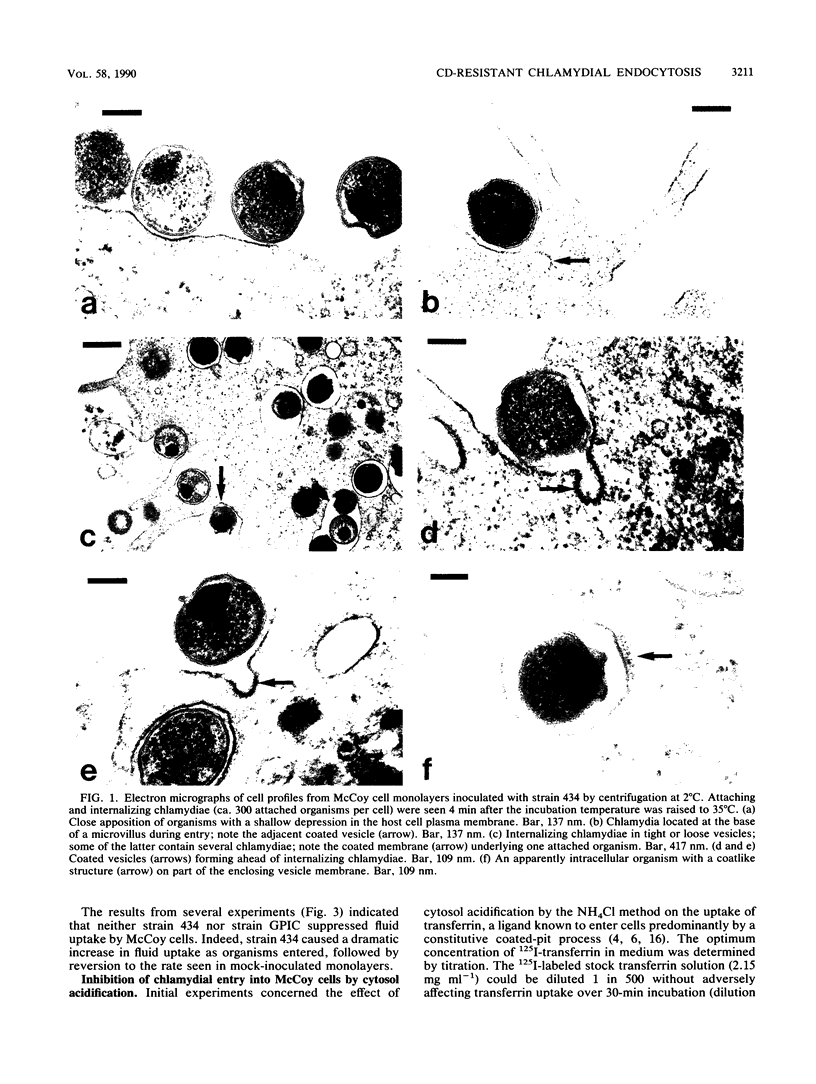
Abstract
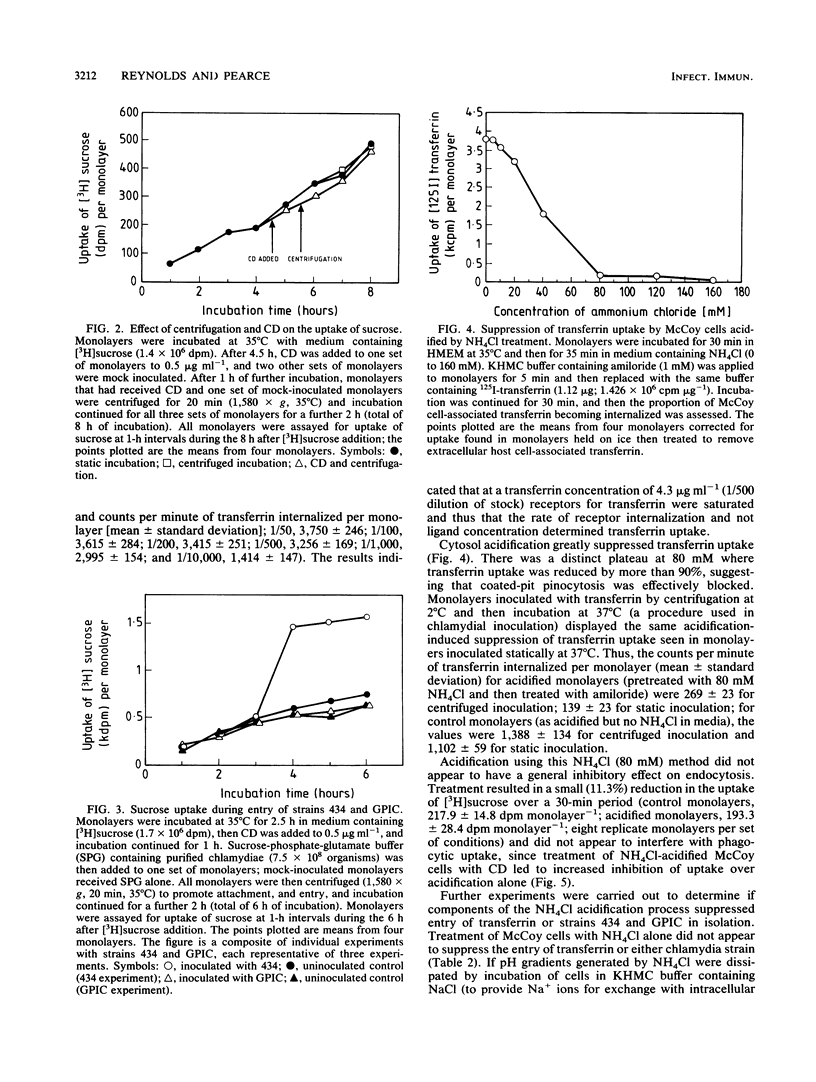
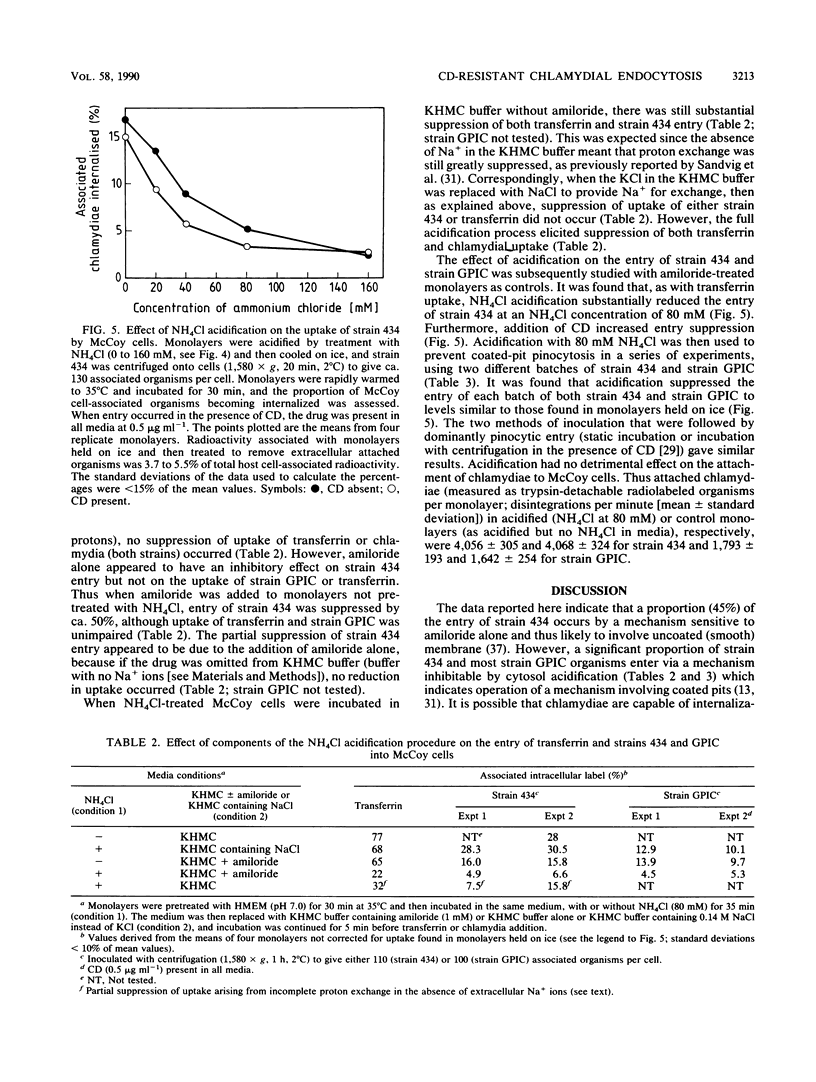
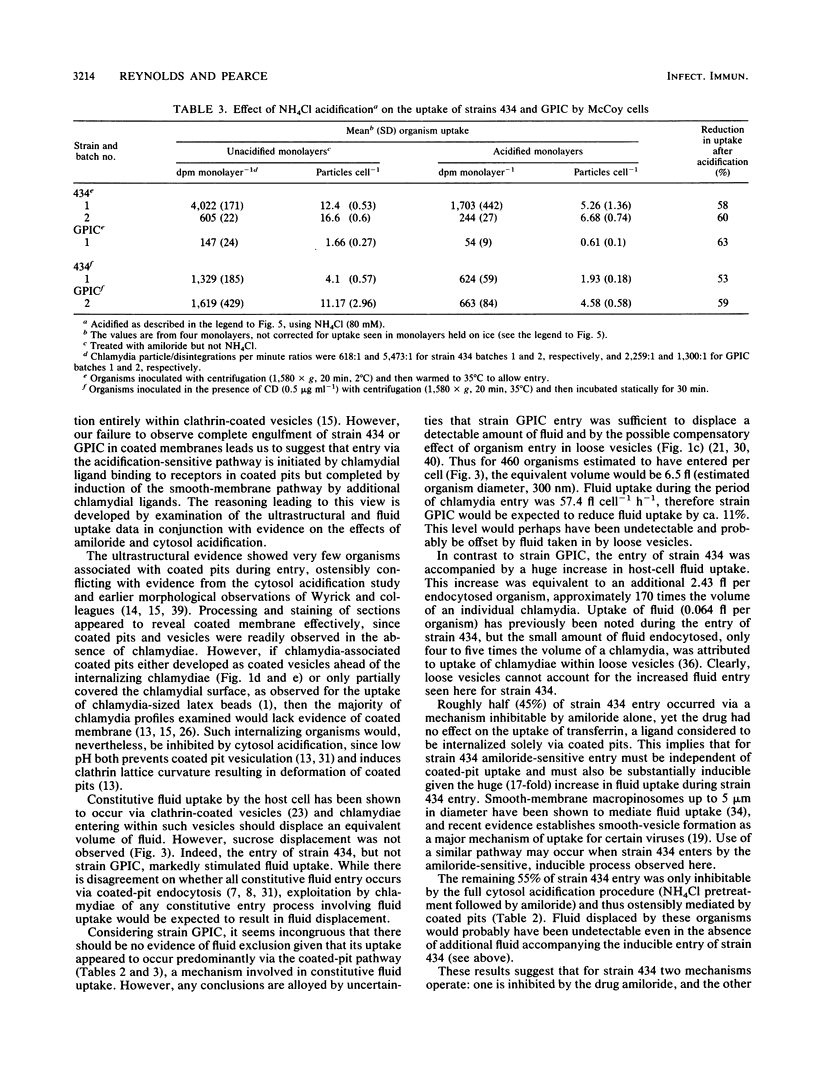
The cytochalasin D-resistant (pinocytic) portion of the entry of two chlamydia strains (Chlamydia trachomatis L2/434/Bu and Chlamydia psittaci GPIC [guinea pig inclusion conjunctivitis]) was examined. By ultrastructural criteria, few organisms of either strain were observed in association with coated host-cell plasma membrane during entry into McCoy cells; this argues against a coated-pit mechanism of entry. When association with a coated membrane was seen, coat material appeared to pinch off ahead of internalizing chlamydiae. However, entry of both strains was substantially reduced by cytosol acidification, a procedure shown to prevent coated-pit vesiculation (K. Sandvig, S. Olsnes, O. W. Petersen, and B. van Deurs, J. Cell Biol. 105:679-689, 1987). No conclusive evidence of displacement of the fluid-phase marker [3H]sucrose from constitutively forming endocytic vesicles was found. Indeed the entry of strain 434 (but not strain GPIC) was accompanied by the influx of a large volume of fluid, suggesting an inducible mechanism. Additionally, entry of strain 434 (but not strain GPIC) was partially inhibitable by amiloride, yet the drug had no effect on the entry of transferrin, a ligand known to enter solely via coated pits. Our findings endorse the view that chlamydial entry can occur via a pathway involving coated pits. However, the unusual morphology of entry and lack of fluid exclusion are consistent with a process whereby although chlamydiae are not fully enclosed by coat material, their entry is dependent on the vesiculation of coated pits. Furthermore, the data support the proposition that a significant proportion of the entry of strain 434 occurs via an inducible pathway independent of coated-pit uptake.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggeler J., Werb Z. Initial events during phagocytosis by macrophages viewed from outside and inside the cell: membrane-particle interactions and clathrin. J Cell Biol. 1982 Sep;94(3):613–623. doi: 10.1083/jcb.94.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan I., Pearce J. H. Association of Chlamydia trachomatis with mammalian and cultured insect cells lacking putative chlamydial receptors. Microb Pathog. 1987 Jan;2(1):63–70. doi: 10.1016/0882-4010(87)90115-x. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Bretscher M. S. Transferrin receptor and its recycling in HeLa cells. EMBO J. 1982;1(3):351–355. doi: 10.1002/j.1460-2075.1982.tb01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Kinetics of phagocytosis of Chlamydia psittaci by mouse fibroblasts (L cells): separation of the attachment and ingestion stages. Infect Immun. 1978 Feb;19(2):607–612. doi: 10.1128/iai.19.2.607-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L., Dautry-Varsat A., Lodish H. F. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J Biol Chem. 1983 Aug 25;258(16):9681–9689. [PubMed] [Google Scholar]
- Cosson P., de Curtis I., Pouysségur J., Griffiths G., Davoust J. Low cytoplasmic pH inhibits endocytosis and transport from the trans-Golgi network to the cell surface. J Cell Biol. 1989 Feb;108(2):377–387. doi: 10.1083/jcb.108.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daukas G., Zigmond S. H. Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J Cell Biol. 1985 Nov;101(5 Pt 1):1673–1679. doi: 10.1083/jcb.101.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. C., Wellner R. B., Cragoe E. J., Jr, Wu H. C. Enhancement of ricin cytotoxicity in Chinese hamster ovary cells by depletion of intracellular K+: evidence for an Na+/H+ exchange system in Chinese hamster ovary cells. J Cell Biol. 1985 Aug;101(2):350–357. doi: 10.1083/jcb.101.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. Effects of cytoplasmic acidification on clathrin lattice morphology. J Cell Biol. 1989 Feb;108(2):401–411. doi: 10.1083/jcb.108.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodinka R. L., Davis C. H., Choong J., Wyrick P. B. Ultrastructural study of endocytosis of Chlamydia trachomatis by McCoy cells. Infect Immun. 1988 Jun;56(6):1456–1463. doi: 10.1128/iai.56.6.1456-1463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodinka R. L., Wyrick P. B. Ultrastructural study of mode of entry of Chlamydia psittaci into L-929 cells. Infect Immun. 1986 Dec;54(3):855–863. doi: 10.1128/iai.54.3.855-863.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C. R., Miller K., Beardmore J. M. Receptor-mediated endocytosis of transferrin and epidermal growth factor receptors: a comparison of constitutive and ligand-induced uptake. J Cell Sci Suppl. 1985;3:173–186. doi: 10.1242/jcs.1985.supplement_3.17. [DOI] [PubMed] [Google Scholar]
- Howard L., Orenstein N. S., King N. W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974 Jan;27(1):102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet C., Ash J. F., Singer S. J. The antibody-induced clustering and endocytosis of HLA antigens on cultured human fibroblasts. Cell. 1980 Sep;21(2):429–438. doi: 10.1016/0092-8674(80)90479-1. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J., Stukenbrok H., Helenius A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J Cell Biol. 1989 Dec;109(6 Pt 1):2721–2729. doi: 10.1083/jcb.109.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C., Wang S., Wentworth B. B., Grayston J. T. Primary isolation of TRIC organisms in HeLa 229 cells treated with DEAE-dextran. J Infect Dis. 1972 Jun;125(6):665–668. doi: 10.1093/infdis/125.6.665. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Blyth W. A., Taverne J. Interactions of TRIC agents with macrophages and BHK-21 cells observed by electron microscopy. J Hyg (Lond) 1973 Sep;71(3):515–528. doi: 10.1017/s0022172400046507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D., Danen R., Bard J. Both species of chlamydia and two biovars of Chlamydia trachomatis stimulate mouse B lymphocytes. J Immunol. 1986 Jun 1;136(11):4249–4254. [PubMed] [Google Scholar]
- Marsh M., Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980 Sep 25;142(3):439–454. doi: 10.1016/0022-2836(80)90281-8. [DOI] [PubMed] [Google Scholar]
- Montesano R., Roth J., Robert A., Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982 Apr 15;296(5858):651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- Prain C. J., Pearce J. H. Ultrastructural studies on the intracellular fate of Chlamydia psittaci (strain guinea pig inclusion conjunctivitis) and Chlamydia trachomatis (strain lymphogranuloma venereum 434): modulation of intracellular events and relationship with endocytic mechanism. J Gen Microbiol. 1989 Jul;135(7):2107–2123. doi: 10.1099/00221287-135-7-2107. [DOI] [PubMed] [Google Scholar]
- Register K. B., Morgan P. A., Wyrick P. B. Interaction between Chlamydia spp. and human polymorphonuclear leukocytes in vitro. Infect Immun. 1986 Jun;52(3):664–670. doi: 10.1128/iai.52.3.664-670.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Petersen O. W., van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987 Aug;105(2):679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. A. Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J Cell Sci. 1989 Sep;94(Pt 1):135–142. doi: 10.1242/jcs.94.1.135. [DOI] [PubMed] [Google Scholar]
- Söderlund G., Kihlström E. Attachment and internalization of a Chlamydia trachomatis lymphogranuloma venereum strain by McCoy cells: kinetics of infectivity and effect of lectins and carbohydrates. Infect Immun. 1983 Dec;42(3):930–935. doi: 10.1128/iai.42.3.930-935.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund G., Kihlström E. Effect of methylamine and monodansylcadaverine on the susceptibility of McCoy cells to Chlamydia trachomatis infection. Infect Immun. 1983 May;40(2):534–541. doi: 10.1128/iai.40.2.534-541.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vretou E., Goswami P. C., Bose S. K. Adherence of multiple serovars of Chlamydia trachomatis to a common receptor on HeLa and McCoy cells is mediated by thermolabile protein(s). J Gen Microbiol. 1989 Dec;135(12):3229–3237. doi: 10.1099/00221287-135-12-3229. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Murray A. Control mechanisms governing the infectivity of Chlamydia trachomatis for HeLa cells: mechanisms of endocytosis. J Gen Microbiol. 1984 Jul;130(7):1765–1780. doi: 10.1099/00221287-130-7-1765. [DOI] [PubMed] [Google Scholar]
- West M. A., Bretscher M. S., Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989 Dec;109(6 Pt 1):2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Harding C., Stahl P. Receptor-mediated endocytosis. Biochem J. 1985 Nov 15;232(1):1–14. doi: 10.1042/bj2320001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrick P. B., Choong J., Davis C. H., Knight S. T., Royal M. O., Maslow A. S., Bagnell C. R. Entry of genital Chlamydia trachomatis into polarized human epithelial cells. Infect Immun. 1989 Aug;57(8):2378–2389. doi: 10.1128/iai.57.8.2378-2389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong E. C., Chi E. Y., Chen W. J., Kuo C. C. Degradation of Chlamydia trachomatis in human polymorphonuclear leukocytes: an ultrastructural study of peroxidase-positive phagolysosomes. Infect Immun. 1986 Aug;53(2):427–431. doi: 10.1128/iai.53.2.427-431.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]