;20(Suppl):20. [PubMed] [Google Scholar]
- 221.Hall R, Duarte-Gardea M, Harlass F. Oral versus vaginal misoprostol for labor induction. Obstet Gynecol. 2002;99(6):1044. doi: 10.1016/s0029-7844(02)01995-6. [DOI] [PubMed] [Google Scholar]
- 222.Widomski D, et al. The prostaglandin analogs, misoprostol and SC-46275, potently inhibit cytokine release from activated human monocytes. Immunopharmacol Immunotoxicol. 1997;19(2):165. doi: 10.3109/08923979709007656. [DOI] [PubMed] [Google Scholar]
- 223.Briner W, House J, O’Leary M. Synthetic prostaglandin E1 misoprostol as a treatment for tinnitus. Arch Otolaryngol Head Neck Surg. 1993;119(6):652. doi: 10.1001/archotol.1993.01880180068013. [DOI] [PubMed] [Google Scholar]
- 224.Akkuzu B, Yilmaz I, Cakmak O, Ozluoglu LN. Efficacy of misoprostol in the treatment of tinnitus in patients with diabetes and/or hypertension. Auris Nasus Larynx. 2004;31(3):226. doi: 10.1016/j.anl.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 225.Naoumova RP, Dunn S, Rallidis L, et al. Prolonged inhibition of cholesterol synthesis explains the efficacy of atorvastatin. J Lipid Res. 1997;38(7):1496. [PubMed] [Google Scholar]
- 226.Gresser U, Gathof BS. Atorvastatin: Gold standard for prophylaxis of myocardial ischemia and stroke - Comparison of the clinical benefit of statins on the basis of randomized controlled endpoint studies. Eur J Med Res. 2004;9(1):1. [PubMed] [Google Scholar]
- 227.Heinonen TM, Stein E, Weiss SR, McKenney JM, Davidson M, Shurzinske L, Black DM. The lipid-lowering effects of atorvastatin, a new HMG-CoA reductase inhibitor: Results of a randomized, double-masked study. Clin Ther. 1996;18(5):853. doi: 10.1016/s0149-2918(96)80045-2. [DOI] [PubMed] [Google Scholar]
- 228.Sugiyama M, et al. Effects of atorvastatin on inflammation and oxidative stress. Heart Vessels. 2005;20(4):133. doi: 10.1007/s00380-005-0833-9. [DOI] [PubMed] [Google Scholar]
- 229.Wang H, Lynch JR, Song P, Yang HJ, Yates RB, Mace B, Warner DS, Guyton JR, Laskowitz DT. Simvastatin and atorvastatin improve behavioral outcome, reduce hippocampal degeneration, and improve cerebral blood flow after experimental traumatic brain injury. Exp Neurol. 2007;206(1):59. doi: 10.1016/j.expneurol.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 230.Olzowy B, Canis M, Hempel JM, Mazurek B, Suckfull M. Effect of atorvastatin on progression of sensorineural hearing loss and tinnitus in the elderly: Results of a prospective, randomized, double-blind clinical trial. Otol Neurotol. 2007;28(4):455. doi: 10.1097/01.mao.0000271673.33683.7b. [DOI] [PubMed] [Google Scholar]
- 231.Wu CT, Wong CS, Yeh CC, Borel CO. Treatment of cerebral vasospasm after subarachnoid hemorrhage - A review. Acta Anaesthesiol Taiwan. 2004;42(4):215–22. [PubMed] [Google Scholar]
- 232.Duley L, Henderson-Smart DJ, Meher S. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev (CD-ROM) 2006;3:CD001449. doi: 10.1002/14651858.CD001449.pub2. [DOI] [PubMed] [Google Scholar]
- 233.Scriabine A, van den Kerckhoff W. Pharmacology of nimodipine. A review. Ann N Y Acad Sci. 1988;522:698. doi: 10.1111/j.1749-6632.1988.tb33415.x. [DOI] [PubMed] [Google Scholar]
- 234.Theopold HM. Nimodipine (Bay e 9736) a new therapy concept in diseases of the inner ear? Laryngo-Rhino-Otol. 1985;64(12):609. [PubMed] [Google Scholar]
- 235.Wang H, Jiang S, Yang W, Han D. Evaluating effects of some medicine on tinnitus with animal behavioral model in rats. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2000;35(5):331–4. [PubMed] [Google Scholar]
- 236.Jastreboff PJ, Brennan JF. Specific effects of nimodipine on the auditory system. Ann N Y Acad Sci. 1988;522:716. [Google Scholar]
- 237.Davies E, Knox E, Donaldson I. The usefulness of nimodipine, an L-calcium channel antagonist, in the treatment of tinnitus. Br J Audiol. 1994;28(3):125–9. doi: 10.3109/03005369409086559. [DOI] [PubMed] [Google Scholar]
- 238.Hutcheon DE, Leonard G. Diuretic and antihypertensive actions of furosemide. J Clin Pharmacol J New Drugs. 1967;7(1):26–33. doi: 10.1002/j.1552-4604.1967.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 239.Shiozaki A, Miyazaki H, Niisato N, et al. Furosemide, a blocker of Na+/K+/2Cl- cotransporter, diminishes proliferation of poorly differentiated human gastric cancer cells by affecting G0/G1 state. J Physiol Sci. 2006;56(6):401. doi: 10.2170/physiolsci.RP010806. [DOI] [PubMed] [Google Scholar]
- 240.Sakaguchi N, Crouch JJ, Lytle C, Schulte BA. Na-K-Cl cotransporter expression in the developing and senescent gerbil cochlea. Hear Res. 1998;118(1–2):114. doi: 10.1016/s0378-5955(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 241.Chen H, Sun D. The role of Na-K-Cl co-transporter in cerebral ischemia. Neurol Res. 2005;27(3):280. doi: 10.1179/016164105X25243. [DOI] [PubMed] [Google Scholar]
- 242.Kolbaev SN, Sharonova IN, Vorobjev VS, Skrebitsky VG. Mechanisms of GABA(A) receptor blockade by millimolar concentrations of furosemide in isolated rat Purkinje cells. Neuropharmacology. 2002;42(7):913. doi: 10.1016/s0028-3908(02)00042-4. [DOI] [PubMed] [Google Scholar]
- 243.Rybak LP, Morizono T. Effect of furosemide upon endolymph potassium concentration. Hear Res. 1982;7(2):223. doi: 10.1016/0378-5955(82)90015-6. [DOI] [PubMed] [Google Scholar]
- 244.Risey JA, Guth PS, Amedee RG. Furosemide distinguishes central and peripheral tinnitus. Int Tinnitus J. 1995;1(2):99–103. [PubMed] [Google Scholar]
- 245.Futaki T, Kitahara M, Morimoto M. A comparison of the furosemide and glycerol tests for Meniere’s disease. With special reference to the bilateral lesion. Acta Oto-Laryngol. 1977;83(3–4):272. doi: 10.3109/00016487709128845. [DOI] [PubMed] [Google Scholar]
- 246.Kuchar DL, O’Rourke MF. High dose furosemide in refractory cardiac failure. Eur Heart J. 1985;6(11):954. doi: 10.1093/oxfordjournals.eurheartj.a061793. [DOI] [PubMed] [Google Scholar]
- 247.Ho KM, Sheridan DJ. Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ - Br Med J (Clin Res Ed) 2006;333(7565):420. doi: 10.1136/bmj.38902.605347.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Huic M, Vrhovac B, Macolic-Sarinic V, Francetic I, Bakran I, Giljanovic S. How safe are bioequivalence studies in healthy volunteers? Therapie. 1996;51(4):410. [PubMed] [Google Scholar]
- 249.Wallach DP. Studies on the GABA pathway. I The inhibition of gamma-aminobutyric acid-alpha-ketoglutaric acid transaminase in vitro and in vivo by U-7524 (amino-oxyacetic acid) Biochem Pharmacol. 1961;5:323. doi: 10.1016/0006-2952(61)90023-5. [DOI] [PubMed] [Google Scholar]
- 250.Kerwin R, Pycock C. Baclofen (beta-p-chlorophenyl-gamma-aminobutyric acid) enhances [3H]gamma-aminobutyric acid (3H-GABA) release from rat globus pallidus in vitro. J Pharm Pharmacol. 1978;30(10):622. doi: 10.1111/j.2042-7158.1978.tb13343.x. [DOI] [PubMed] [Google Scholar]
- 251.Bobbin RP, Gondra MI. Effect of intracochlear aminooxyacetic acid on cochlear potentials and endolymph composition. Ann Otol Rhinol Laryngol. 1975;84(2 Pt 1):192. doi: 10.1177/000348947508400209. [DOI] [PubMed] [Google Scholar]
- 252.Reed HT, Meltzer J, Crews P, Norris CH, Quine DB, Guth PS. Amino-oxyacetic acid as a palliative in tinnitus. Arch Otolaryngol. 1985;111(12):803. doi: 10.1001/archotol.1985.00800140047008. [DOI] [PubMed] [Google Scholar]
- 253.Guth PS, Risey J, Briner W, et al. Evaluation of amino-oxyacetic acid as a palliative in tinnitus. Ann Otol Rhinol Laryngol. 1990;99(1):74. doi: 10.1177/000348949009900113. [DOI] [PubMed] [Google Scholar]
- 254.McCabe BF. Central aspects of drugs for motion sickness and vertigo. Adv Otorhinolaryngol. 1973;20:458. doi: 10.1159/000393116. [DOI] [PubMed] [Google Scholar]
- 255.Kranke P, et al. The efficacy and safety of transdermal scopolamine for the prevention of postoperative nausea and vomiting: A quantitative systematic review. Anesth Analg. 2002;95(1):133. doi: 10.1097/00000539-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 256.Miranda HF, Silva WI, Wolstenholme WW, Cuevas N. Selective antagonism by pirenzepine and N-methyl-scopolamine on muscarinic receptor subtypes of the rat vas deferens. Gen Pharmacol. 1988;19(3):417. doi: 10.1016/0306-3623(88)90039-0. [DOI] [PubMed] [Google Scholar]
- 257.Dohanich GP, Johnson AE, Nock B, McEwen BS, Feder HH. Distribution of cholinergic muscarinic binding sites in guinea-pig brain as determined by in vitro autoradiography of [3H]N-methyl scopolamine binding. Eur J Pharmacol. 1985;119(1–2):9. doi: 10.1016/0014-2999(85)90315-2. [DOI] [PubMed] [Google Scholar]
- 258.Metherate R, Ashe JH, Weinberger NM. Acetylcholine modifies neuronal acoustic rate-level functions in guinea pig auditory cortex by an action at muscarinic receptors. Synapse (NY) 1990;6(4):364. doi: 10.1002/syn.890060409. [DOI] [PubMed] [Google Scholar]
- 259.Whipple MR, Drescher DG. Muscarinic receptors in the cochlear nucleus and auditory nerve of the guinea pig. J Neurochem. 1984;43(1):192. doi: 10.1111/j.1471-4159.1984.tb06696.x. [DOI] [PubMed] [Google Scholar]
- 260.Wallhausser-Franke E, Cuautle-Heck B, Wenz G, Langner G, Mahlke C. Scopolamine attenuates tinnitus-related plasticity in the auditory cortex. NeuroReport. 2006;17(14):1487. doi: 10.1097/01.wnr.0000230504.25102.26. [DOI] [PubMed] [Google Scholar]
- 261.Lobarinas E, Sun W, Stolzberg D, Lu J, Salvi R. Human brain imaging of tinnitus and animal models. Semin Hear. 2008;29(4):333–49. doi: 10.1055/s-0028-1095893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Timmerman H. Calcium modulation and clinical effect. Profile of cyclandelate. Drugs. 1987;33(Suppl 2):1. doi: 10.2165/00003495-198700332-00003. [DOI] [PubMed] [Google Scholar]
- 263.Siniatchkin M, Gerber WD, Vein A. Clinical efficacy and central mechanisms of cyclandelate in migraine: A double-blind placebo-controlled study. Funct Neurol. 1998;13(1):47. [PubMed] [Google Scholar]
- 264.Middleton B, Middleton A, White DA, Bell GD. Dietary cyclandelate decreases pre-established atherosclerosis in the rabbit. Atherosclerosis. 1984;51(2–3):171. doi: 10.1016/0021-9150(84)90165-5. [DOI] [PubMed] [Google Scholar]
- 265.Holstein N. Ginkgo special extract EGb 761 in tinnitus therapy. An overview of results of completed clinical trials. Fortschr Med Orig. 2001;118(4):157–64. [PubMed] [Google Scholar]
- 266.Huang MH, Huang CC, Ryu SJ, Chu NS. Sudden bilateral hearing impairment in vertebrobasilar occlusive disease. Stroke. 1993;24(1):132. doi: 10.1161/01.str.24.1.132. [DOI] [PubMed] [Google Scholar]
- 267.Memin Y. Perceived efficacy of cyclandelate in the treatment of cochleovestibular and retinal disturbances related to cerebrovascular insufficiency. A study in general practice comprising 2772 patients. Drugs. 1987;33(Suppl 2):120. doi: 10.2165/00003495-198700332-00022. [DOI] [PubMed] [Google Scholar]
- 268.Hester TO, Theilman G, Green W, Jones RO. Cyclandelate in the management of tinnitus: A randomized, placebo- controlled study. Otolaryngol Head Neck Surg. 1998;118(3 Pt 1):329. doi: 10.1016/S0194-59989870310-9. [DOI] [PubMed] [Google Scholar]
- 269.Memo M, Battaini F, Spano PF, Trabucchi M. Sulpiride and the role of dopaminergic receptor blockade in the antipsychotic activity of neuroleptics. Acta Psychiatr Scand. 1981;63(4):314. doi: 10.1111/j.1600-0447.1981.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 270.Imafuku J. The characterization of [3H]sulpiride binding sites in rat striatal membranes. Brain Res. 1987;402(2):331. doi: 10.1016/0006-8993(87)90040-0. [DOI] [PubMed] [Google Scholar]
- 271.Jastreboff PJ, Hazell JWP. A neurophysiological approach to tinnitus: Clinical implications. Br J Audiol. 1993;27(1):7–17. doi: 10.3109/03005369309077884. [DOI] [PubMed] [Google Scholar]
- 272.Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: Evidence for limbic system links and neural plasticity. Neurology. 1998;50(1):114. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- 273.Larson ER, Ariano MA. D3 and D2 dopamine receptors: Visualization of cellular expression patterns in motor and limbic structures. Synapse (NY) 1995;20(4):325. doi: 10.1002/syn.890200406. [DOI] [PubMed] [Google Scholar]
- 274.Lopez-Gonzalez MA, Esteban-Ortega F. Tinnitus dopaminergic pathway. Ear noises treatment by dopamine modulation. Med Hypotheses. 2005;65(2):349. doi: 10.1016/j.mehy.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 275.Lopez-Gonzalez MA, Santiago AM, Esteban-Ortega F. Sulpiride and melatonin decrease tinnitus perception modulating the auditolimbic dopaminergic pathway. J Otolaryngol. 2007;36(4):213. doi: 10.2310/7070.2007.0018. [DOI] [PubMed] [Google Scholar]
- 276.Lincoln GA, Tortonese DJ. Does melatonin act on dopaminergic pathways in the mediobasal hypothalamus to mediate effects of photoperiod on prolactin secretion in the ram? Neuroendocrinology. 1995;62(5):425. doi: 10.1159/000127032. [DOI] [PubMed] [Google Scholar]
- 277.Nguyen-Legros J, Chanut E, Versaux-Botteri C, Simon A, Trouvin JH. Dopamine inhibits melatonin synthesis in photoreceptor cells through a D2-like receptor subtype in the rat retina: Biochemical and histochemical evidence. J Neurochem. 1996;67(6):2514. doi: 10.1046/j.1471-4159.1996.67062514.x. [DOI] [PubMed] [Google Scholar]
- 278.Lopez-Gonzalez MA, Moliner-Peiro F, Alfaro-Garcia J, Esteban-Ortega F. Sulpiride plus hydroxyzine decrease tinnitus perception. Auris Nasus Larynx. 2007;34(1):23. doi: 10.1016/j.anl.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 279.Seftel AD. Phosphodiesterase type 5 inhibitor differentiation based on selectivity, pharmacokinetic, and efficacy profiles. Clin Cardiol. 2004;27(4, Suppl 1):I14. doi: 10.1002/clc.4960271305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ravipati G, McClung JA, Aronow WS, Peterson SJ, Frishman WH. Type 5 phosphodiesterase inhibitors in the treatment of erectile dysfunction and cardiovascular disease. Cardiol Rev. 2007;15(2):76. doi: 10.1097/01.crd.0000233904.77128.49. [DOI] [PubMed] [Google Scholar]
- 281.Rashid A. The efficacy and safety of PDE5 inhibitors. Clin Cornerstone. 2005;7(1):47. doi: 10.1016/s1098-3597(05)80048-1. [DOI] [PubMed] [Google Scholar]
- 282.Mazurek B, Haupt H, Szczepek AJ, Sandmann J, Gross J, Klapp BF, Kiesewetter H, Kalus U, Stoever T, Caffier PP. Evaluation of vardenafil for the treatment of subjective tinnitus: A controlled pilot study. J Negat Results Biomed. 2009;8(1):3. doi: 10.1186/1477-5751-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Mahadevan S, Park Y. Multifaceted therapeutic benefits of Ginkgo biloba L. : Chemistry, efficacy, safety, and uses. J Food Sci. 2008;73(1):R14. doi: 10.1111/j.1750-3841.2007.00597.x. [DOI] [PubMed] [Google Scholar]
- 284.Morgenstern C, Biermann E. The efficacy of Ginkgo special extract EGb 761 in patients with tinnitus. Int J Clin Pharmacol Ther. 2002;40(5):188. doi: 10.5414/cpp40188. [DOI] [PubMed] [Google Scholar]
- 285.Jastreboff PJ, Zhou S, Jastreboff MM, Kwapisz U, Gryczynska U. Attenuation of salicylate-induced tinnitus by Ginkgo biloba extract in rats. Audiol Neuro-Otol. 1997;2(4):197. doi: 10.1159/000259244. [DOI] [PubMed] [Google Scholar]
- 286.Coles R. Trial of an extract of Ginkgo biloba (EGB) for tinnitus and hearing loss [letter] Clin Otolaryngol. 1988;13(6):501. [PubMed] [Google Scholar]
- 287.Holgers KM, Axelsson A, Pringle I. Ginkgo biloba extract for the treatment of tinnitus. Audiology. 1994;33(2):85. doi: 10.3109/00206099409071870. [DOI] [PubMed] [Google Scholar]
- 288.Smith PF, Zheng Y, Darlington CL. Ginkgo biloba extracts for tinnitus: More hype than hope? J Ethnopharmacol. 2005;100(1–2):95. doi: 10.1016/j.jep.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 289.Drew S, Davies E. Effectiveness of Ginkgo biloba in treating tinnitus: Double blind, placebo controlled trial. BMJ - Br Med J (Clin Res Ed) 2001;322(7278):73. doi: 10.1136/bmj.322.7278.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Patterson MB, Balough BJ. Review of pharmacological therapy for tinnitus. Int Tinnitus J. 2006;12(2):149–59. [PubMed] [Google Scholar]
- 291.Rejali D, Sivakumar A, Balaji N. Ginkgo biloba does not benefit patients with tinnitus: A randomized placebo-controlled double-blind trial and meta-analysis of randomized trials. Clin Otolaryngol Allied Sci. 2004;29(3):226. doi: 10.1111/j.1365-2273.2004.00814.x. [DOI] [PubMed] [Google Scholar]
- 292.Jan JE, Reiter RJ, Wasdell MB, Bax M. The role of the thalamus in sleep, pineal melatonin production, and circadian rhythm sleep disorders. J Pineal Res. 2009;46(1):1. doi: 10.1111/j.1600-079X.2008.00628.x. [DOI] [PubMed] [Google Scholar]
- 293.Seggie J, Werstiuk ES, Grota L. Lithium and circadian patterns of melatonin in the retina, hypothalamus, pineal and serum. Prog Neuro-Psychopharmacol Biol Psychiatry. 1987;11(2–3):325. doi: 10.1016/0278-5846(87)90077-7. [DOI] [PubMed] [Google Scholar]
- 294.Berra B, Rizzo AM. Melatonin: Circadian rhythm regulator, chronobiotic, antioxidant and beyond. Clin Dermatol. 2009;27(2):202. doi: 10.1016/j.clindermatol.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 295.Jou MJ, Peng TI, Yu PZ, Jou SB, Reiter RJ, Chen JY, Wu HY, Chen CC, Hsu LF. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J Pineal Res. 2007;43(4):389. doi: 10.1111/j.1600-079X.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- 296.Lopez-Gonzalez MA, Guerrero JM, Rojas F, Delgado F. Ototoxicity caused by cisplatin is ameliorated by melatonin and other antioxidants. J Pineal Res. 2000;28(2):73. doi: 10.1034/j.1600-079x.2001.280202.x. [DOI] [PubMed] [Google Scholar]
- 297.Bas E, Martinez-Soriano F, Lainez JM, Marco J. An experimental comparative study of dexamethasone, melatonin and tacrolimus in noise-induced hearing loss. Acta Oto-Laryngol. 2009;129(4):385. doi: 10.1080/00016480802566279. [DOI] [PubMed] [Google Scholar]
- 298.Rosenberg SI, Silverstein H, Rowan PT, Olds MJ. Effect of melatonin on tinnitus. Laryngoscope. 1998;108(3):305. doi: 10.1097/00005537-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 299.Megwalu UC, Finnell JE, Piccirillo JF. The effects of melatonin on tinnitus and sleep. Otolaryngol Head Neck Surg. 2006;134(2):210. doi: 10.1016/j.otohns.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 300.McFadden SL, Ding D, Burkard RF, Jiang H, Reaume AG, Flood DG, Salvi RJ. Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129, CD-1 mice. J Comp Neurol. 1999;413(1):101. [PubMed] [Google Scholar]
- 301.Kaltenbach JA, Rachel JD, Mathog TA, Zhang J, Falzarano PR, Lewandowski M. Cisplatin-induced hyperactivity in the dorsal cochlear nucleus and its relation to outer hair cell loss: Relevance to tinnitus. J Neurophysiol. 2002;88(2):699. doi: 10.1152/jn.2002.88.2.699. [DOI] [PubMed] [Google Scholar]
- 302.Rubio ME, Juiz JM. Chemical anatomy of excitatory endings in the dorsal cochlear nucleus of the rat: Differential synaptic distribution of aspartate aminotransferase, glutamate, and vesicular zinc. J Comp Neurol. 1998;399(3):341. doi: 10.1002/(sici)1096-9861(19980928)399:3<341::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 303.Zirpel L, Parks TN. Zinc inhibition of group I mGluR-mediated calcium homeostasis in auditory neurons. J Assoc Res Otolaryngol. 2001;2(2):180–7. doi: 10.1007/s101620010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Shambaugh GE., Jr Zinc for tinnitus, imbalance, and hearing loss in the elderly. Am J Otol. 1986;7(6):476. [PubMed] [Google Scholar]
- 305.Gersdorff M, Robillard T, Stein F, Declaye X, Van der Bemden S. The zinc sulfate overload test in patients suffering from tinnitus associated with low serum zinc. Preliminary report. Acta Oto-Rhino-Laryngol Belg. 1987;41(3):498. [PubMed] [Google Scholar]
- 306.Coelho CB, Tyler R, Hansen M. Zinc as a possible treatment for tinnitus. Prog Brain Res. 2007;166:279. doi: 10.1016/S0079-6123(07)66026-9. [DOI] [PubMed] [Google Scholar]
- 307.Ochi K, Kinoshita H, Kenmochi M, Nishino H, Ohashi T. Zinc deficiency and tinnitus. Auris Nasus Larynx. 2003;30(Suppl):S25. doi: 10.1016/s0385-8146(02)00145-1. [DOI] [PubMed] [Google Scholar]
- 308.Arda HN, Tuncel U, Akdogan O, Ozluoglu LN. The role of zinc in the treatment of tinnitus. Otol Neurotol. 2003;24(1):86. doi: 10.1097/00129492-200301000-00018. [DOI] [PubMed] [Google Scholar]
- 309.Paaske PB, Pedersen CB, Kjems G, Sam IL. Zinc therapy of tinnitus. A placebo-controlled study. Ugeskr Laeg. 1990;152(35):2473. [PubMed] [Google Scholar]
- 310.Yetiser S, Tosun F, Satar B, Arslanhan M, Akcam T, Ozkaptan Y. The role of zinc in management of tinnitus. Auris Nasus Larynx. 2002;29(4):329. doi: 10.1016/s0385-8146(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 311.Levine RA, Nam EC, Melcher J. Somatosensory pulsatile tinnitus syndrome: somatic testing identifies a pulsatile tinnitus subtype that implicates the somatosensory system. Trends Amplif. 2008;12(3):242–53. doi: 10.1177/1084713808321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Kirchmann M, Thomsen LL, Olesen J. Basilar-type migraine: Clinical, epidemiologic, and genetic features. Neurology. 2006;66(6):880. doi: 10.1212/01.wnl.0000203647.48422.dd. [DOI] [PubMed] [Google Scholar]
- 313.Weissman JL, Hirsch BE. Imaging of tinnitus: A review. Radiology. 2000;216(2):342. doi: 10.1148/radiology.216.2.r00au45342. [DOI] [PubMed] [Google Scholar]
- 314.Branstetter BFT, Weissman JL. The radiologic evaluation of tinnitus. Eur Radiol. 2006;16(12):2792. doi: 10.1007/s00330-006-0306-2. [DOI] [PubMed] [Google Scholar]
- 315.Pinchoff RJ, Burkard RF, Salvi RJ, Coad ML, Lockwood AH. Modulation of tinnitus by voluntary jaw movements. Am J Otol. 1998;19(6):785. [PubMed] [Google Scholar]
- 316.Simmons R, Dambra C, Lobarinas E, Stocking C, Salvi R. Head, neck and eye movements that modulate tinnitus. Sem Hear. 2008;29:361–70. doi: 10.1055/s-0028-1095895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Hanley PJ, Davis PB, Paki B, Quinn SA, Bellekom SR. Treatment of tinnitus with a customized, dynamic acoustic neural stimulus: Clinical outcomes in general private practice. Ann Otol Rhinol Laryngol. 2008;117(11):791. doi: 10.1177/000348940811701101. [DOI] [PubMed] [Google Scholar]
- 318.Henry JA, Schechter MA, Zaugg TL, et al. Clinical trial to compare tinnitus masking and tinnitus retraining therapy. Acta Oto-Laryngol. 2006;(Suppl 556):64. doi: 10.1080/03655230600895556. [DOI] [PubMed] [Google Scholar]
- 319.Henry JA, Schechter MA, Zaugg TL, et al. Outcomes of clinical trial: Tinnitus masking versus tinnitus retraining therapy. J Am Acad Audiol. 2006;17(2):104–32. doi: 10.3766/jaaa.17.2.4. [DOI] [PubMed] [Google Scholar]
- 320.Dobie RA, Sullivan MD, Katon WJ, Sakai CS, Russo J. Antidepressant treatment of tinnitus patients. Interim report of a randomized clinical trial. Acta Oto-Laryngol. 1992;112(2):242. doi: 10.1080/00016489.1992.11665412. [DOI] [PubMed] [Google Scholar]
- 321.Paul AK, Lobarinas E, Simmons R, et al. Metabolic imaging of rat brain during pharmacologically-induced tinnitus. NeuroImage. 2009;44(2):312. doi: 10.1016/j.neuroimage.2008.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]


