Abstract
The majority of trauma patients (>90%) do not require any blood product transfusion and their mortality is <1%. However, 3% to 5% of civilian trauma patients will receive a massive transfusion (MT), defined as >10 units of packed red blood cells (PRBC) in 24 hours. In addition, more than 25% of these patients will arrive to emergency departments with evidence of trauma-associated coagulopathy. With this combination of massive blood loss and coagulopathy, it has become increasingly more common to transfuse early the trauma patients and with a combination of PRBC, plasma, and platelets. Given the inherent uncertainties common early in the care of patients with severe injuries, the efficient administration of massive amounts of PRBC and clotting factors tends to work best in a predefined, protocol driven system. Our purpose here is to (1) define the problem of massive hemorrhage and coagulopathy in the trauma patient, (2) identify which group of patients this type of protocol should be applied, (3) describe the extensive coordination required to implement this multispecialty MT protocol, (4) explain in detail how the MT was developed and implemented, and (5) emphasize the need for a robust performance improvement or quality improvement process to monitor the implementation of such a protocol and to help identify problems and deliver feedback in a “real-time” fashion. The successful implementation of such a complex process can only be accomplished in a multispecialty setting. Input and representation from departments of Trauma, Critical Care, Anesthesiology, Transfusion Medicine, and Emergency Medicine are necessary to successfully formulate (and implement) such a protocol. Once a protocol has been agreed upon, education of the entire nursing and physician staff is equally essential to the success of this effort. Once implemented, this process may lead to improved clinical outcomes and decreased overall blood utilization with extremely small wastage of vital blood products.
Keywords: Trauma, Exsanguination, Hemorrhage, Massive transfusion, Protocol
Trauma is the leading cause of death in the age group of 1 year to 44 years.1 Hemorrhagic shock and exsanguination are responsible for a large number of these deaths, accounting for >80% of deaths in the operating room and ~50% of deaths in the first 24 hours after injury.2–5 Fortunately, only 3% to 5% of civilian trauma patients at admissions will require a massive transfusion (MT), defined as 10 or more units of packed red blood cells (PRBC) in the first 24 hours.6–8 More importantly, this group of patients consumes 75% of all blood products transfused at busy urban trauma centers.9 Several authors have demonstrated improved outcomes by using predefined ratios of blood products, early in the care of these patients with severe injuries.7,10–16 Rapid processing and preparation of such a large amount of blood and blood products in a short period of time required significant planning and prior coordination of personnel and dedicated resources to ensure delivery of these products in an immediate and sustained fashion.
Previous descriptions of the coagulopathy from trauma were based on laboratory data from the operating room, and the conclusion was that abnormal coagulation laboratory values were not found in the first hours after injury and were associated with dilution. However, recent studies have shown that at least 25% of patients arrive at the trauma center already coagulopathic and that these patients are at a markedly higher risk of mortality.14,17,18 Trauma-associated coagulopathy is a separate entity characterized by nonsurgical bleeding that can occur with or without appropriate concentrations of coagulation factors.19 Therefore, it has become paramount to have strategies in place to directly address this coagulopathy in the patient with severe injuries.6
An increasing number of institutions have demonstrated that a small (but not insignificant) portion of the trauma population will require a massive amount of blood products in a rapid fashion.20–23 In light of this, it is essential that trauma centers have an established mechanism to deliver these products quickly and in the correct amounts to these critically injured patients. Several groups have shown that MT protocols can be successfully implemented and have a significant positive impact on trauma outcomes.10,12,24,25 Damage control resuscitation (DCR) is a team effort that requires teamwork, communication, and collaboration. The goal is to organize a group of individuals to think and act as a team with a common goal.26,27
The damage control concept was initially described by Stone et al.28 as an alternative approach in the management of the exsanguinating trauma patient who becomes cold and coagulopathic during laparotomy. Rotondo et al.,29 in 1993, applied the term to this surgical resuscitation strategy and delineated damage control into three separate and distinct phases. Phase 1 consists of the abbreviated laparotomy, with the addressing of life-threatening hemorrhage and gross bowel spillage. Phase 2 involves the restoration of the patients to “normal” physiology through correction of acidosis, hypothermia, and trauma-associated coagulopathy.29 Phase 3 involves the return to the operating room for definitive repair and reconstruction of injuries temporized during phase 1. Phase 3 occurs after restoration of “normal” physiology is achieved. The concept of DCR evolved out of this same approach and is composed of three basic components: (1) permissive hypotension-palpable distal pulses in an awake patient, (2) minimizing crystalloid-based resuscitation strategies, and (3) the immediate release and administration of predefined blood products (PRBC, plasma, and platelets) in ratios similar to that of whole blood.6,22,30 This aggressive delivery of blood products begins before any laboratory defined anemia or coagulopathy.6,31,32 Damage control hematology defines the process of delivering large amounts of blood products in an efficient manner in patients who have been identified as having life-threatening hemorrhage.10,33,34
CREATION
A damage control hematology protocol should be in place to address the patient who has significant acute blood loss and arrives at the trauma center already coagulopathic. A multidisciplinary team, including specialists from the emergency medicine, trauma, critical care, transfusion medicine, nursing, pathology, hematology, and anesthesia departments, should be involved in the creation of this protocol.10,33,34 The Blood Bank (BB) should function and be viewed as more than just a warehouse where blood products are stored and orders placed. Johansson35 has stated this very clearly, describing the evolution of their process “from provider to partner.” To this aim, experts in transfusion medicine must be active participants in the resuscitation of the massively bleeding patient, despite the odd hours when these trauma patients usually arrive. Although the numbers are improving around the world, there are relatively few centers that have MT protocols in place and few of the existing protocols are based on quality data.8 It is our purpose here to describe the creation of such a protocol.
The Purpose of a MT Protocol
The purpose of such a protocol is to provide blood products to hemodynamically unstable trauma patients in an immediate and sustained manner. To accomplish this, one must have immediately available for the release of predefined blood components that allow for uniform and rapid access to a massive amount of blood products. In the absence of a predefined MT protocol, access to the appropriate volume and ratios of blood products may be significantly delayed. The layers of potential delay are too numerous to list here but include physical ordering of the blood, communication, and decision-making between involved parties, and the sending of laboratory samples and timely receipt of their results. Failure to immediately address and treat the evolving coagulopathy so often observed in these patients may lead to a worsening of their coagulopathy, possibly even to exsanguination and death. The rapid infusion of the correct ratio of products not only reduces the chances of developing (or decrease the severity of) trauma-associated coagulopathy but also has been shown to improve survival and decrease overall usage of blood.7,10,12,24
Creating the Multidisciplinary Team
As stated earlier, to make this system work requires the cooperation and input of multiple specialties.10,33 The trauma patient will rapidly move through the system from emergency department (ED) to the operating room and if still alive, finally settle in to the intensive care unit. Physicians from each of these departments should be actively involved in the development of MT protocols. It is also essential that personnel from the BB are involved from the protocols inception; this should include personnel from the hematology and pathology departments as well as BB technicians and managers.
Identifying Optimal Ratios
There is a paucity of literature (and even less data) available to help clinicians organize and develop a data-driven institutional MT protocol. This is a reflection of the lack of such protocols in place both in the United States and around the world.8 Many existing protocols were developed based on what little was available at the time of their conceptualization (military experience and animal data).10–12,21,24 Using these as guides, these groups used available data to guide not only the optimal ratio of blood products to be transfused, but also how much to transfuse, how best to deliver the products, and how the protocol should be activated.18,21,31,32,36,37
To date, however, there are no prospective data informing clinicians of the optimal ratio of blood products for the MT trauma patient. In fact, no class 1 data and very little class 2 data were available. Given the difficulty associated with performing a randomized controlled trial in a group of exsanguinating patients, several authors have attempted to define the optimal transfusion regimen in the absence of such a study design. Hirshberg et al.31 created a computer-based hemodilution model to simulate the exsanguinating patient and found that current resuscitation protocols severely underestimated the need for clotting factor replacement.31 On the basis of their findings, the authors recommended aiming for a ratio of plasma to PRBC of 2:3 and a ratio of platelets to PRBC of 8:10. Ho et al.38also attempted to define the proper ratio of plasma to PRBC by developing a pharmacokinetic mathematical model to simulate the coagulopathy seen in trauma patients. This group recommended the equivalent of whole blood be transfused to avoid development or worsening of coagulopathy during the initial resuscitation of an exsanguinating patient. On the basis of their ongoing work, this same group has recently advocated that patients with severe injuries receive at least 1 unit of plasma and platelets for every red blood cell transfusion or more simply put 1:1:1.39 This ratio is similar to what has been proposed for DCR by the US military in the exsanguinating victim in combat.6,7,40
An exhaustive review of the literature demonstrated no class 1 data (and little class 2 evidence) describing the ideal ratio to transfuse to the trauma patient with exsanguinating hemorrhage.7,8,11,12,15,16,23,30,41,42 Based on what was available, however, it seemed that ratios of at least 2:3 for plasma: PRBC and 1:5 for apheresis platelets: PRBC seemed justifiable and these were incorporated into the MT protocols at several institutions.10 In one particular study, patients receiving plasma: RBC at a ratio of 2:3 or greater and apheresis platelets: RBC at a ratio of 1:5 or greater were noted to have a lower 30-day mortality when compared with patients receiving less than these ratios. Patients achieving ratios of 1:1 did not reduce mortality any further than that observed for 2:3.13 This was similar to what Kashuk et al.43 showed in a 5-year retrospective review of 133 patients. However, it is worth noting that only 45 patients in the study by Gunter et al.13 and 11 patients in the study by Kashuk et al.43 achieved plasma: RBC ratio of 1:1, and their findings may represent a type II error.
The clinical practice described by Beekley40 advocates transfusing on a 1:1:1 ratio, essentially trying to recreate the transfusion of whole blood. Duchesne et al.11 recently evaluated their 4-year experience of patients who required a MT at their urban level I trauma center. The authors found that those resuscitated with plasma to RBC ratio of 1:1 had a distinct survival advantage over those with a ratio of 1:4. Holcomb et al.41 recently reported their findings from a multicenter, retrospective study of 466 massively transfused civilian trauma patients. The authors demonstrated that patients receiving higher ratios (>1:2) of plasma and platelets to PRBC had decreased truncal hemorrhage and increased survival at 6 hours, 24 hours, and 30 days. In an evaluation of the German Trauma Registry, Maegele et al.42 evaluated outcomes in 713 critically injured patients who received a MT. They saw the greatest reduction in 24 hours and 30-day mortality in the patients who achieved a high ratio of plasma to PRBC. Sperry et al.16 recently evaluated 415 blunt trauma patients within the “Glue Grant” database who received 8 units of PRBC in 12 hours. The authors demonstrated that in those patients who achieved a ratio of FFP: PRBC >1:1.5, a significantly lower mortality rate was observed in the first 48 hours.
Protocol Activation and Delivery of Products
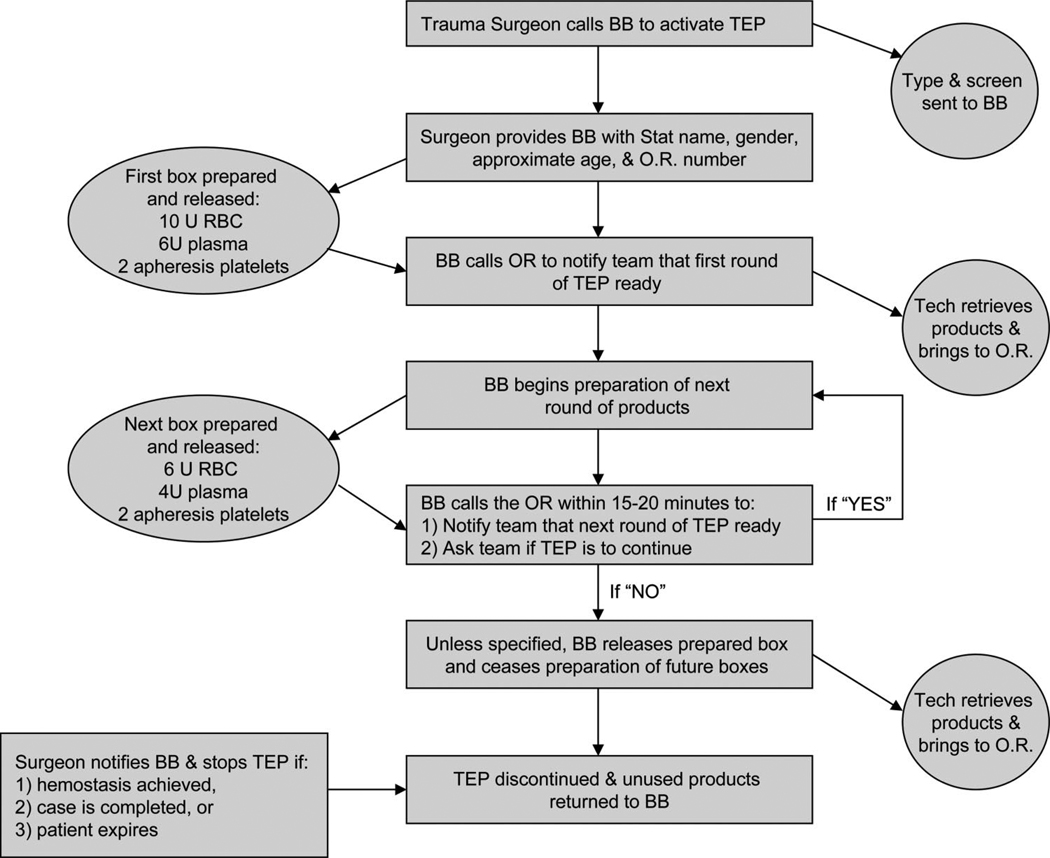
The next set of challenges involves exactly who receives these increased ratios and exactly how the products would be delivered. Activation of most MT protocols is restricted to the attending trauma surgeon (or in some cases, the attending anesthesiologist). This is usually accomplished with a phone call directly to the BB. The attending makes this decision based on the clinical data available in the trauma resuscitation area. Activation of these protocols does not usually rely on laboratory values because points of care coagulation tests are not available in most EDs.10–12,24 At a minimum, however, a type and screen should be sent from the ED to allow the BB to convert released components over to type-specific products as soon as possible. Typically, the attending surgeon passes along to the BB the appropriate demographic data (trauma “code” name, approximate age, and gender) and the location to which the first “cooler” should be delivered. An example of the “flow” of such a protocol is illustrated in Figure 1 The BB then executes the MT protocol by providing a cooler with 6 units of universal donor plasma and 10 units of uncrossmatched PRBC. Rapid release of these products is possible by keeping several units (4–6) of thawed universal donor (AB) plasma on hand at all times. In addition, 2 units of apheresis platelets are released with the cooler (attached to transparent bag outside the cooler) or shortly thereafter. This cooler should meet the patient in the operating room or arrive shortly after. The BB should then begin preparation on the next “cooler” of products. When each cooler of products are ready, the BB should contact the trauma and or operating room team that the next “cooler” is en route and inquire as to whether the protocol (and preparation of further products) should proceed. This procedure should continue the delivery of each round of products. At the completion of the operation, the trauma team should designate a responsible member to ensure that any unused blood products are quickly returned to the BB to prevent wastage.10
Figure 1.
Flow diagram of massive transfusion protocol activation.
Adjuncts
Recombinant-Activated Factor VIIa
Initial reports from the Israeli military on their use of recombinant factor VIIa (rFVIIa) in seven patients with severe injuries in 2001 were quite positive, noting cessation of diffuse bleeding and decreased blood product usage.44 A recent review of the preclinical and clinical data available for the use of factor VIIa showed that the drug to be safe and possibly effective in the treatment of trauma-associated coagulopathy.45 The US military has evaluated patients from the joint theater trauma registry and found that patients who underwent MT and received rfVIIa early in their course had decreased 30-day mortality.46 Boffard et al.47 performed the only randomized study evaluating the use of factor VIIa in the exsanguinating trauma patient. The authors noted a reduction in the amount of blood transfused (in blunt trauma patients) but did not find a mortality benefit. Given its controversial nature (not to mention its costs), the use of this agent in trauma patients at many institutions remains restricted and is not incorporated into their MT protocols.10–12 However, some centers such as R. Adams Cowley Shock Trauma in Baltimore use “low-dose” rfVIIa in trauma patients with evidence of coagulopathy. The investigators recently evaluated 81 consecutive patients at their institution who received “low-dose” (1.2 mg) factor VIIa and noted a significant reduction in prothrombin time and usage of PRBC and plasma.48
Autotransfusion Devices or “Cell-Saver” Techniques
Two decades ago, Timberlake and McSwain49 and Ozmen and McSwain50 from Tulane showed that the use of an autotransfusion device (such as a cell-saver) was safe and effective in patients with intra-abdominal contamination and hemoperitoneum. Smith et al.51 recently noted that intraoperative blood salvage is not only safe but that application of such devices is associated with a marked decrease in the use of banked blood. Bowley et al.52 demonstrated the efficacy of using intraoperative blood salvage in patients who had suffered penetrating abdominal trauma, demonstrating a 45% reduction in the use of banked blood. In a randomized controlled trial by the same group, there was no difference in the intraoperative blood salvage group when compared with controls regarding postoperative sepsis, survival, coagulopathy, and requirement for clotting factors.52 Given the proven reduction in the use of the precious commodity of banked blood, we would recommend that those centers with the capability to provide this adjunct “around the clock” strongly consider the addition of this valuable tool to their MT protocol.
Age of Blood in Protocol
The exact role (and potential impact) of the age of stored blood on clinical outcomes in patients with severe injuries remains controversial. Although in storage, PRBC undergo a series of predictable changes that result in a dramatic left shift of the hemoglobin-oxygen dissociation curve. Among these is a reduction in RBC deformability and depletion of adenosine triphosphate and 2, 3-diphosphoglycerol. Stored RBC also will show an increase in aggregation, adhesion, and inflammatory mediators. The proposed clinical effect of these changes is an increase in transfusion-related acute lung injury, multisystem organ failure, infections, and death.53–55 There have been 10 observational studies in trauma patients looking at the effects of stored blood on outcomes. The data from these studies consistently showed an increase in organ failure, acute respiratory distress syndrome, mortality, and infections.56–64 Unfortunately, the average age of transfused RBC in the United States is 21 days whereas that of banked blood transfused in current combat theaters in Southwest Asia is >30 days.65–67 There is no class 1 data on the effects of transfusion of large amounts of old blood in patients with severe injuries. Existing observational and retrospective studies do suggest that those patients who receive >4 units of blood will most likely benefit from the transfusion of young blood (<14 days old). With the lack of randomized controlled trials available, we have not mandated that patients with MT get young blood. Given the potential benefits of using young blood in the population with acute injuries, its use in this setting should be considered during development of an institution’s MT protocol.
IMPLEMENTATION
The process to develop and implement a MT protocol is quite time consuming and labor intensive. To put the time course in perspective, in the spring of 2005, Vanderbilt University’s Transfusion Committee convened a subcommittee to directly address the concerns over the process of rapid delivery of large amounts of blood products in uniform and predefined way. The committee was tasked to improve access to the products, in hopes of reducing mortality and decreasing overall blood product utilization. This team was composed of faculty from the Division of Trauma, Department of Anesthesiology, the Department of Pathology/Transfusion Medicine, and the Department of Hematology. After approval, the protocol was implemented in February 2006, almost 1 full calendar year from the beginning of the overall process.10 Bormanis33 recently described a similar multispecialty process in the development of a MT protocol for hospital wide use.
Before implementation, a comprehensive educational campaign should be undertaken (directed at all involved specialties and disciplines) instructing individuals and groups as to how the protocol will be activated and used. This should be done through academic detailing of leaders in the different specialties and through formal presentations at multiple educational conferences with faculty, house staff, nursing, and ancillary services. At our institution, we encountered problems with infusion of the products in a timely fashion.68 Some faculty would select specific blood products from the cooler for transfusion rather than administer the predefined ratio and number of products. This provider-related issue improved dramatically with aggressive academic detailing, invited lectures from national opinion leaders, and through the dissemination to faculty and staff of our initial findings of improved outcomes with compliant protocol activations. These findings included a dramatic reduction in 30-day mortality (86.7% vs. 45.0%; p < 0.001) and reduction in 24-hour PRBC utilization (13.7 units vs. 19.5 units; p = 0.01) when the protocol is followed.13
MATURATION
Outcomes
From the inception of their institution’s MT protocol, Cotton et al.10,68,69 began prospectively collecting data on all protocol activations and entered these into a performance improvement (PI) database. Each case was closely monitored in a “real-time” fashion for the first year and evaluated on a quarterly basis by their multidisciplinary PI team. After the first year, the authors published a retrospective cohort study of all MT protocol activations (69 patients) and compared them with a pre-MT protocol cohort of trauma patients who received MT (70 patients).10 Given similar injuries, these was a 74% reduction in the odds of mortality in MT patients with the implementation of the protocol. After introduction of their MT protocol, investigators at Parkland Hospital in Dallas noted a significant decrease in PRBC, plasma, and platelet use.24 In addition, the release time for products was dramatically reduced to <10 minutes for the first “cooler” and the time between the first and second “coolers” was reduced from 42 to 18 minutes. However, the authors failed to detect a difference in mortality during the time period of the study.
Given rising concerns of infectious complications and lung injury in patients exposed to large volumes of blood products, Cotton et al.68 examined the complications after injury in the 2-year post-MT protocol group (125 patients) and compared with that of the 2-year preprotocol cohort (141 patients). The authors demonstrated a reduction in pneumonia, pulmonary failure, open abdomens, and abdominal compartment syndrome after MT protocol implementation. In addition, sepsis and multiorgan failure were also lower, and there was a significant increase in ventilator-free days in the protocol patients. Consistent with their previous findings, patients receiving the protocol had higher survival and received less blood products overall when compared with the preprotocol cohort.68
The Need for PI
We recommend that all MT protocol activations undergo review by a multidisciplinary PI committee for compliance and need for “real-time” protocol adjustments. Reports on all activations should be created and these data should be given to the MT protocol liaisons (preferably at least one Trauma faculty member) who then investigate or evaluate each case from a surgical and early resuscitation perspective. Anesthesia representatives should then query the case and provide their input and evaluation of intraoperative management and operating room issues. The group, on a monthly or on a quarterly basis, should then review these cases. After these, PI meetings, structured and directed educational conferences, Grand Rounds presentations, and individual provider education may then be performed. In addition to case-by-case issues and variability issues, outcome-related protocol components should also be evaluated for compliance (Table 1).68
TABLE 1.
Trauma Exsanguination Protocol PI/QI Audit Filters
|
TEP, trauma exsanguination protocol.
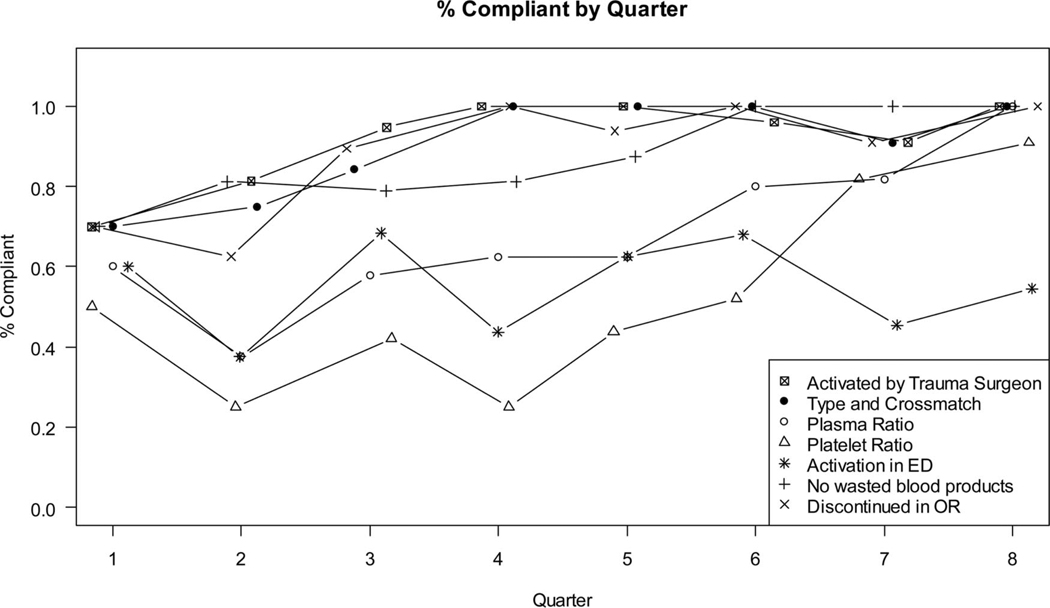
Cotton et al.68 recently examined their protocol’s PI database to evaluate the impact of patient, provider, and system factors on outcomes and protocol compliance. All protocol patients were grouped according to full compliance or noncompliance (at least one protocol violation). The primary outcome of interest was 30-day mortality. In addition to univariate and multivariate analyses, an interrupted time series analyses was conducted to evaluate the impact of educational interventions on protocol compliance. Full compliance of all PI measures over the entire period occurred in only 27% of activations. There were no differences in demographics, injury severity, or physiologic scores between patients with compliant protocol activations and those that were noncompliant. Of note, full compliance was an independent predictor of 30-day survival. Individually, ED activation of the protocol and achievement of predefined plasma: PRBC and platelets: PRBC ratios were independent predictors of 30-day survival. In addition, discontinuation of the protocol at the completion of the case was associated with a reduction in wasted products.
Full compliance improved from 20% in the first quarter to more than 50% in the eighth quarter. More important, each of the PI measures demonstrated a significant improvement in compliance during the study period with the exception of ED activation of the protocol, by the attending trauma surgeon (Fig. 2).68 Given the impact of ED activation on mortality and overall product use, we set out through our PI process to improve early activation of the protocol. Unfortunately, numerous educational efforts have failed to improve beyond the 50% mark. Because of the lack of uniformity in the activation of the protocol, we have actively worked on developing an objective scoring system to augment the clinical acumen of the trauma faculty.
Figure 2.
Compliance of seven audit filters by quarter.
Identifying MT Patients
On the surface, such a protocol may not seem worth-while as it is likely to only benefit about 3% to 5% of the population with injuries at a busy level trauma center.6,7 As we have shown above, it may also be difficult for clinicians to rapidly identify this group of patients. Although there are currently no uniform activation criteria for such protocols, several groups have developed scoring systems (using a variety of anatomic, physiologic, and laboratory variables) to correctly identify the patient who will likely require a MT.69–71 Although each of these scoring systems is quite accurate, the majority of scores require laboratory data and injury severity assessment. The Trauma-Associated Severe Hemorrhage score uses seven independent variables to identify patients who will require a MT. These include systolic blood pressure, gender, hemoglobin, fluid on ultrasound, pulse, base excess, and extremity or pelvic fractures. The McLaughlin score consists of four components: heart rate >105 bpm, systolic blood pressure >110 mm Hg, pH <7.25, and hematocrit <32%.
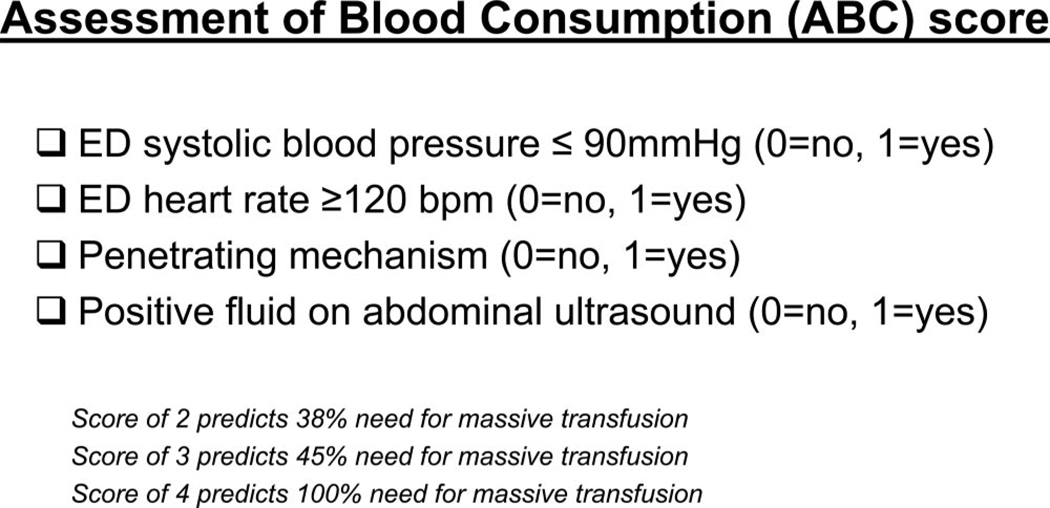
Given the limited access to point-of-care testing at most institutions, a scoring system was developed that relies only on data (physiology and mechanism of injury) readily available during the primary survey. The Assessment of Blood Consumption score correctly identifies those individuals who will require a MT 85% of the time (Fig. 3).72 As with other scores, the ABC uses arrival tachycardia (>120 bpm) and hypotension (<90 mm Hg). In addition, positive fluid on ultrasound and penetrating mechanism of injury are used to determine the risk for MT. Of these, positive fluid on ultrasound was the most predictive for the receipt of MT (odds ratio, 8.2).72 However, the presence of two or more of these four parameters is advocated as a trigger for initiating an institution’s MT protocol.
Figure 3.
ABC score to predict the need for massive transfusion.
Inaba et al.73 noted that ED transfusion of uncrossmatched PRBC is associated with the MT of products. Similarly, we found that when uncross-matched blood is used while still in the ED, patients are at increased risk of receiving MT of not only PRBC but also plasma and platelets during the first 6 hours after arrival.74 In light of this, we have used a system to prompt our trauma faculty to activate our institution’s MT protocol when uncross-matched products are requested while in the trauma bay. Regardless of the scoring system used, it is critical that each of these scoring systems should be used to augment, not replace, a trauma attending’s clinical decision making. These scoring systems must be prospectively validated before widespread recommendations for use should occur.
CONCLUSIONS
Up to 5% of civilian trauma patients will require MT. This group of patients is likely to be coagulopathic at admission and require transfusion of large amounts of blood products in a relatively short period of time. MT protocols are associated with improved survival in patients with exsanguinating hemorrhage. Much of this improvement in survival has been attributed to increased plasma and platelet to PRBC ratios. Recent data suggest that a well-defined protocol delivering products in prespecified ratios and volumes is critical to the observed reductions in mortality.10,69
We encourage all involved with the care of trauma patients to take an active role in the development of a MT protocol and help define how their particular institution will deliver the desired products efficiently. This article describes, in a step-by-step fashion, how this can occur. The key component necessary for the successful development and implementation is communication between the multispecialty team. Without the input and agreement from the other specialties involved, these protocols are doomed to failure. Once a MT protocol is in place, it is imperative that the results are monitored via a local PI process.68 This will allow each institution to modify their process accordingly. With a team effort, damage control hematology can improve patient outcomes and reduce overall blood product use.
REFERENCES
- 1.Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg. 2007;31:1507–1511. doi: 10.1007/s00268-007-9087-2. [DOI] [PubMed] [Google Scholar]
- 2.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Acosta JA, Yang JC, Winchell RJ, et al. Lethal injuries and time to death in a level I trauma center. J Am Coll Surg. 1998;186:528–533. doi: 10.1016/s1072-7515(98)00082-9. [DOI] [PubMed] [Google Scholar]
- 4.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 suppl):S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 5.Demetriades D, Murray J, Charalambides K, et al. Trauma fatalities: time and location of hospital deaths. J Am Coll Surg. 2004;198:20–26. doi: 10.1016/j.jamcollsurg.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 7.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 8.Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006;60(6 suppl):S91–S96. doi: 10.1097/01.ta.0000199549.80731.e6. [DOI] [PubMed] [Google Scholar]
- 9.Como JJ, Dutton RP, Scalea TM, Edelman BB, Hess JR. Blood transfusion rates in the care of acute trauma. Transfusion. 2004;44:809–813. doi: 10.1111/j.1537-2995.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- 10.Cotton BA, Gunter OL, Isbell J, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–1182. doi: 10.1097/TA.0b013e31816c5c80. discussion 1182-1173. [DOI] [PubMed] [Google Scholar]
- 11.Duchesne JC, Hunt JP, Wahl G, et al. Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma. 2008;65:272–276. doi: 10.1097/TA.0b013e31817e5166. discussion 276–278. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez EA, Moore FA, Holcomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 13.Gunter OL, Jr, Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008;65:527–534. doi: 10.1097/TA.0b013e3181826ddf. [DOI] [PubMed] [Google Scholar]
- 14.Niles SE, McLaughlin DF, Perkins JG, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64:1459–1463. doi: 10.1097/TA.0b013e318174e8bc. discussion 1463-1455. [DOI] [PubMed] [Google Scholar]
- 15.Stinger HK, Spinella PC, Perkins JG, et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;64(2 suppl):S79–S85. doi: 10.1097/TA.0b013e318160a57b. discussion S85. [DOI] [PubMed] [Google Scholar]
- 16.Sperry JL, Ochoa JB, Gunn SR, et al. An FFP:PRBC transfusion ratio ≥1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–993. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 17.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 18.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 19.Hess JR, Lawson JH. The coagulopathy of trauma versus disseminated intravascular coagulation. J Trauma. 2006;60(6 suppl):S12–S19. doi: 10.1097/01.ta.0000199545.06536.22. [DOI] [PubMed] [Google Scholar]
- 20.Hess JR, Zimrin AB. Massive blood transfusion for trauma. Curr Opin Hematol. 2005;12:488–492. doi: 10.1097/01.moh.0000177828.85904.70. [DOI] [PubMed] [Google Scholar]
- 21.Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003;17:223–231. doi: 10.1016/s0887-7963(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 22.Tieu BH, Holcomb JB, Schreiber MA. Coagulopathy: its pathophysiology and treatment in the injured patient. World J Surg. 2007;31:1055–1064. doi: 10.1007/s00268-006-0653-9. [DOI] [PubMed] [Google Scholar]
- 23.Fox CJ, Gillespie DL, Cox ED, et al. The effectiveness of a damage control resuscitation strategy for vascular injury in a combat support hospital: results of a case control study. J Trauma. 2008;64(2 suppl):S99–S106. doi: 10.1097/TA.0b013e3181608c4a. discussion S106–S107. [DOI] [PubMed] [Google Scholar]
- 24.O’Keeffe T, Refaai M, Tchorz K, Forestner JE, Sarode R. A massive transfusion protocol to decrease blood component use and costs. Arch Surg. 2008;143:686–690. doi: 10.1001/archsurg.143.7.686. discussion 690-681. [DOI] [PubMed] [Google Scholar]
- 25.Repine TB, Perkins JG, Kauvar DS, Blackborne L. The use of fresh whole blood in massive transfusion. J Trauma. 2006;60(6 suppl):S59–S69. doi: 10.1097/01.ta.0000219013.64168.b2. [DOI] [PubMed] [Google Scholar]
- 26.McConaughey E. Crew resource management in healthcare: the evolution of teamwork training and MedTeams. J Perinat Neonatal Nurs. 2008;22:96–104. doi: 10.1097/01.JPN.0000319095.59673.6c. [DOI] [PubMed] [Google Scholar]
- 27.Helmreich RL, Merritt AC, Wilhelm JA. The evolution of Crew Resource Management training in commercial aviation. Int J Aviat Psychol. 1999;9:19–32. doi: 10.1207/s15327108ijap0901_2. [DOI] [PubMed] [Google Scholar]
- 28.Stone HH, Strom PR, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg. 1983;197:532–535. doi: 10.1097/00000658-198305000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotondo MF, Schwab CW, McGonigal MD, et al. ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35:375–382. discussion 382-373. [PubMed] [Google Scholar]
- 30.Beekley AC. Damage control resuscitation: a sensible approach to the exsanguinating surgical patient. Crit Care Med. 2008;36:S267–S274. doi: 10.1097/CCM.0b013e31817da7dc. [DOI] [PubMed] [Google Scholar]
- 31.Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma. 2003;54:454–463. doi: 10.1097/01.TA.0000053245.08642.1F. [DOI] [PubMed] [Google Scholar]
- 32.Lucas CE, Ledgerwood AM, Saxe JM, Dombi G, Lucas WF. Plasma supplementation is beneficial for coagulation during severe hemorrhagic shock. Am J Surg. 1996;171:399–404. doi: 10.1016/S0002-9610(97)89618-3. [DOI] [PubMed] [Google Scholar]
- 33.Bormanis J. Development of a massive transfusion protocol. Transfus Apher Sci. 2008;38:57–63. doi: 10.1016/j.transci.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Stainsby D, MacLennan S, Hamilton PJ. Management of massive blood loss: a template guideline. Br J Anaesth. 2000;85:487–491. doi: 10.1093/bja/85.3.487. [DOI] [PubMed] [Google Scholar]
- 35.Johansson PI. The blood bank: from provider to partner in treatment of massively bleeding patients. Transfusion. 2007;47(2 suppl):176S–181S. doi: 10.1111/j.1537-2995.2007.01381.x. discussion 182S–183S. [DOI] [PubMed] [Google Scholar]
- 36.Lucas CE. Resuscitation of the injured patient: the three phases of treatment. Surg Clin North Am. 1977;57:3–15. doi: 10.1016/s0039-6109(16)41130-8. [DOI] [PubMed] [Google Scholar]
- 37.Cinat ME, Wallace WC, Nastanski F, et al. Improved survival following massive transfusion in patients who have undergone trauma. Arch Surg. 1999;134:964–968. doi: 10.1001/archsurg.134.9.964. discussion 968–970. [DOI] [PubMed] [Google Scholar]
- 38.Ho AM, Dion PW, Cheng CA, et al. A mathematical model for fresh frozen plasma transfusion strategies during major trauma resuscitation with ongoing hemorrhage. Can J Surg. 2005;48:470–478. [PMC free article] [PubMed] [Google Scholar]
- 39.Ho AM, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation? Am J Surg. 2005;190:479–484. doi: 10.1016/j.amjsurg.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 40.Beekley AC. Damage control resuscitation: a sensible approach to the exsanguinating surgical patient. Crit Care Med. 2008;36(7 suppl):S267–S274. doi: 10.1097/CCM.0b013e31817da7dc. [DOI] [PubMed] [Google Scholar]
- 41.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 42.Maegele M, Lefering R, Paffrath T, et al. Red-blood-cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiple injury: a retrospective analysis from the Trauma Registry of the Deutsche Gesellschaft fur Unfallchirurgie. Vox Sang. 2008;95:112–119. doi: 10.1111/j.1423-0410.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 43.Kashuk JL, Moore EE, Johnson JL, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–270. doi: 10.1097/TA.0b013e31817de3e1. discussion 270-261. [DOI] [PubMed] [Google Scholar]
- 44.Martinowitz U, Kenet G, Segal E, et al. Recombinant activated factor VII for adjunctive hemorrhage control in trauma. J Trauma. 2001;51:431–438. doi: 10.1097/00005373-200109000-00002. discussion 438–439. [DOI] [PubMed] [Google Scholar]
- 45.Holcomb JB. Use of recombinant activated factor VII to treat the acquired coagulopathy of trauma. J Trauma. 2005;58:1298–1303. doi: 10.1097/01.ta.0000169871.29748.95. [DOI] [PubMed] [Google Scholar]
- 46.Spinella PC, Perkins JG, McLaughlin DF, et al. The effect of recombinant activated factor VII on mortality in combat-related casualties with severe trauma and massive transfusion. J Trauma. 2008;64:286–293. doi: 10.1097/TA.0b013e318162759f. discussion 293-284. [DOI] [PubMed] [Google Scholar]
- 47.Boffard KD, Riou B, Warren B, et al. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005;59:8–15. doi: 10.1097/01.ta.0000171453.37949.b7. discussion 15–18. [DOI] [PubMed] [Google Scholar]
- 48.Stein DM, Dutton RP, Hess JR, Scalea TM. Low-dose recombinant factor VIIa for trauma patients with coagulopathy. Injury. 2008;39:1054–1061. doi: 10.1016/j.injury.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 49.Timberlake GA, McSwain NE., Jr Autotransfusion of blood contaminated by enteric contents: a potentially life-saving measure in the massively hemorrhaging trauma patient? J Trauma. 1988;28:855–857. doi: 10.1097/00005373-198806000-00026. [DOI] [PubMed] [Google Scholar]
- 50.Ozmen V, McSwain NE, Jr, Nichols RL, Smith J, Flint LM. Autotransfusion of potentially culture-positive blood (CPB) in abdominal trauma: preliminary data from a prospective study. J Trauma. 1992;32:36–39. doi: 10.1097/00005373-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Smith LA, Barker DE, Burns RP. Autotransfusion utilization in abdominal trauma. Am Surg. 1997;63:47–49. [PubMed] [Google Scholar]
- 52.Bowley DM, Barker P, Boffard KD. Intraoperative blood salvage in penetrating abdominal trauma: a randomised, controlled trial. World J Surg. 2006;30:1074–1080. doi: 10.1007/s00268-005-0466-2. [DOI] [PubMed] [Google Scholar]
- 53.Card RT. Red cell membrane changes during storage. Transfus Med Rev. 1988;2:40–47. doi: 10.1016/s0887-7963(88)70030-9. [DOI] [PubMed] [Google Scholar]
- 54.Napolitano LM, Corwin HL. Efficacy of red blood cell transfusion in the critically ill. Crit Care Clin. 2004;20:255–268. doi: 10.1016/j.ccc.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Tinmouth A, Fergusson D, Yee IC, et al. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 56.Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg. 2005;140:432–438. doi: 10.1001/archsurg.140.5.432. discussion 438–440. [DOI] [PubMed] [Google Scholar]
- 57.Claridge JA, Sawyer RG, Schulman AM, McLemore EC, Young JS. Blood transfusions correlate with infections in trauma patients in a dose-dependent manner. Am Surg. 2002;68:566–572. [PubMed] [Google Scholar]
- 58.Offner PJ, Moore EE, Biffl WL, Johnson JL, Silliman CC. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711–716. doi: 10.1001/archsurg.137.6.711. discussion 716–717. [DOI] [PubMed] [Google Scholar]
- 59.Silverboard H, Aisiku I, Martin GS, Adams M, Rozycki G, Moss M. The role of acute blood transfusion in the development of acute respiratory distress syndrome in patients with severe trauma. J Trauma. 2005;59:717–723. [PubMed] [Google Scholar]
- 60.Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620–624. discussion 624–625. [PubMed] [Google Scholar]
- 61.Keller ME, Jean R, LaMorte WW, Millham F, Hirsch E. Effects of age of transfused blood on length of stay in trauma patients: a preliminary report. J Trauma. 2002;53:1023–1025. doi: 10.1097/00005373-200211000-00037. [DOI] [PubMed] [Google Scholar]
- 62.Weinberg JA, McGwin G, Jr, Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008;65:279–282. doi: 10.1097/TA.0b013e31817c9687. discussion 282-274. [DOI] [PubMed] [Google Scholar]
- 63.Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 64.Murrell Z, Haukoos JS, Putnam B, Klein SR. The effect of older blood on mortality, need for ICU care, and the length of ICU stay after major trauma. Am Surg. 2005;71:781–785. doi: 10.1177/000313480507100918. [DOI] [PubMed] [Google Scholar]
- 65.Spinella PC, Perkins JG, Grathwohl KW, et al. Risks associated with fresh whole blood and red blood cell transfusions in a combat support hospital. Crit Care Med. 2007;35:2576–2581. doi: 10.1097/01.CCM.0000285996.65226.A9. [DOI] [PubMed] [Google Scholar]
- 66.Spinella PC, Perkins JG, Grathwohl KW, et al. Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J Trauma. 2008;64(2 suppl):S69–S77. doi: 10.1097/TA.0b013e318160ba2f. discussion S77-S68. [DOI] [PubMed] [Google Scholar]
- 67.Corwin HL, Gettinger A, Pearl RG, et al. The CRIT Study: anemia and blood transfusion in the critically ill—current clinical practice in the United States. Crit Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 68.Cotton BA, Au BK, Nunez TC, Gunter OL, Robertson AM, Young PP. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009;66:41–48. doi: 10.1097/TA.0b013e31819313bb. discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 69.McLaughlin DF, Niles SE, Salinas J, et al. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008;64(2 suppl):S57–S63. doi: 10.1097/TA.0b013e318160a566. discussion S63. [DOI] [PubMed] [Google Scholar]
- 70.Schreiber MA, Perkins J, Kiraly L, et al. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007;205:541–545. doi: 10.1016/j.jamcollsurg.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 71.Yucel N, Lefering R, Maegele M, et al. Trauma Associated Severe Hemorrhage (TASH)-Score: probability of mass transfusion as surrogate for life threatening hemorrhage after multiple trauma. J Trauma. 2006;60:1228–1236. doi: 10.1097/01.ta.0000220386.84012.bf. discussion 1236-1227. [DOI] [PubMed] [Google Scholar]
- 72.Nunez TC, Voskresensky IV, Dossett LA, et al. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? J Trauma. 2009;66:346–352. doi: 10.1097/TA.0b013e3181961c35. [DOI] [PubMed] [Google Scholar]
- 73.Inaba K, Teixeira PG, Shulman I, et al. The impact of uncross-matched blood transfusion on the need for massive transfusion and mortality: analysis of 5,166 uncross-matched units. J Trauma. 2008;65:1222–1226. doi: 10.1097/TA.0b013e31818e8ff3. [DOI] [PubMed] [Google Scholar]
- 74.Cotton BA, Dossett LA, Au BK, Nunez TC, Robertson AM, Young PP. Room for (performance) improvement: provider-related factors associated with poor outcomes in massive transfusion. J Trauma. 2009;67:1004–1012. doi: 10.1097/TA.0b013e3181bcb2a8. [DOI] [PubMed] [Google Scholar]