Abstract
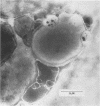
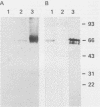
Leishmania major antigen-liposomes prepared as dehydration-rehydration vesicles (DRV) and composed of equimolar amounts of L-alpha-distearoyl phosphatidylcholine and cholesterol confer high-level host-protective immunity against virulent homologous challenge to susceptible BALB/c mice. Physical and antigenic characterization of these protective liposomes is described. Both empty and L. major antigen-DRV were multilamellate and heterogeneous in size, ranging from 0.10 to 2.00 microns. Although the liposomes were made by using a crude mixture of promastigote antigens, lipophosphoglycan covered the liposome surface; this was demonstrated by immunogold electron microscopy. Application of sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot (immunoblot) analysis revealed preferential entrapment of the 63-kilodalton promastigote protease (gp63) into the DRV. We suggest that our L. major antigen-DRV merit further study because of their preferential entrapment of these two host protective antigens together with their long in vivo half-life. In addition, this report illustrates that intravenous or subcutaneous immunization of BALB/c mice with the same limited subset of protective antigens, predominantly lipophosphoglycan and gp63, within DRV liposomes leads to either protection and low splenic interleukin-3 production or to nonprotection and high splenic interleukin-3 production, respectively. This was consistent with our hypothesis that differential antigen presentation after administration of the same immunogen by the intravenous or the subcutaneous route results in differential T-cell activation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bomford R. The comparative selectivity of adjuvants for humoral and cell-mediated immunity. II. Effect on delayed-type hypersensitivity in the mouse and guinea pig, and cell-mediated immunity to tumour antigens in the mouse of Freund's incomplete and complete adjuvants, alhydrogel, Corynebacterium parvum, Bordetella pertussis, muramyl dipeptide and saponin. Clin Exp Immunol. 1980 Feb;39(2):435–441. [PMC free article] [PubMed] [Google Scholar]
- Bordier C., Etges R. J., Ward J., Turner M. J., Cardoso de Almeida M. L. Leishmania and Trypanosoma surface glycoproteins have a common glycophospholipid membrane anchor. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5988–5991. doi: 10.1073/pnas.83.16.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. The promastigote surface protease of Leishmania. Parasitol Today. 1987 May;3(5):151–153. doi: 10.1016/0169-4758(87)90199-2. [DOI] [PubMed] [Google Scholar]
- Bottomly K., Janeway C. A., Jr Antigen presentation by B cells. Nature. 1989 Jan 5;337(6202):24–24. doi: 10.1038/337024a0. [DOI] [PubMed] [Google Scholar]
- Bouvier J., Etges R. J., Bordier C. Identification and purification of membrane and soluble forms of the major surface protein of Leishmania promastigotes. J Biol Chem. 1985 Dec 15;260(29):15504–15509. [PubMed] [Google Scholar]
- Champsi J., McMahon-Pratt D. Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect Immun. 1988 Dec;56(12):3272–3279. doi: 10.1128/iai.56.12.3272-3279.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. S., Chang K. P. Monoclonal antibody affinity purification of a Leishmania membrane glycoprotein and its inhibition of leishmania-macrophage binding. Proc Natl Acad Sci U S A. 1986 Jan;83(1):100–104. doi: 10.1073/pnas.83.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P. Leishmania donovani-macrophage binding mediated by surface glycoproteins/antigens: characterization in vitro by a radioisotopic assay. Mol Biochem Parasitol. 1981 Nov;4(1-2):67–76. doi: 10.1016/0166-6851(81)90030-x. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Studies on the capacity of B cells to serve as antigen-presenting cells. J Immunol. 1981 Mar;126(3):1075–1079. [PubMed] [Google Scholar]
- DeTolla L. J., Scott P. A., Farrell J. P. Single gene control of resistance to cutaneous leishmaniasis in mice. Immunogenetics. 1981;14(1-2):29–39. doi: 10.1007/BF00344297. [DOI] [PubMed] [Google Scholar]
- Feng Z. Y., Louis J., Kindler V., Pedrazzini T., Eliason J. F., Behin R., Vassalli P. Aggravation of experimental cutaneous leishmaniasis in mice by administration of interleukin 3. Eur J Immunol. 1988 Aug;18(8):1245–1251. doi: 10.1002/eji.1830180815. [DOI] [PubMed] [Google Scholar]
- Handman E., Ceredig R., Mitchell G. F. Murine cutaneous leishmaniasis: disease patterns in intact and nude mice of various genotypes and examination of some differences between normal and infected macrophages. Aust J Exp Biol Med Sci. 1979 Feb;57(1):9–29. doi: 10.1038/icb.1979.2. [DOI] [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Greenblatt C. L., Goding J. W. An amphipathic sulphated glycoconjugate of Leishmania: characterization with monoclonal antibodies. EMBO J. 1984 Oct;3(10):2301–2306. doi: 10.1002/j.1460-2075.1984.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Schnur L. F., Spithill T. W., Mitchell G. F. Passive transfer of Leishmania lipopolysaccharide confers parasite survival in macrophages. J Immunol. 1986 Dec 1;137(11):3608–3613. [PubMed] [Google Scholar]
- Hoover D. L., Nacy C. A. Macrophage activation to kill Leishmania tropica: defective intracellular killing of amastigotes by macrophages elicited with sterile inflammatory agents. J Immunol. 1984 Mar;132(3):1487–1493. [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Genetically determined susceptibility to Leishmania tropica infection is expressed by haematopoietic donor cells in mouse radiation chimaeras. Nature. 1980 Nov 13;288(5787):161–162. doi: 10.1038/288161a0. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Kaushal D. C., Carter R. Radioiodination of parasite antigens with 1,3,4,6-tetrachloro-3 alpha, 6 alpha-diphenylglycoluril (IODOGEN): studies with zygotes of Plasmodium gallinaceum. J Protozool. 1982 Feb;29(1):114–117. doi: 10.1111/j.1550-7408.1982.tb02891.x. [DOI] [PubMed] [Google Scholar]
- Kahl L. P., McMahon-Pratt D. Structural and antigenic characterization of a species- and promastigote-specific Leishmania mexicana amazonensis membrane protein. J Immunol. 1987 Mar 1;138(5):1587–1595. [PubMed] [Google Scholar]
- Kahl L. P., Scott C. A., Lelchuk R., Gregoriadis G., Liew F. Y. Vaccination against murine cutaneous leishmaniasis by using Leishmania major antigen/liposomes. Optimization and assessment of the requirement for intravenous immunization. J Immunol. 1989 Jun 15;142(12):4441–4449. [PubMed] [Google Scholar]
- Khan M. M., Strober S., Melmon K. L. Regulatory effects of mast cells on lymphoid cells: the role of histamine type 1 receptors in the interaction between mast cells, helper T cells and natural suppressor cells. Cell Immunol. 1986 Nov;103(1):41–53. doi: 10.1016/0008-8749(86)90066-3. [DOI] [PubMed] [Google Scholar]
- Kirby C., Clarke J., Gregoriadis G. Cholesterol content of small unilamellar liposomes controls phospholipid loss to high density lipoproteins in the presence of serum. FEBS Lett. 1980 Mar 10;111(2):324–328. doi: 10.1016/0014-5793(80)80819-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lelchuk R., Carrier M., Kahl L., Liew F. Y. Distinct IL-3 activation profile induced by intravenous versus subcutaneous routes of immunization. Cell Immunol. 1989 Sep;122(2):338–349. doi: 10.1016/0008-8749(89)90082-8. [DOI] [PubMed] [Google Scholar]
- Lelchuk R., Graveley R., Liew F. Y. Susceptibility to murine cutaneous leishmaniasis correlates with the capacity to generate interleukin 3 in response to leishmania antigen in vitro. Cell Immunol. 1988 Jan;111(1):66–76. doi: 10.1016/0008-8749(88)90051-2. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E., Spithill T. W. Vaccination against cutaneous leishmaniasis in mice using nonpathogenic cloned promastigotes of Leishmania major and importance of route of injection. Aust J Exp Biol Med Sci. 1984 Apr;62(Pt 2):145–153. doi: 10.1038/icb.1984.14. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F. The way ahead for vaccines and vaccination: symposium summary. Vaccine. 1988 Apr;6(2):200–205. doi: 10.1016/s0264-410x(88)80029-x. [DOI] [PubMed] [Google Scholar]
- Modabber F. A model for the mechanism of sensitivity of BALB/c mice to L. major and premunition in leishmaniasis. Ann Inst Pasteur Immunol. 1987 Sep-Oct;138(5):781–786. doi: 10.1016/s0769-2625(87)80038-7. [DOI] [PubMed] [Google Scholar]
- Orlandi P. A., Jr, Turco S. J. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1987 Jul 25;262(21):10384–10391. [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Russell D. G., Talamas-Rohana P. Leishmania and the macrophage: a marriage of inconvenience. Immunol Today. 1989 Oct;10(10):328–333. doi: 10.1016/0167-5699(89)90188-6. [DOI] [PubMed] [Google Scholar]
- Russell D. G., Talamas-Rohana P., Zelechowski J. Antibodies raised against synthetic peptides from the Arg-Gly-Asp-containing region of the Leishmania surface protein gp63 cross-react with human C3 and interfere with gp63-mediated binding to macrophages. Infect Immun. 1989 Feb;57(2):630–632. doi: 10.1128/iai.57.2.630-632.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Wilhelm H. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J Immunol. 1986 Apr 1;136(7):2613–2620. [PubMed] [Google Scholar]
- Scott P., Pearce E., Natovitz P., Sher A. Vaccination against cutaneous leishmaniasis in a murine model. II. Immunologic properties of protective and nonprotective subfractions of soluble promastigote extract. J Immunol. 1987 Nov 1;139(9):3118–3125. [PubMed] [Google Scholar]
- Segal A. W., Gregoriadis G., Black C. D. Liposomes as vehicles for the local release of drugs. Clin Sci Mol Med. 1975 Aug;49(2):99–106. doi: 10.1042/cs0490099. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Wills E. J., Richmond J. E., Slavin G., Black C. D., Gregoriadis G. Morphological observations on the cellular and subcellular destination of intravenously administered liposomes. Br J Exp Pathol. 1974 Aug;55(4):320–327. [PMC free article] [PubMed] [Google Scholar]
- Senior J., Crawley J. C., Gregoriadis G. Tissue distribution of liposomes exhibiting long half-lives in the circulation after intravenous injection. Biochim Biophys Acta. 1985 Mar 29;839(1):1–8. doi: 10.1016/0304-4165(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Senior J., Gregoriadis G. Stability of small unilamellar liposomes in serum and clearance from the circulation: the effect of the phospholipid and cholesterol components. Life Sci. 1982 Jun 14;30(24):2123–2136. doi: 10.1016/0024-3205(82)90455-6. [DOI] [PubMed] [Google Scholar]
- Steinman R. M. Dendritic cells. Transplantation. 1981 Mar;31(3):151–155. [PubMed] [Google Scholar]
- Stingl G., Tamaki K., Katz S. I. Origin and function of epidermal Langerhans cells. Immunol Rev. 1980;53:149–174. doi: 10.1111/j.1600-065x.1980.tb01043.x. [DOI] [PubMed] [Google Scholar]
- Tony H. P., Parker D. C. Major histocompatibility complex-restricted, polyclonal B cell responses resulting from helper T cell recognition of antiimmunoglobulin presented by small B lymphocytes. J Exp Med. 1985 Jan 1;161(1):223–241. doi: 10.1084/jem.161.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tümer A., Kirby C., Senior J., Gregoriadis G. Fate of cholesterol-rich liposomes after subcutaneous injection into rats. Biochim Biophys Acta. 1983 Oct 4;760(1):119–125. doi: 10.1016/0304-4165(83)90132-0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- de Ibarra A. A., Howard J. G., Snary D. Monoclonal antibodies to Leishmania tropica major: specificities and antigen location. Parasitology. 1982 Dec;85(Pt 3):523–531. doi: 10.1017/s0031182000056304. [DOI] [PubMed] [Google Scholar]