Abstract
A novel locus DFNB90 was mapped to 7p22.1-p15.3 by carrying out a genome scan in a multigenerational consanguineous family from Pakistan with autosomal recessive nonsyndromic hearing impairment (ARNSHI).DFNB90 is the eighth ARNSHI locus mapped to chromosome 7. A multipoint LOD score of 4.0 was obtained at a number of SNP marker loci spanning from rs1468996 (chromosome 7: 5.7 Mb) tors957960 (chromosome 7: 18.8 Mb). The 3-unit support interval and the region of homozygosity for DFNB90 spans from markers rs1553960 (chromosome 7: 4.9 Mb) to rs206198 (chromosome 7: 20.3 Mb). Candidate genes ACTB, BZW, OCM, MACC1, NXPH1, PRPS1L1, RAC1 and RPA3, which lie within the DFNB90 region, were sequenced and no potentially causal variants were identified.
Key Words: DFNB90, 7p22.1-p15.3, Autosomal recessive, Nonsyndromic hearing impairment, DFNA5, DFNB44
Introduction
To date, approximately 150 nonsyndromic hearing impairment (NSHI) genes have been localized, and about 90 of them display autosomal recessive (AR) inheritance. Fourty-one genes have been identified for ARNSHI (see also the Hereditary Hearing Loss Homepage). The discovery of new NSHI genes should improve our understanding of the mechanism of hearing as well as aid in the development of diagnostic and therapeutic interventions for hearing impairment (HI).
This article describes the mapping of the DFNB90 locus to chromosome 7p22.1-p15.3 in a multigenerational consanguineous Pakistani family with ARNSHI. Although already seven ARNSHI loci have been mapped to chromosome 7 for which three genes have been identified, SLC26A5[1] (MIM 60493), SLC26A4[2] (MIM 605646) and HGF[3] (MIM 142409), there is no overlap between this new locusand previously reported loci.
Materials and Methods
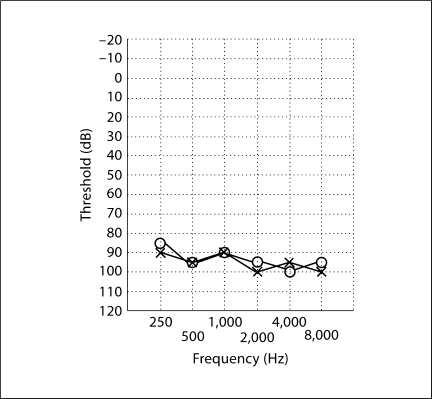
The Institutional Review Boards of the Quaid-I-Azam University and the Baylor College of Medicine and Affiliated Hospitals approved the research protocol prior to study initiation. Informed consent was obtained from all family members who participated in this study. Family 4437 is a multigenerational consanguineous kindred which displays clear evidence of ARNSHI (fig. 1). The ARNSHI in this family is bilateral, symmetric and prelingual. An audiogram from individual IV-1 displays severe-to-profound impairment at all frequencies (fig. 2). Careful physical examination, including Romberg testing, gait assessment and fundoscopy, were performed to rule out vestibular or syndromic features. Perinatal, maternal and personal medical history regarding infections, ototoxic drug use, traumas and noise exposure did not indicate a possible environmental cause for HI.
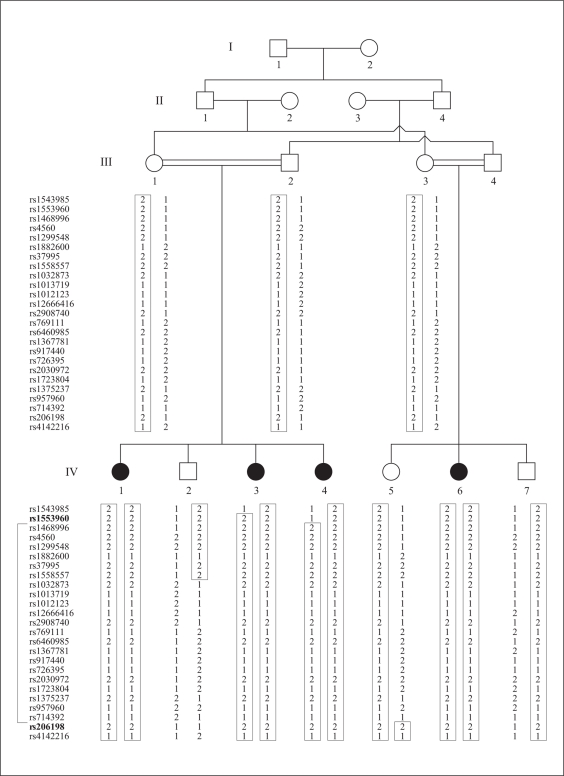
Fig. 1.
Pedigree drawing of family 4437 with ARNSHI. Closed symbols represent individuals with ARNSHI. Open symbols denote hearing subjects. Informative SNP marker genotypes are placed beneath each symbol of the corresponding individual. The SNP markers for each genotype are listed in the left-hand column. The markers shown in bold delimit 3-unit support interval.
Fig. 2.
Audiogram of individual IV-1 of family 4437. Air conduction testing is marked using circles for the right ear and crosses for the left ear. HI was bilateral, symmetric and severe-to-profound involving all frequencies.
Venous blood was obtained from ten family members, including four individuals who are hearing-impaired. Genomic DNA was extracted following a standard protocol [4]. A whole genome scan was carried out at the Center for Inherited Disease Research (CIDR) using the Illumina HumanLinkage-12 panel which contains 6,090 SNP marker loci.
The genotype data were screened for Mendelian incompatibilities using PEDCHECK [5], while MERLIN [6] was used to assess the data for occurrence of double recombination events over short genetic distances, which are most likely due to genotyping error. MLINK of the FASTLINK package [7] was used to perform a two-point linkage analysis, while ALLEGRO1.2c [8] was used for a multipoint linkage analysis. An AR mode of inheritance with complete penetrance and a disease allele frequency of 0.001 were used in the analysis. Marker allele frequencies were estimated from observed and reconstructed genotypes of founders from pedigree 4437 and 50 additional Pakistani families that underwent a genome scan at the same time at the CIDR. For the multipoint analysis, genetic map distances were based on the Build 36 version of the Rutgers combined linkage-physical map of the human genome [9]. For those SNP loci which were not included on the Rutgers map, the sequence-based physical map (Build 36) was used as a reference to determine the physical positions of SNP marker loci and then interpolation was used to determine the genetic map distance.
In addition to carrying out a multipoint linkage analysis using ALLEGRO1.2c, a linkage analysis was also carried out using MERLIN, due to concerns that the multipoint LOD score might be inflated due to intermarker linkage disequilibrium (LD). MERLIN was used to analyze clusters of markers that had no intermarker recombination using haplotype frequencies that were estimated from family 4437 and 50 additional families which were genotyped at the same time. Haplotype reconstruction was performed using SIMWALK2 [10,11].
Primers were designed to cover the exons and 5′ promoter region of eight genes (ACTB, BZW, OCM, MACC1, NXPH1, PRPS1L1, RAC1 and RPA3) using Primer3 software [12]. DNA samples from one hearing (III-1) and two HI (IV-3 and IV-6) pedigree members (fig. 1) were diluted, PCR-amplified, then purified with ExoSAP-IT (USB Corp., Cleveland, Ohio, USA). Sequencing of exons and splice site regions was performed with the BigDye Terminator v3.1 Cycle Sequencing Kit and Applied Biosystems 3730 DNA Analyzer (Applera Corp., Foster City, Calif., USA). The DNA sequences were assembled and analyzed through the Sequencher software V4.9 (Gene Codes Corp., Ann Arbor, Mich., USA).
Results
No genotyping errors were detected through the occurrence of Mendelian inconsistencies or double recombination events occurring over short genetic map distances. A maximum two-point LOD score of 2.5 (θ = 0) was observed at marker rs957960 (chromosome 7: 18.84 Mb) (table 1). Using ALLEGRO1.2c, a maximum multipoint LOD score of 4.0 was obtained for 20 marker loci located on chromosome 7 (see table 1). Incorporating LD in the analysis using MERLIN did not change the results. The 3-unit support interval, which spans 26.5 cM, lies between markers rs1553960 (4.87 Mb) and rs206198 (20.34 Mb) and contains 15.47 Mb of sequence (table 1).
Table 1.
LOD scores for the DFNB90 locus
| SNP markera,b | Genetic positionc | Physical positiond | Multipoint LOD scoree | Two-point LOD score at θ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.01 | 0.05 | 0.10 | 0.20 | 0.30 | 0.40 | ||||
| rs1543985 | 1.94 | 1,179,653 | −∞ | −∞ | −1.6 | −0.4 | −0.0 | 0.2 | 0.1 | 0.0 |
| rs1553960 | 8.10 | 4,866,621 | −∞ | −∞ | 0.5 | 1.0 | 1.0 | 0.8 | 0.4 | 0.1 |
| rs1468996 | 10.40 | 5,700,983 | 4.0 | 2.4 | 2.4 | 2.2 | 1.9 | 1.3 | 0.7 | 0.2 |
| rs4560 | 10.40 | 6,029,809 | 4.0 | 1.0 | 1.0 | 0.9 | 0.8 | 0.6 | 0.4 | 0.2 |
| rs1299548 | 12.54 | 7,268,818 | 4.0 | 1.1 | 1.1 | 1.0 | 0.9 | 0.6 | 0.4 | 0.2 |
| rs1882600 | 12.54 | 7,328,048 | 4.0 | 1.1 | 1.0 | 1.0 | 0.8 | 0.6 | 0.4 | 0.2 |
| rs37995 | 14.73 | 7,995,659 | 4.0 | 1.6 | 1.6 | 1.4 | 1.3 | 0.9 | 0.6 | 0.2 |
| rs1558557 | 14.73 | 8,275,518 | 4.0 | 1.4 | 1.3 | 1.2 | 1.1 | 0.8 | 0.5 | 0.2 |
| rs1032873 | 18.81 | 9,618,805 | 4.0 | 1.7 | 1.6 | 1.5 | 1.3 | 1.0 | 0.6 | 0.2 |
| rs1013719 | 19.78 | 10,521,275 | 4.0 | 0.9 | 1.0 | 0.8 | 0.7 | 0.5 | 0.3 | 0.1 |
| rs1012123 | 19.78 | 10,652,456 | 4.0 | 1.0 | 1.0 | 0.9 | 0.8 | 0.6 | 0.3 | 0.1 |
| rs12666416 | 21.94 | 12,008,942 | 4.0 | 1.6 | 1.6 | 1.4 | 1.3 | 0.9 | 0.6 | 0.2 |
| rs2908740 | 21.94 | 12,025,775 | 4.0 | 1.6 | 1.6 | 1.5 | 1.3 | 0.9 | 0.6 | 0.2 |
| rs769111 | 21.94 | 12,026,331 | 4.0 | 1.6 | 1.6 | 1.5 | 1.3 | 0.9 | 0.6 | 0.2 |
| rs6460985 | 23.73 | 12,890,081 | 4.0 | 1.5 | 1.5 | 1.3 | 1.2 | 0.9 | 0.5 | 0.2 |
| rs1367781 | 26.18 | 14,529,570 | 4.0 | 1.9 | 1.8 | 1.7 | 1.5 | 1.0 | 0.6 | 0.3 |
| rs917440 | 27.97 | 15,690,278 | 4.0 | 1.5 | 1.5 | 1.8 | 1.2 | 0.9 | 0.5 | 0.2 |
| rs726395 | 27.97 | 15,701,240 | 4.0 | 1.5 | 1.5 | 1.4 | 1.2 | 0.9 | 0.5 | 0.2 |
| rs2030972 | 27.99 | 16,025,705 | 4.0 | 1.2 | 1.1 | 1.0 | 0.9 | 0.6 | 0.4 | 0.1 |
| rs1723804 | 29.05 | 16,750,469 | 4.0 | 1.8 | 1.8 | 1.7 | 1.4 | 1.0 | 0.6 | 0.3 |
| rs1375237 | 30.05 | 17,532,778 | 4.0 | 1.4 | 1.3 | 1.2 | 1.9 | 0.8 | 0.5 | 0.2 |
| rs957960 | 31.19 | 18,843,933 | 4.0 | 2.5 | 2.5 | 2.2 | 2.0 | 1.4 | 0.8 | 0.3 |
| rs714392 | 32.23 | 19,577,674 | 3.8 | 1.1 | 1.1 | 1.0 | 0.9 | 0.6 | 0.4 | 0.1 |
| rs206198 | 34.63 | 20,338,283 | −4.5 | 1.9 | 1.9 | 1.8 | 1.7 | 1.2 | 0.8 | 0.3 |
| rs4142216 | 34.64 | 20,707,692 | −1.4 | 1.0 | 1.0 | 1.0 | 0.8 | 0.6 | 0.4 | 0.7 |
Markers shown in bold flank the region of homozygosity.
Uninformative SNP markers are not displayed.
Genetic map position from the Rutgers combined linkage-physical map of the human genome, Build 36.
Physical map position from Build 36 of the human reference sequence.
Multipoint LOD scores obtained from ALLEGRO12c.
The region of homozygosity overlaps with the 3-unit support interval. A recombination event between SNP markers rs1553960 and rs1468996 was observed in the affected child IV-4, which delimited the upper boundary of the region of homozygosity. The lower boundary of the region of homozygosity is delimited by the recombination event between SNP markers rs714392 and rs206198 in child IV-5. The region of homozygosity resides between the markers rs1553960 (4.87 Mb) and rs206198 (20.34 Mb; fig. 1). The locus DFNB90 was designated by the HUGO Nomenclature Committee.
Within the region of homozygosity, there are 72 genes which include hypothetical proteins. No functional sequence variants were identified in the eight sequenced candidate genes.
Discussion
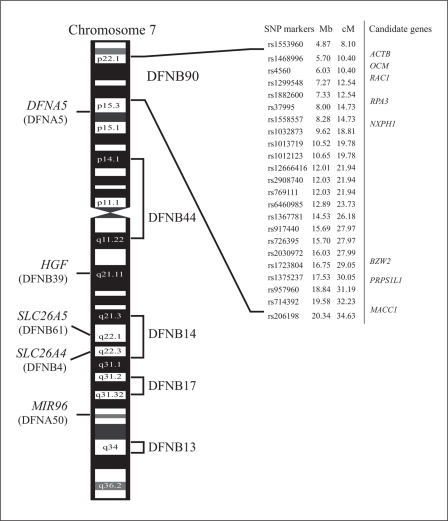
Six ARNSHI loci have been mapped to the long arm of chromosome 7 (7q): DFNB4 (7q31) [13], DFNB13 (7q34-q36) [14], DFNB14 (7q31) [15], DFNB17 (7q31) [16], DFNB39 (7q11.22-q21.12) [17], and DFNB61 (7q22.1) [1]. Extending from the short to the long arm of chromosome 7 lies DFNB44 (7p14.1-q11.22) [18], which is 17.2 Mb centromeric to DFNB90 (fig. 3). For these ARNSHI loci, only the genes SLC26A5 for DFNB61 [1], SLC26A4 for DFNB4 [2] and HGF for DFNB39 [3] have been identified. Additionally, on chromosome 7p lies the autosomal dominant (AD) locus DFNA5, which is centromeric to DFNB90 by 4.4 Mb, and on chromosome 7q is DFNA50 [19], which lies between DFNB17 and DFNB13. The gene for DFNA5 has been identified and bears the same name, DFNA5[20]. For DFNA50, the microRNA miR-96 [21] was shown to be responsible for progressive ADNSHI. Mapping of ARNSHI in family 4437 to the 7p22.1-p15.3 makes DFNB90 the tenth NSHI locus to be localized to chromosome 7.
Fig. 3.
Designated genetic interval of DFNB90 and other DFNB/DFNA genes and loci on chromosome 7.
Among the eight genes which were sequenced within the DFNB90 region, the PRPS1L1 gene encodes a highly homologous protein Phosphoribosyl Pyrophosphate Synthetase 1-Like 1 (MIM 611566) to the two subunits of Phosphoribosyl Pyrophosphate Synthetase 1 (PRPS1; MIM 311850). PRPS1 is the cause of X-linked NSHI (DFN2)[22]; Charcot-Marie-Tooth disease 5 [23], a syndromic form of auditory and optic neuropathy; Arts syndrome [24], which is characterized by mental retardation, motor problems, optic atrophy and hearing impairment; and phosphoribosyl pyrophosphate synthetase superactivity [25], for which the phenotype includes gout from hyperuricemia and neurodevelopmental abnormalities including sensorineural deafness.
The Metastasis-Associated gene in Colon Cancer 1 (MACC1; MIM 612646) is believed to be a key regulator of the hepatocyte growth factor (HGF; MIM 142409) and the HGF receptor (HGFR or MET; MIM164860) pathway [26]. HGF was reported to be the gene responsible for the ARNSHI locus DFNB39 [3].
Six of the sequenced genes (Actin, Beta (ACTB; MIM 102630), Basic Leucine Zipper and W2 Domains 2 (BZW2), Neuroxophilin (NXPH1; MIM 604639), Oncomodulin (OCM; MIM 164795), Replication Protein A3 (RPA3; MIM 179837) and RAS-related C3 botulinum toxin substrate 1 (RAC1; MIM 602048)) have all been shown to be expressed in the fetal human cochlea [27]. Additionally, the ACTB gene was reported to cause a form of juvenile-onset dystonia that is accompanied by sensorineural hearing loss [28,29]. The sequence of the ACTB gene is also 93% identical to Actin, Gamma 1 [30] (ACTG1; MIM 102560) which is the cause of one form of ADNSHI.
The OCM[31]gene is exclusively expressed in outer hair cells of the cochlea and also in the apical inner hair cells. In the outer hair cells, protein expression was observed in the nucleus, cytoplasm and cuticular plate, but not in the stereocilia.
The identification of the DFNB90 gene should provide additional insight into the genetic etiology of ARNSHI and the mechanism of hearing.
Acknowledgements
We wish to thank the family members for their invaluable participation and cooperation. This work was made possible through grants from the Higher Education Commission, Pakistan, (to W.A.) and the National Institutes of Health (NIH) – National Institute of Deafness and Other Communication Disorders grant DC03594 (to S.M.L.). Genotyping services were provided by the CIDR through a fully funded federal contract from the NIH to The Johns Hopkins University, contract No. N01-HG-65403.
The URLs for data presented herein are as follows:
· Hereditary Hearing Loss Homepage,
http://hereditaryhearingloss.org/
· Online Mendelian Inheritance in Man (OMIM),
http://www.ncbi.nlm.nih.gov/omim/
· UCSC genome browser, http://genome.ucsc.edu/
References
- 1.Liu XZ, Ouyang XM, Xia XJ, Zheng J, Pandya A, Li F, Du LL, Welch KO, Petit C, Smith RJ, Webb BT, Yan D, Arnos KS, Corey D, Dallos P, Nance WE, Chen ZY. Prestin, a cochlear motor protein, is defective in non-syndromic hearing loss. Hum Mol Genet. 2003;12:1155–1162. doi: 10.1093/hmg/ddg127. [DOI] [PubMed] [Google Scholar]
- 2.Li XC, Everett LA, Lalwani AK, Desmukh D, Friedman TB, Green ED, Wilcox ER. A mutation in pds causes non-syndromic recessive deafness. Nat Genet. 1998;18:215–217. doi: 10.1038/ng0398-215. [DOI] [PubMed] [Google Scholar]
- 3.Schultz JM, Khan SN, Ahmed ZM, Riazuddin S, Waryah AM, Chhatre D, Starost MF, Ploplis B, Buckley S, Velasquez D, Kabra M, Lee K, Hassan MJ, Ali G, Ansar M, Ghosh M, Wilcox ER, Ahmad W, Merlino G, Leal SM, Friedman TB, Morell RJ. Noncoding mutations of hgf are associated with nonsyndromic hearing loss, dfnb39. Am J Hum Genet. 2009;85:25–39. doi: 10.1016/j.ajhg.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimberg J, Nawoschik S, Belluscio L, McKee R, Turck A, Eisenberg A. A simple and efficient non-organic procedure for the isolation of genomic DNA from blood. Nucleic Acids Res. 1989;17:8390. doi: 10.1093/nar/17.20.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Connell JR, Weeks DE. Pedcheck: A program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin – rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- 7.Cottingham RW, Jr, Idury RM, Schaffer AA. Faster sequential genetic linkage computations. Am J Hum Genet. 1993;53:252–263. [PMC free article] [PubMed] [Google Scholar]
- 8.Gudbjartsson DF, Jonasson K, Frigge ML, Kong A. Allegro, a new computer program for multipoint linkage analysis. Nat Genet. 2000;25:12–13. doi: 10.1038/75514. [DOI] [PubMed] [Google Scholar]
- 9.Matise TC, Chen F, Chen W, De La Vega FM, Hansen M, He C, Hyland FC, Kennedy GC, Kong X, Murray SS, Ziegle JS, Stewart WC, Buyske S. A second-generation combined linkage physical map of the human genome. Genome Res. 2007;17:1783–1786. doi: 10.1101/gr.7156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weeks DE, Sobel E, O'Connell JR, Lange K. Computer programs for multilocus haplotyping of general pedigrees. Am J Hum Genet. 1995;56:1506–1507. [PMC free article] [PubMed] [Google Scholar]
- 11.Sobel E, Lange K. Descent graphs in pedigree analysis: Applications to haplotyping, location scores, and marker-sharing statistics. Am J Hum Genet. 1996;58:1323–1337. [PMC free article] [PubMed] [Google Scholar]
- 12.Rozen S, Skaletsky H. Primer3 on the www for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin CT, Weiss S, Farrer LA, De Stefano AL, Adair R, Franklyn B, Kidd KK, Korostishevsky M, Bonne-Tamir B. Linkage of congenital, recessive deafness (dfnb4) to chromosome 7q31 and evidence for genetic heterogeneity in the middle eastern druze population. Hum Mol Genet. 1995;4:1637–1642. doi: 10.1093/hmg/4.9.1637. [DOI] [PubMed] [Google Scholar]
- 14.Mustapha M, Chardenoux S, Nieder A, Salem N, Weissenbach J, el-Zir E, Loiselet J, Petit C. A sensorineural progressive autosomal recessive form of isolated deafness, dfnb13, maps to chromosome 7q34-q36. Eur J Hum Genet. 1998;6:245–250. doi: 10.1038/sj.ejhg.5200177. [DOI] [PubMed] [Google Scholar]
- 15.Mustapha M, Salem N, Weil D, el-Zir E, Loiselet J, Petit C. Identification of a locus on chromosome 7q31, dfnb14, responsible for prelingual sensorineural non-syndromic deafness. Eur J Hum Genet. 1998;6:548–551. doi: 10.1038/sj.ejhg.5200261. [DOI] [PubMed] [Google Scholar]
- 16.Greinwald JH, Jr, Wayne S, Chen AH, Scott DA, Zbar RI, Kraft ML, Prasad S, Ramesh A, Coucke P, Srisailapathy CR, Lovett M, Van Camp G, Smith RJ. Localization of a novel gene for nonsyndromic hearing loss (dfnb17) to chromosome region 7q31. Am J Med Genet. 1998;78:107–113. [PubMed] [Google Scholar]
- 17.Wajid M, Abbasi AA, Ansar M, Pham TL, Yan K, Haque S, Ahmad W, Leal SM. Dfnb39, a recessive form of sensorineural hearing impairment, maps to chromosome 7q11.22-q21.12. Eur J Hum Genet. 2003;11:812–815. doi: 10.1038/sj.ejhg.5201041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansar M, Chahrour MH, Amin Ud Din M, Arshad M, Haque S, Pham TL, Yan K, Ahmad W, Leal SM. Dfnb44, a novel autosomal recessive non-syndromic hearing impairment locus, maps to chromosome 7p14.1-q11.22. Hum Hered. 2004;57:195–199. doi: 10.1159/000081446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modamio-Hoybjor S, Moreno-Pelayo MA, Mencia A, del Castillo I, Chardenoux S, Morais D, Lathrop M, Petit C, Moreno F. A novel locus for autosomal dominant nonsyndromic hearing loss, dfna50, maps to chromosome 7q32 between the dfnb17 and dfnb13 deafness loci. J Med Genet. 2004;41:e14. doi: 10.1136/jmg.2003.012500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Laer L, Huizing EH, Verstreken M, van Zuijlen D, Wauters JG, Bossuyt PJ, Van de Heyning P, McGuirt WT, Smith RJ, Willems PJ, Legan PK, Richardson GP, Van Camp G. Nonsyndromic hearing impairment is associated with a mutation in dfna5. Nat Genet. 1998;20:194–197. doi: 10.1038/2503. [DOI] [PubMed] [Google Scholar]
- 21.Mencia A, Modamio-Hoybjor S, Redshaw N, Morin M, Mayo-Merino F, Olavarrieta L, Aguirre LA, del Castillo I, Steel KP, Dalmay T, Moreno F, Moreno-Pelayo MA. Mutations in the seed region of human mir-96 are responsible for nonsyndromic progressive hearing loss. Nat Genet. 2009;41:609–613. doi: 10.1038/ng.355. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Han D, Li J, Han B, Ouyang X, Cheng J, Li X, Jin Z, Wang Y, Bitner-Glindzicz M, Kong X, Xu H, Kantardzhieva A, Eavey RD, Seidman CE, Seidman JG, Du LL, Chen ZY, Dai P, Teng M, Yan D, Yuan H. Loss-of-function mutations in the prps1 gene cause a type of nonsyndromic x-linked sensorineural deafness, dfn2. Am J Hum Genet. 2010;86:65–71. doi: 10.1016/j.ajhg.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Sohn KM, Shy ME, Krajewski KM, Hwang M, Park JH, Jang SY, Won HH, Choi BO, Hong SH, Kim BJ, Suh YL, Ki CS, Lee SY, Kim SH, Kim JW. Mutations in prps1, which encodes the phosphoribosyl pyrophosphate synthetase enzyme critical for nucleotide biosynthesis, cause hereditary peripheral neuropathy with hearing loss and optic neuropathy (cmtx5) Am J Hum Genet. 2007;81:552–558. doi: 10.1086/519529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Brouwer AP, Williams KL, Duley JA, van Kuilenburg AB, Nabuurs SB, Egmont-Petersen M, Lugtenberg D, Zoetekouw L, Banning MJ, Roeffen M, Hamel BC, Weaving L, Ouvrier RA, Donald JA, Wevers RA, Christodoulou J, van Bokhoven H. Arts syndrome is caused by loss-of-function mutations in prps1. Am J Hum Genet. 2007;81:507–518. doi: 10.1086/520706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roessler BJ, Nosal JM, Smith PR, Heidler SA, Palella TD, Switzer RL, Becker MA. Human x-linked phosphoribosylpyrophosphate synthetase superactivity is associated with distinct point mutations in the prps1 gene. J Biol Chem. 1993;268:26476–26481. [PubMed] [Google Scholar]
- 26.Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I, Birchmeier W, Schlag PM. Macc1, a newly identified key regulator of hgf-met signaling, predicts colon cancer metastasis. Nat Med. 2009;15:59–67. doi: 10.1038/nm.1889. [DOI] [PubMed] [Google Scholar]
- 27.Skvorak AB, Weng Z, Yee AJ, Robertson NG, Morton CC. Human cochlear expressed sequence tags provide insight into cochlear gene expression and identify candidate genes for deafness. Hum Mol Genet. 1999;8:439–452. doi: 10.1093/hmg/8.3.439. [DOI] [PubMed] [Google Scholar]
- 28.Gearing M, Juncos JL, Procaccio V, Gutekunst CA, Marino-Rodriguez EM, Gyure KA, Ono S, Santoianni R, Krawiecki NS, Wallace DC, Wainer BH. Aggregation of actin and cofilin in identical twins with juvenile-onset dystonia. Ann Neurol. 2002;52:465–476. doi: 10.1002/ana.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Procaccio V, Salazar G, Ono S, Styers ML, Gearing M, Davila A, Jimenez R, Juncos J, Gutekunst CA, Meroni G, Fontanella B, Sontag E, Sontag JM, Faundez V, Wainer BH. A mutation of beta-actin that alters depolymerization dynamics is associated with autosomal dominant developmental malformations, deafness, and dystonia. Am J Hum Genet. 2006;78:947–960. doi: 10.1086/504271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu M, Yang T, Wei S, DeWan AT, Morell RJ, Elfenbein JL, Fisher RA, Leal SM, Smith RJ, Friderici KH. Mutations in the gamma-actin gene (actg1) are associated with dominant progressive deafness (dfna20/26) Am J Hum Genet. 2003;73:1082–1091. doi: 10.1086/379286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakaguchi N, Henzl MT, Thalmann I, Thalmann R, Schulte BA. Oncomodulin is expressed exclusively by outer hair cells in the organ of corti. J Histochem Cytochem. 1998;46:29–40. doi: 10.1177/002215549804600105. [DOI] [PubMed] [Google Scholar]