Abstract
RAD51 recombinase polymerizes at the site of double-strand breaks (DSBs) where it performs DSB repair. The loss of RAD51 causes extensive chromosomal breaks, leading to apoptosis. The polymerization of RAD51 is regulated by a number of RAD51 mediators, such as BRCA1, BRCA2, RAD52, SFR1, SWS1, and the five RAD51 paralogs, including XRCC3. We here show that brca2-null mutant cells were able to proliferate, indicating that RAD51 can perform DSB repair in the absence of BRCA2. We disrupted the BRCA1, RAD52, SFR1, SWS1, and XRCC3 genes in the brca2-null cells. All the resulting double-mutant cells displayed a phenotype that was very similar to that of the brca2-null cells. We suggest that BRCA2 might thus serve as a platform to recruit various RAD51 mediators at the appropriate position at the DNA–damage site.
Author Summary
Mutations in BRCA1 and BRCA2 predispose hereditary breast and ovarian cancer. Such mutations sensitize to chemotherapeutic agents, including camptothecin, cisplatin, and poly(ADP-ribose) polymerase (PARP) inhibitor, since RAD51 mediators including both BRCA proteins promote repair of DNA lesions induced by these drugs. Little is known of the functional relationships among RAD51, BRCA2, and other RAD51 mediators, because no brca2-null cells were available. Furthermore, the phenotype of sws1 mutants has not been documented. We here disrupted every known RAD51 mediator and analyzed the phenotype of the resulting mutants in both BRCA2-deficient and -proficient backgrounds. The understanding of the function of individual RAD51 mediators and their functional interactions will contribute to the accurate prediction of anti-cancer therapy efficacy.
Introduction
Homologous recombination (HR) maintains genome integrity by accurately repairing double-strand breaks (DSBs) that arise during the mitotic cell cycle or are induced by radiotherapy [1], [2]. HR also plays an important role in releasing the replication forks that stall at damaged template DNA strands [3], [4]. Thus, effective HR makes tumor cells tolerant to the chemotherapeutic agents that damage DNA and stall replicative DNA polymerases. Such chemotherapeutic agents include cis-diaminedichloroplatinum(II) (cisplatin), camptothecin, and poly(ADP-ribose) polymerase (PARP) inhibitors, including olaparib (AstraZeneca). Cisplatin is a crosslinking agent that generates intra- and inter-strand crosslinks and thereby stalls replicative DNA polymerases. Camptothecin inhibits the ligation of single-strand breaks (SSBs) that are formed during the normal functioning of topoisomerase 1. Resulting unrepaired SSBs are converted to DSBs upon replication. Similarly, PARP inhibitors interfere with SSB repair [5]. Since HR plays a major role in repairing DNA lesions generated by camptothecin, cisplatin, and PARP inhibitors [6], measuring HR efficiency in individual malignant tumors may help predict the efficacy of these chemotherapeutic treatment for each tumor [7]–[9].
HR-dependent DSB repair is accomplished by the following step-wise reactions [10]. DSBs are processed by the Mre11/Rad50/Nbs1 complex and the CtIP, Exo1 and DNA2 nucleases to develop 3′ single-strand DNA (ssDNA) tails [11]–[17]. RAD51, an essential recombinase, polymerizes on these ssDNAs, leading to the formation of nucleoprotein filaments. These filaments undergo homology search and subsequent invasion into homologous duplex DNA to form a D-loop structure, where they serve as a primer for DNA synthesis [18], [19]. After the extended end is displaced from the D-loop, it anneals to its partner-end to complete DSB repair. We know that RAD51 plays a key role in HR in vertebrate cells, as inactivation of RAD51 results in the accumulation of chromosomal breaks in mitotic cells and inhibits the completion of even a single cell cycle [1], [2]. The polymerization of RAD51 at damage sites is strictly regulated by a number of accessory factors (hereafter called RAD51 mediators), including the five RAD51 paralogs, SWS1, RAD52, SFR1, BRCA1, BRCA2, and PALB2 [3], [20]–[27]. The functional relationships of these RAD51 mediators are poorly understood, because cells deficient in multiple RAD51 mediators have not been established.
BRCA2 was originally identified as a tumor suppressor, as germline mutation of the BRCA2 gene results in a high risk of developing breast, ovarian, pancreatic, prostatic, and male breast cancer [3], [20], [28], [29]. BRCA2 is recruited to processed DSBs, and facilitates the assembly of RAD51 at the single-strand tail. The middle of the BRCA2 protein has eight BRC repeats, comprising 26 amino acids. Biochemical studies have revealed that individual BRC repeats prompt the loading of RAD51 on ssDNA [30], [31]. Since no brca2-null cells have been established, the function of BRCA2 has been postulated from the phenotypic analysis of mice carrying an allele, extending from the N-terminus to the BRC3 motif (hereafter brca2tr) that encodes a truncated form of BRCA2. Cells derived from brca2tr mice and brca2tr DT40 cells are able to proliferate and exhibit increased sensitivity to ionizing radiation, camptothecin, and cisplatin [32], [33]. It remains unclear whether brca2 null cells display the same phenotype as do rad51 cells, or a milder phenotype.
The roles of the RAD51 mediators have been characterized by phenotypic analysis of their mutants. Mammalian brca1-deficient cells show normal focus formation of the RPA ssDNA binding protein but diminished RAD51 focus formation at DSBs, indicating that BRCA1 facilitates the polymerization of RAD51 after the resection of DSBs [15], [34]. DT40 cells deficient in any one of the five RAD51 paralogs show a very similar phenotype, including compromised RAD51 focus formation and the same degree of DNA damage sensitivity [35], [36], suggesting that these five proteins form a functional unit in the promotion of RAD51 polymerization in which each RAD51 paralog is essential for its function. No biochemical studies have yet defined the molecular mechanisms underlying the promotion of RAD51 assembly by BRCA1, the RAD51 paralogs, or SWS1. SWS1 is another RAD51 mediator [26], and sws1-deficient vertebrate cells have not yet been reported. SFR1 was originally identified in fission yeast [25], and its role in in vitro HR reactions [37], [38] as well as the phenotypic analysis of sfr1-deficient mice have been recently reported [39]. The biochemical character of full-length human BRCA2 has been recently documented, whereas no biochemical studies have defined its functional interaction with other RAD51 mediators or the molecular mechanisms underlying the promotion of RAD51 assembly by BRCA1, the RAD51 paralogs, or SWS1 [40]–[42].
In this paper, we addressed the function of Rad51 mediators and their relationship in DNA-damage responses. We generated the single Rad51 mediator mutant cells, including brca2 null, sfr1 and sws1 deficient cells from DT40 chicken cell line [43]. We also disrupted the BRCA1, one of the RAD51 paralogs (XRCC3), RAD52, RAD54, SWS1, and SFR1 in brca2 null deficient DT40 cells. The phenotypes of these cells were analyzed to reveal hierarchical relationship of RAD51, BRCA2, and the other RAD51 mediators, where RAD51 is able to operate HR without BRCA2 while BRCA2 is required for the functioning of the other RAD51 mediators. Hence, BRCA2 might serve as a platform to recruit various RAD51 mediators at the appropriate position of DNA-damage sites. Our study sheds light on the functional relationship of RAD51 and every known RAD51 mediators for the first time, and thereby significantly contributes to the development of effective anti-cancer therapies.
Results
Loss of SFR1 or SWS1 has a limited impact on DNA–damage responses
To analyze SFR1 and SWS1, we disrupted the SFR1 and SWS1 genes in DT40 cells (Figure 1A–1D). Table 1 summarizes the selection marker genes we used to disrupt genes in this study. The resulting sfr1 and sws1 mutant clones proliferated with nearly normal kinetics (Figure 2) and exhibited an increase in cellular sensitivity to cisplatin. The sfr1 mutant was sensitive also to camptothecin and olaparib (Figure 3). Both mutants showed a slight but significant decrease in ionizing-radiation-induced RAD51 focus formation (Figure 4). We conclude that SFR1 and SWS1 indeed work as RAD51 mediators, though their contribution to HR-dependent repair is less significant than that of BRCA1, BRCA2, and the RAD51 paralogs.
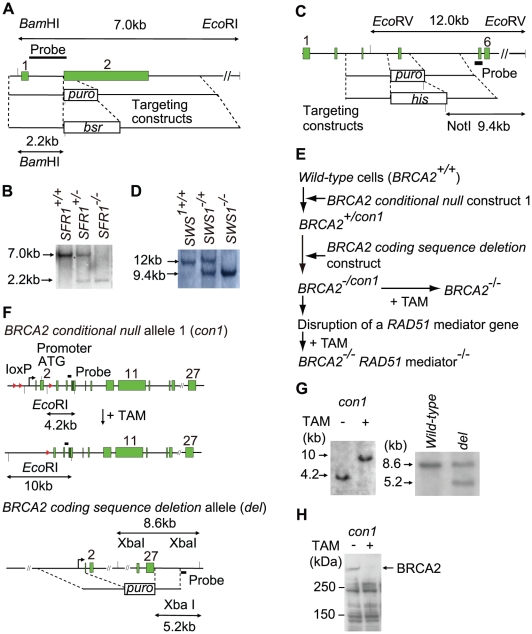
Figure 1. Gene disruption of the SFR1, SWS1, and BRCA2 loci.
(A) Schematic representation of the endogenous SFR1 locus and gene-disruption constructs carrying the puro or bsr selection marker gene. The solid boxes represent exons, and numbers right above boxes represent exon numbers. Relevant BamHI and EcoRI sites are indicated. (B) Southern-blot analysis of genomic DNA digested by both BamHI and EcoRI was performed using the probe DNA shown in (A). Positions of hybridizing fragments of wild-type (WT) and targeted loci are indicated. (C) Schematic representation of the endogenous SWS1 locus and gene-disruption constructs carrying the puro or his selection marker gene. Relevant EcoRV and NotI sites are indicated. (D) Southern-blot analysis of genomic DNA digested by EcoRV and NotI was performed with the probe DNA shown in (C). (E) Experimental methods to generate BRCA2−/− and RAD51mediator−/−/BRCA2−/− cells. We generated BRCA2−/− cells from conditional mutant BRCA2−/con1 cells. In the minus allele of the BRCA2−/con1 cells, the whole coding sequence is deleted (hereafter called the coding sequence deletion allele). The structures of the conditional-null allele-1 (con1) is shown in (F). Treatment of BRCA2−/con1 cells with 4-OH tamoxifen (TAM) led to the generation of BRCA2−/− cells. To generate RAD51mediator−/−/BRCA2−/− cells, we disrupted one of the RAD51mediator genes in BRCA2−/con1 cells. Exposure of the resulting RAD51mediator−/−/BRCA2−/con1 cells to TAM led to the generation of RAD51mediator−/−/BRCA2−/− cells. (F) Schematic representation of BRCA2 conditional-null allele and the brca2-null allele wherein the whole coding sequences are deleted. The conditional-null allele-1 (con1) shown on top was described previously [33]; the structure of the coding sequence deletion allele is shown in the second row. Treatment of the BRCA2−/con1 cells with TAM causes deletion of the promoter and initiation codon. The relevant EcoRI sites in the conditional-null allele-1, the relevant XbaI sites in the coding sequence deletion allele, and the position of the probes used in the Southern-blot analysis (G) are indicated. The solid boxes and arrowheads represent the exons and loxP signals, respectively. (G) Southern-blot analysis of the conditional allele (left) and the other coding sequence deletion (−) allele (right) in BRCA2−/con1 cells with (+) or without (−) TAM treatment. Southern-blot analysis of EcoRI or XbaI-digested genomic DNA was performed with the probe DNA shown in (F). (H) Western-blot analysis to verify the loss of BRCA2 protein in BRCA2−/− cells derived from BRCA2−/con1 cells.
Table 1. DT40 mutants used in this study.
| Cell line | Selection marker for gene disruption | Reference or source |
| sws1 | puro/his | This study |
| sfr1 | bsr/puro | This study |
| brca2-null | hyg/his | This study |
| rad52 | bsr/his | [69] |
| rad54 | neo/his | [70] |
| xrcc3 | bsr/his | [71] |
| brca1 | puro/his | [46] |
| brca2tr | hyg/his | [33] |
| sws1/brca2-null | puro/his | This study |
| sfr1/brca2-null | bsr/puro/his | This study |
| rad52/brca2-null | hyg/puro/his | This study |
| xrcc3/brca2-null | hyg/bsr/puro/his | This study |
| rad54/brca2-null | neo/bsr/puro/his | This study |
| brca1/brca2-null | hyg/bsr/puro/his | This study |
Figure 2. Decreased cellular proliferation in brca2-null cells.
(A) Growth curve for cells of the indicated genotype. (B) The relative rate of cell growth per 8 hours (a single cell cycle for wild-type cells) plotted for cells carrying the indicated genotypes. Each value represents the averaged results from three separate experiments. Error bars represent standard deviation. (C) Cell-cycle distribution of brca2-null cells that were pulse-labeled with BrdU for 10 minutes and subsequently stained with FITC-conjugated anti-BrdU antibody (Y-axis, log scale) and propidium iodide (PI) (X-axis, linear scale). The upper gate indicates cells incorporating BrdU (S phase), the lower middle gate indicates G1 cells, and the lower-right gate indicates G2/M cells. The sub G1 fraction (lower-left gate) indicates dead cells. The number in each gate indicates the percentage of gated events.
Figure 3. Cellular tolerance to camptothecin, cisplatin, and olaparib.
(A) Cells of the indicated genotype were exposed to camptothecin for 72 hours, a period during which wild-type cells are able to divide nine times in the absence of exogenous DNA damage. The X-axis represents the concentration of camptothecin and the Y-axis represents the relative number of surviving cells at 72 hours. The vertical dotted lines show LC50 values (the concentration of camptothecin that reduces cellular survival to 50% relative to cellular survival without camptothecin treatment). Relative LC50 values of camptothecin (B), cisplatin (C), and Poly(ADP-ribose) polymerase inhibitor olaparib (D and E) are shown. Values shown are mean ± SD.
Figure 4. γ-ray–induced Rad51 subnuclear foci in RAD51-mediator mutant cells.
(A) Immuno-staining of irradiated wild-type and mutant DT40 clones using anti-RAD51 antibody. Cells were fixed 3 hours after irradiation with 4Gy γ-rays. Bar, 10 µm. (B) Quantification of RAD51 foci in individual cells of the indicated genotype. Data shown are the means of three experiments. Error bars indicate standard deviation. Statistical analysis was performed using the t test. * P<0.01 compared to wild-type.
The brca2-null mutant is capable of proliferating
To create brca2-null cells, we generated compound heterozygous mutant cells (hereafter called BRCA2−/con1 cells) (Figure 1E). The whole coding sequence was deleted in the minus (−) allele of the -/conditional-null allele-1 (-/con1) genotype of the BRCA2−/con1 cells (Coding sequence deletion allele in Figure 1F). We conditionally deleted the con1 allele of the BRCA2−/con1 cells by adding tamoxifen, which activated the chimeric Cre recombinase [28] and thereby eliminated the promoter and coding sequences, including exons 1 and 2 of the con1 BRCA2 allelic gene (BRCA2 conditional-null allele-1 in Figure 1F). At day two of continuous tamoxifen treatment, the vast majority of cells lost the intact BRCA2 gene in the conditional allele, and a substantial fraction of cells began to die. To our surprise, we were able to reproducibly establish clonally expanding cells wherein the conditional BRCA2 allele was deleted (BRCA2−/− cells, hereafter called brca2-null cells) from 10 to 20% of the tamoxifen-treated populations. We verified deletion of the BRCA2 allele in the established clones by Southern-blot (Figure 1G) and western-blot (Figure 1H) analysis. The ability of the brca2-deleted cells to proliferate is in marked contrast to the immediate cell death observed in rad51-deleted cells [1]. The plating efficiency of the brca2-null clones was around 20%, which is significantly lower than that of the wild-type (100%) and brca2tr (60%) cells [33].
One obvious concern with this experiment was that expression of the N-terminal-truncated BRCA2 protein in the Cre-mediated deletion lines could allow for residual function. We therefore created a second version of the conditionally inactivated BRCA2 allele, wherein sequences spanning from the promoter to intron 12 could be eliminated by induction of Cre (BRCA2 conditional-null allele-2 in Figure S1). We exposed the resulting compound heterozygous mutant cells to tamoxifen and confirmed reproducible establishment of BRCA2-deleted clones (Text S1). This second brca2 conditional-null allele supported proliferation with generation times very similar to those of the first version of the brca2-null cells (data not shown). The more extensively deleted brca2 clones showed a phenotype indistinguishable from that of the smaller deletion clones, indicating that both deletions confer the null phenotype.
BRCA2 contributes to genome maintenance to a lesser extent than does RAD51
We assessed the proliferative properties of brca2-null clones by monitoring their growth curve (Figure 2A and 2B) and cell cycle (Figure 2C). Wild-type cells doubled every 8 hours and increased in cell number by 64 times over 48 hours. The brca2-null cells increased by 17 times over 48 hours (Figure 2A), calculated as a 1.6-fold increase over 8 hours (1.66 = 17) (Figure 2B). The number of brca1, brca2tr, and xrcc3 clones [36] increased 1.6, 1.9, and 1.8 fold, respectively, over eight hours. The reduced growth kinetics of the brca2-null cells is partly due to apoptosis in a substantial fraction of the cell population, as evidenced by the accumulation of cells in a sub-G1 fraction (Figure 2C).
To understand the cause of this cell death, we measured the number of chromosome breaks in mitotic cells. Forty-six chromosomal aberrations were detectable in 100 mitotic brca2-null cells, larger than the number of aberrations observed in any other RAD51-mediator mutants (Table 2). We therefore conclude that spontaneously arising DSBs resulted in cell death in a fraction of brca2-null cells, thus accounting for the reduced growth kinetics (Figure 2A and 2B). The viability of the brca2-null cells reveals that BRCA2 plays a less important role than does RAD51 in the maintenance of genome integrity [1]. The high number of spontaneously arising chromosomal breaks in the brca2-null cells (Table 2) shows that BRCA2 plays a more fundamental role in genome maintenance than do any of the other RAD51 mediators.
Table 2. Spontaneous chromosomal aberrations.
| Chromatid-type | Chromosome-type | |||||
| Cell line | Gaps | Breaks | Gaps | Breaks | Exchange | Total |
| wild-type | 1 | 1 | 1 | 0 | 0 | 3 |
| xrcc3 | 1 | 1 | 4 | 6 | 0 | 12 |
| rad54 | 1.7 | 0 | 1.7 | 0 | 0.7 | 4.1 |
| brca1 | 6 | 10 | 0 | 6 | 2 | 24 |
| brca2-null | 16 | 10 | 2 | 18 | 0 | 46 |
| brca2tr | 4 | 4 | 3 | 3 | 2 | 16 |
| brca1/brca2-null | 0 | 24 | 2 | 13 | 0 | 39 |
Spontaneous chromosomal aberration of indicated genotypes. Data are the numbers of aberrations per 100 cells. At least 100 mitotic cells were analyzed for each genotype.
brca2-null cells displayed a stronger phenotype than the brca1, brca2tr, and rad51 paralog mutant clones
We analyzed cellular tolerance to camptothecin, cisplatin, and olaparib by measuring cellular survival at 72 hours (7–9 cell cycles) after continuous exposure to these agents in a liquid medium. We did not use the conventional colony-formation assay for this analysis, because the plating efficiency of the brca2-null cells was only 20%, 5-fold lower than that of wild-type cells. Figure 3A presents an example of cellular sensitivity to camptothecin, a DNA-damaging agent. Subsequent figures illustrate the sensitivity of each mutant, assessed by LC50 values, i.e., the dose that reduces cell survival to 50% relative to the LC50 value of wild-type cells, which is defined as 100% (Figure 3B–3E).
In the cellular-survival analysis, the brca2-null cells showed an increased sensitivity to camptothecin (Figure 3B), cisplatin (Figure 3C), and olaparib (Figure 3D and 3E). Moreover, sensitivity to cisplatin and olaparib was higher with the brca2-null cells than for any of the other HR mutant cells, including the brca1, rad52, rad54, and xrcc3 clones (Figure 3). We therefore conclude that BRCA2 plays a more important role in HR-dependent repair than do the other RAD51 mediators, including BRCA1, RAD52, RAD54, the RAD51 paralogs, SFR1, and SWS1.
The less prominent phenotype of the brca2tr cells compared to the brca2-null cells indicates that the BRCA2 BRC3-truncated protein retains significant residual HR function. Although brca1 cells were less sensitive to cisplatin and olaparib than were brca2-null cells, the brca1-null cells exhibited a slightly higher sensitivity to camptothecin than did the brca2-null cells (Figure 3A). The greater contribution of BRCA1 to cellular tolerance to camptothecin might be attributable to the role played by BRCA1 in DNA-damage responses other than HR, such as collaborative action with CtIP to eliminate covalently bound oligo-peptides from DSBs [15].
We next measured the frequency of HR-dependent repair of I-Sce1-mediated DSBs in a recombination substrate, SCneo, inserted into the OVALBUMIN locus [44], [45] (Table 3). The frequency of HR-dependent DSB repair was calculated as the number of neomycin-resistant (neo+) colonies relative to the number of plated cells. The frequency of HR in the brca2-null, brca2tr, and brca1 cells was decreased by 1.5×104-, 1.5×102-, and 4.5×103-fold, respectively, compared with wild-type cells. We conclude that the brca2-null cells retain residual HR activity, which may account for their viability even in the complete absence of BRCA2.
Table 3. Double strand break-induced gene conversion frequency.
| Cell line | Relative recombination frequency ± SE | Range of neo+ colonies per 1×107 transfected cells (range) |
| wild-type | 1.0±0.8 | 1.1∼2.5×105 |
| xrcc3 | 1.3±0.1×10−1 | 1.3∼2.0×104 |
| brca1 | 2.2±0.4×10−4 | 20∼36 |
| brca2-null | 6.8±0.9×10−5 | 4∼11 |
| brca2tr | 6.6±4.2×10−3 | 347∼1493 |
| sws1/brca2-null | 7.1±1.5×10−5 | 8∼15 |
| sfr1/brca2-null | 5.6±0.2×10−5 | 6∼13 |
| rad52/brca2-null | 4.4±0.2×10−5 | 5∼10 |
| xrcc3/brca2-null | 3.0±1.1×10−5 | 2∼13 |
| rad54/brca2-null | 7.6±1.6×10−5 | 7∼20 |
| brca1/brca2-null | 1.9±0.5×10−5 | 1∼7 |
Analysis of HR frequency induced by I-Sce1-induced DSBs in an artificial substrate. SCneo-puro was stably integrated into the OVALBUMIN locus of indicated genotypes by gene-targeting. 1.0×107 cells of each genotype were transfected with 30 µg of either I-Sce1 expression vector or pBluescript KS, and subsequently selected using a neomycin analog (G418). The ratio of recombination frequency was calculated by comparing the number of G418 resistant clones in the mutant cell lines to that in the wild-type cell line. Only results obtained using the I-Sce1 expression vector are shown.
Since BRCA2 promotes the loading of RAD51 at damage sites, we measured RAD51 focus formation at 3 hours after ionizing radiation. The number of RAD51 foci was reduced but not eliminated in the brca1 and brca2tr clones, compared with wild-type cells (Figure 4). These findings are consistent with previous observations [33], [46]. By contrast, we hardly detected any RAD51 focus formation in the brca2-null cells. In conclusion, the BRCA2 protein plays a key role in the efficient recruitment of RAD51 to DNA-damage sites, but is not essential for every HR reaction.
BRCA2 is required for the effective participation of BRCA1, RAD52, SFR1, SWS1, and XRCC3 in HR
The idea that RAD51 carries out HR even without BRCA2 led us to investigate whether or not other RAD51 mediators substitute for BRCA2 in the promotion of RAD51-dependent HR. To this end, we deleted the BRCA1, RAD52, SFR1, SWS1, and XRCC3 genes in the conditional brca2-null background, then inactivated the BRCA2 gene by treating the cells with tamoxifen (Figure 1E). We also disrupted the RAD54 gene in the conditional brca2-null background (Table 1). The RAD54 protein promotes HR after the assembly of RAD51 at DNA-damage sites [47]. To our surprise, we were able to reproducibly establish all resulting double mutants, although a substantial fraction (∼30%) of the brca2-null cells died each cell cycle.
The growth kinetics for the brca1/brca2-null, rad52/brca2-null, sfr1/brca2, sws1/brca2-null, and xrcc3/brca2-null double-mutant clones was similar to those of the brca2-null single mutant (Figure 2). Taking the very severe phenotype of brca1 cells into account, the viability of the brca1/brca2-null cells was surprising. The cloning efficiency of the brca1/brca2-null cells was slightly higher than that of the brca2-null single-mutant cells (30% compared to 20%). Accordingly, the number of spontaneous chromosomal aberrations in the brca1/brca2-null cells was consistently slightly lower than that in the brca2-null cells (Table 2). In summary, although the loss of either BRCA1 or BRCA2 greatly increased the number of spontaneous chromosomal breaks, inactivation of BRCA1 in the brca2-null cells resulted in a slight reduction in the severity of the brca2 phenotype.
An early study shows rad52/xrcc3 double-mutant cells are synthetic lethal and exhibit numerous chromosomal breaks [24], whereas we here found that the brca2/rad52 and brca2/xrcc3 double-mutant cells were viable. Thus, the synthetic lethality might be attributable to BRCA2 mediated formation of toxic HR intermediates, because the brca2/xrcc3 cells exhibit spontaneously arising isochromatid breaks, where two sisters are broken at the same site due to defective completion of HR [48]. To test this hypothesis, we conditionally inactivated the BRCA2 gene in the rad52/xrcc3 cells (Text S1). We found that the inactivation of the BRCA2 gene indeed rescued the rad52/xrcc3 cells (Figure S2). This observation indicates that the synthetic lethality of the rad52/xrcc3 cells does not argue against the idea that the functioning of RAD52 and XRCC3 depends on BRCA2. Likewise, the formation of toxic HR intermediates might explain the apparent discrepancy between the viability of rad52/brca2-null DT40 cells and the mortality caused by shRNA mediated depletion of RAD52 in brca2 deficient mammalian cells [49], as the latter cells express a residual amount of RAD52 and truncated BRCA2 proteins perhaps leading to the formation of toxic HR intermediates.
We next measured the sensitivity of the brca1/brca2-null, rad52/brca2-null, rad54/brca2-null, sfr1/brca2-null, sws1/brca2-null, and xrcc3/brca2-null double-mutant clones to camptothecin, cisplatin, and olaparib (Figure 3 and Figure 5). Remarkably, inactivation of any gene did not increase cellular sensitivity to the three damaging agents by more than two-fold. This observation indicates that the contribution made by BRCA1, the RAD51 paralogs, RAD52, RAD54, SFR1, and SWS1 to HR depends mostly on BRCA2. Interestingly, the loss of BRCA1, SFR1, and SWS1 somewhat increased the cellular tolerance of the brca2-null cells to cisplatin. Similarly, the loss of SWS1 increased the cellular tolerance of the brca2-null cells to camptothecin and olaparib. This increased tolerance was not accompanied by the upregulation of RAD51 focus formation (data not shown). We therefore suggest that, in the absence of BRCA2, SWS1 has a moderately antagonistic effect on HR-dependent repair. By contrast, the loss of RAD52 and XRCC3 significantly increased the cellular sensitivity of the brca2-null cells to olaparib. In summary, BRCA2 is required for all the analyzed RAD51 mediators to function, and the functional relationships between BRCA2 and the other RAD51 mediators in HR-mediated repair differ slightly depending on the type of DNA damage.
Figure 5. Effect of brca2 deletion on sws1-, sfr1-, rad52-, rad54-, xrcc3-, and brca1-deficient cells.
Cellular sensitivities to the indicated DNA damaging agents were analyzed using the same method as in Figure 3. The LC50 values are shown in Figure 3B–3E.
Discussion
In this study, we established brca2-null cells as well as cells deficient in each of the RAD51 mediators. We show that BRCA2 plays a more important role in the promotion of both RAD51 polymerization at DNA-damage sites and HR-dependent repair than does any other RAD51 mediator, including BRCA1, the RAD51 paralogs, RAD52, SFR1, and SWS1. The ability of brac2-null cells to proliferate is in marked contrast with the immediate cell death that occurs upon depletion of RAD51 [1]. Therefore, RAD51 is able to perform HR even in the absence of BRCA2. To explore the question of which RAD51 mediators might substitute for BRCA2 in the promotion of RAD51-dependent HR repair, we inactivated the RAD51 mediators in brca2-null cells. Loss of any one of the other RAD51 mediators did not further reduce the viability of brca2-null cells. In a related study, we also found that the brca2-null mutant and the palb2/brca2-null double mutant showed the same phenotype with respect to both spontaneous chromosomal aberrations and increased sensitivity to DNA-damaging agents (manuscript in preparation). Thus we conclude that BRCA1, PALB2, the RAD51 paralogs, RAD52, SFR1, and SWS1 all require BRCA2 to contribute to HR.
BRCA2 plays a major role in the recruitment of RAD51 to DNA–damage sites, but is not essential for every HR reaction
Data on Ustilago maydis [50] and Arabidopsis thaliana [51] suggest that BRCA2 might be essential for RAD51 to function in any HR reaction. However, we here report that RAD51 can form HR products even in brca2-null cells, indicating that RAD51 plays a more important role than BRCA2 in HR. This hierarchy between RAD51 and BRCA2 is supported by previous reports of experiments with mice, as rad51 null embryos died earlier (∼E6.5) than did BRCA2 null (∼E8.5) embryos [52], [53]. The viability of brca2-null DT40 cells is consistent with the clonal expansion of BRCA2-deficient cells derived from mammary epithelial lineage-specific or T cell lineage-specific BRCA2-null-deficient mice [54], [55]. Adding to these findings, we here show solid evidence that vertebrate RAD51 is capable of functioning in the absence of BRCA2.
Collaboration between BRCA1 and BRCA2 is required for efficient HR
The phenotypic analysis of brca1, brca2-null, and brca1/brca2-null clones, combining with the previous study of rad51-null cells, reveals the functional relationship described as follows. The capability of HR was dramatically diminished when either BRCA1 or BRCA2 was absent, indicating that the collaboration of BRCA2 and BRCA1 is required for efficient HR events. brca2-null cells exhibited more prominent defects in HR than did brca1-null cells, indicating that BRCA2 can function in HR independently of BRCA1. Moreover, BRCA2′s contribution to the repair of cisplatin-induced interstrand crosslinks is more significant than BRCA1, which is likely attributable to the fact that BRCA2, but not BRCA1, functions in the Fanconi anemia repair pathway [56]. BRCA1 has additional functions other than in HR, such as mediating the damage checkpoint and processing DSBs [15], [57]. The fact that rad51-null cells have a considerably stronger phenotype than brca2-null cells indicates that RAD51 could still perform HR-dependent repair in brca2-null cells.
The phenotypic similarities between the brca2-null and the brca1/brca2-null clones indicate that BRCA1 contributes to HR by collaborating with BRCA2. Presumably, the two BRCA proteins form a functional unit and collaborate intimately to load RAD51 at damage sites. This idea is supported by the fact that BRCA1 physically associates with BRCA2 through the PALB2 protein [58]. However, this idea is challenged by recent studies that suggest that BRCA1 plays a role in the resection of DSBs [14], [59]. One possible scenario is that the complex formation of BRCA1 and BRCA2 may allow for close collaboration between the BRCA1-dependent resection of DSBs and the subsequent loading of RAD51 on the resulting 3′ overhang. Such an interaction interface might be shared by the E. coli RecBCD complex, which serves as the DSB resection complex and also interacts directly with RecA following chi site recognition [60]. In summary, the phenotypic analysis of brca1, brca2-null, and beca1/brca2-null DT40 clones demonstrates that BRCA1 controls RAD51 in HR, mainly through collaboration with BRCA2.
BRCA2 is required for BRCA1, PALB-2, the RAD51 paralogs, SFR1, and SWS1 to promote HR
Our study reveals that rad52/brca2-null, sfr1/brca2-null, sws1/brca2-null, and xrcc3/brca2-null clones exhibit a phenotype very similar to that of brca2-null cells (Figure 5). In a separate study, we conformed phenotypic similarity between brca2-null and palb2/brca2-null clones (data not shown). We therefore suggest that, like BRCA1, PALB2, the RAD51 paralogs, RAD52, SFR1, and SWS1 are also able to participate in HR, mostly depending on BRCA2. One possible scenario is that BRCA2 is recruited to DNA-damage sites through PALB2 or by directly interacting with the junction between the duplex DNA and the single-strand sequences [61]. BRCA2 might thus serve as a platform to recruit various RAD51 mediators to the appropriate positions of DNA-damage sites (Figure 6).
Figure 6. Model of BRCA2 dependent regulation of various RAD51 mediators at DNA–damage sites.
In the absence of BRCA2, RAD51 polymer assembles at DNA damage sites (top), while BRCA2 significantly promotes the polymerization of RAD51 (bottom). This promotion is attributable to the requirement of BRCA2 for the appropriate localization of other RAD51 mediators at DNA damage sites. Accordingly, the loss of BRCA2 may result in the abnormal functioning of some RAD51 mediators, for example, SWS1. This scenario explains why the loss of SWS1 increased cellular tolerance to camptothecin, cisplatin, and olaparib only when BRCA2 was absent (Figure 3).
Applications for clinical research
DT40 is a unique cell line that offers a panel of DNA-repair-deficient isogenic mutants derived from a stable parental line. DT40 cells have several characteristics that affect cellular responses to anti-cancer agents. First, DT40 appears, for unknown reasons, to possess a significantly higher HR efficiency than any mammalian cell line [43]. The efficient HR in DT40 cells is prominent particularly in HR between diverged homologous sequences such as Immunoglobulin V gene diversification [62] and gene targeting, where the selection marker genes of gene-disruption constructs may interfere with HR as heterologous sequences. Second, like many cancer cells, DT40 lacks the functional p53, and as a result has no G1/S damage checkpoint [63]. In addition, 70% of the DT40 cell cycle takes place in the S phase. Thus, DNA damage at any phase of the cell cycle may have a direct impact on DNA replication. These characteristics, specific to DT40, suggest that a defect in DNA repair associated with DNA replication, including HR-mediated DNA repair, may display a more prominent phenotype in DT40 cells than in other cell lines that have a longer G1 phase and/or a normal G1/S checkpoint. Bearing this in mind, DT40 is revealed as a unique and valuable tool and has been used extensively to explore the role of individual HR factors responsible for cancer therapy.
Materials and Methods
Cell culture and DNA transfection
Cells were aquired and cultured as described previously [1], [43]. All mutants were isolated from single colonies. DNA transfection and selection were performed as described previously [43], [64]. Details of the cell lines used in this study are shown in Table 1.
Generation of SFR1−/− DT40 cells
To disrupt the SFR1 gene, we generated SFR1-puro and SFR1-bsr disruption constructs by combining two genomic PCR products with the puro- or bsr-selection-marker cassette. Genomic DNA sequences were amplified using the 5′-CCCGGTACTGAGGGGTGCGATTGCTTGCAGG-3′ and 5′-CCCTTAGAGTTGCACTCATTGGCTAAAG-3′ primers for the upstream arm, and the 5′-GGCTCAAACTGGTCAAGATGTACCGATCTAAGG-3′ and 5′-CCACCAGCATCCACTAAAGGGCAAGGAACG-3′ primers for the downstream arm. Amplified PCR products were cloned into pCR2.1-TOPO vector (Invitrogen). The 1.7 kb fragment of the upstream arm was cloned into the KpnI site of pCR2.1 containing the 3.0 kb downstream arm. Marker-gene cassettes were inserted at the BamHI site of the resulting plasmid.
To generate SFR1−/− cells, SFR1-puro and SFR1-bsr disruption constructs lineralized with NotI were transfected sequentially by electroporation (Bio-Rad). The genomic DNA of the transfectants was digested with both BamHI and EcoRI, and gene-targeting events were confirmed by Southern blot analysis. The probe was prepared from a PCR-amplification of DT40 genomic DNA using primers 5′-GAACAGCACCACGCAATTCA-3′ and 5′-CCTTAGAGTTGCACTCATTGG-3′.
Cloning of SFR1 cDNA
Chicken SFR1 cDNA was isolated by PCR amplification of the primary cDNAs using the 5′-GTTGAGATGGAGGAAGCAGCGTGTGGTAAA-3′ and 5′-CACCACTCAATTCCACTTCAAAGAG-3′ primers. The gene bank accession number of the chicken SFR1 gene is XM-001234167.
Generation of SWS1−/− DT40 cells
To disrupt the SWS1 gene, we generated SWS1 gene-disruption constructs containing the 2.6 kb upstream and the 3.0 kb downstream genomic fragments. The 2.6 kb fragment was PCR-amplified using the 5′-ggggacaactttgtatagaaaagttgTTCTTACGTCACTCCAGAAGAACA-3′ and 5′-ggggactgcttttttgtacaaacttgCCAAGTCTGTGAATCGCAGAAGCA-3′ primers. The 3.0 kb fragment was PCR-amplified using the 5′-ggggacagctttcttgtacaaagtggAATTCCAAGCAGTTCCACATCTCT-3′ and 5′-ggggacaactttgtataataaagttgGTATGGCTCCTGTCAGGTTAGAGT-3′ primers. Note that the underlined sequences denote the recognition sequences in the Gateway system (Invitrogen). Using the MultiSite Gateway system with pENTR-lox-his, pENTR-lox-puro and pDEST-DTA-MLS [65], a floxed his or puro gene was inserted between the upstream and downstream arms on a plasmid carrying a diphtheria toxin A (DT-A) gene, thus yielding the two targeting vectors, SWS1-his/loxP and SWS1-puro/loxP.
To generate SWS1−/− cells, SWS1-his/loxP and SWS1-puro/loxP gene-disruption constructs linearized with AscI were transfected sequentially into DT40 cells (Bio-Rad). The genomic DNA of the transfectants was digested with both EcoRV and NotI, and gene-targeting events were confirmed by Southern-blot analysis. The probe was prepared by PCR-amplification of chicken genomic DNA using the 5′-GCTCGCAGGAACACAACTCCTT-3′ and 5′-GTACAGGAGTGTTTCTCTGCGG-3′ primers.
Cloning of SWS1 cDNA
The gene bank accession numbers for the human and chicken Sws1 genes are XP-058899 and XP-415841, respectively. RT-PCR of DT40 transcripts was done using the 5′-CGCGTCGACATGGATAGCACCTTACCAGCT-3′ and 5′-CGCGGATCCTCATCCTTCATCCTCTTCCTC-3′ primers.
Generation of BRCA2−/− DT40 cells
The brca2-null mutant cells were generated as follows (Figure 1). We inserted conditional brca2 heterozygous cells (BRCA2+/con1) harboring two loxP signals into the other allele upstream of the promoter and downstream of exon 2. Construction of the BRCA2 conditional-null targeting vector was carried out as described previously [33]. To delete the intact allele of the BRCA2+/con1 cells, we constructed a targeting vector to delete all exons of the BRCA2 gene. The ∼6. kb and ∼3.5 kb fragments at the BRCA2 locus [66] were amplified from DT40 genomic DNA by using the 5′-CCGCTCGAGTTTTGTTAGTTGTGAGATGTG-3′ and 5′-TTATCGGGGCTTTGTCAGCTTTAGCTTCTC-3′ primers and the 5′-CGGAGTTGAATAATGGTACATTTCTGGCAC-3′ and 5′-GTTGAATTTGAAACTGGCTGAACAGAAGAG-3′ primers, respectively. Both fragments were cloned into TOPO-pCRXL cloning vector (Invitrogen, Carlsbad, California) to make the topo/6.0 kb and topo/3.5 kb vectors. The ∼5.2 kb NotI fragment from the topo/6.0 kb vector was inserted into the NotI site in the multicloning site of the topo/3.5 kb vector, resulting in the pUpper/Lower vector. Finally, a loxP-flanked puro-resistance cassette was inserted into the BglII site in the pUpper/Lower vector. The resulting targeting construct was transfected into the BRCA2+/con1 cells followed by selection with puromycin. The genomic DNA of the transfectants was digested with XbaI, and gene-targeting events were confirmed by Southern-blot analysis with a probe that was amplified from genomic DNA using the 5′-ATCCATGTCACTGTTGACATCCTGACTGCC-3′ and 5′-AGATACAAACCCAATGGGAAGCCAGGTGTG-3′ primers. The bands detected by the probe were 8.6 kb from the wild-type allele and 5.2 kb from the targeted allele.
Upon exposure of the BRCA2−/con1 cells to tamoxifen, an estrogen antagonist, nucleotide sequences, including promoter and coding sequences encoding the initiation codon to the 67th amino acid, were excised by a chimeric Cre recombinase fused to the estrogen-receptor ligand-binding domain [24], leading to the complete disruption of the BRCA2 gene.
Disruption of individual HR genes in BRCA2−/− DT40 cells
To disrupt RAD51 mediator genes in BRCA2−/− DT40 cells, we disrupted each gene in the BRCA2−/con1 cells (Table 1). We exposed the resulting RAD51 mediator−/−/BRCA2−/con1 cells to tamoxifen and isolated the RAD51 mediator−/−/BRCA2−/− cells.
Western blot analysis
Western blotting was performed as previously described [33]. The rabbit polyclonal primary antibody, which recognizes the N-terminal 203 amino acids of chicken BRCA2, was diluted 1∶100 with blocking buffer. The anti-rabbit IgG HRP conjugated antibody was diluted 1∶5000 with blocking buffer.
Flow-cytometric analysis
To measure growth kinetics, cells were counted daily using flow-cytometric analysis, as described previously [7]. To measure cell-cycle distribution, cells (5×105/ml) were labeled for 10 minutes with 20 µmol/L 5-bromo-2′-deoxyuridine (BrdU) and subsequently harvested. Harvested cells were fixed and analyzed as previously described [7].
Measurement of cellular sensitivity to camptothecin, cisplatin, and olaparib in liquid culture
To measure cellular survival, cells (1.5×103–1.5×104) were incubated in 1 ml culture medium per well containing various concentrations of the DNA-damaging agents. At 72 hours, the ATP in the cellular lysates was measured to assess the number of live cells. The camptothecin (TopoGen Inc., Colombus, OH) and olaparib (AstraZeneca) were diluted with DMSO, and the cisplatin (Nihonkayaku, Tokyo, Japan) was diluted with PBS. To measure the sensitivity of the DT40 cell lines to these agents, cells were continuously exposed to various concentrations of the drug and the number of cells was measured at 72 hours. At least three independent experiments were carried out. Sensitivity was calculated by dividing the number of cells treated with the drug by the number of untreated cells [8].
Measurement of ATP to assess cellular sensitivity to DNA damaging agents
To assess cell numbers after treatment with the genotoxic reagents, we measured the amount of ATP in the whole cell lysate [67].
Visualization of RAD51 foci
Cells were harvested at 3 hours after gamma irradiation. Cells were spun onto slides using a Shandon Cytospin 3 centrifuge (Shandon, Pittsburgh, Pa.). Staining and visualization of RAD51 foci were carried out as previously described [34] using rabbit polyclonal antibody, which recognizes human RAD51, at a dilution of 1∶500 (Calbiochem, San Diego, CA San Diego, CA), and Alexa Fluor 488 goat anti-human IgG antibody at a dilution of 1∶1000 (Molecular Probes Inc., Eugene, OR [34]).
Analysis of chromosomal aberrations
Measurement of chromosomal aberrations was performed as described previously [68].
Measurement of HR frequencies for I-Sce1–induced DSB repair
Measurement of recombination frequencies for I-SceI-induced DSB repair was performed as described previously [34], [44]. Modified SCneo was inserted into the previously described OVALBUMIN gene construct and targeted into the OVALBUMIN locus in wild-type, xrcc3, brca1, brca2, and brca2tr DT40 clones. For transient transfections, 1×107 cells were suspended in 0.5 ml of phosphate-buffered saline, mixed with 30 µg of I-SceI expression vector (pCBASce) or pBluescript KS without linearization, and electroporated at 250 V, 960 microfarads. At 24 hours after electroporation, the cells were plated in 96-well plates with or without 2.0 mg/ml neomycin analog (G418). The cells were grown for 7 to 10 days, after which formed colonies were counted. HR frequency was calculated by dividing the number of neomycin-resistant colonies by the number of plated cells.
Statistical analysis
Survival data were log-transformed giving approximate normality. Analysis of covariance (ANCOVA) was used to test for differences in the linear dose-response curves between wild-type and a series of mutant cells or brca2-null cells and a series of double-knockout mutant cells. Viability of the DT40 cells was estimated using regressing curves. Regression-curve equations were used to calculate LC50 (50% lethal concentration) values. Relative LC50 values were normalized according to the LC50 value of the parental wild-type cells.
Supporting Information
Generation of BRCA2−/− (ver.2) cells. (A) Experimental methods to generate BRCA2−/− (ver.2) cells. (B) Schematic representation of the BRCA2 conditional-null allele-2. The conditional-null allele-2 (con2) was generated by targeting the his selection-marker gene flanked by two loxP signals (ploxP-his) in intron 12 of the BRCA2 conditional allele 1. Treatment of the BRCA2−/con2 cells causes deletion from the promoter to exon 11, encoding all BRC motifs. The relevant XhoI sites in the conditional-null allele-1 and the position of the probe used in the Southern-blot analysis are indicated. The solid boxes and arrowheads represent the exons and loxP signals, respectively.
(EPS)
Proliferation and spontaneous chromosomal aberrations of brca2/rad52/xrcc3 triple knockout cells. (A) Growth kinetics of the indicated cell cultures in the absence (right) and presence (left) of TAM. Each value represents the averaged results from two separate clones. (B) Cell viability was assessed by flow cytometric analysis of PI uptake and forward scatter (FSC) representing the cell size after tamoxifen (TAM) treatment 4 days. A fixed number of plastic beads was added before flow cytometric analysis to calibrate cell number. Cells falling in the R1 and R2 gates identify dead and viable cells, respectively, and numbers given show their percentages. (C) Spontaneous chromosomal aberrations in cells with indicated genotype after tamoxifen (TAM) treatment 6 days. The data shown in the histogram indicate the types and numbers of chromosomal breaks in 50 analyzed mitotic cells. Two breaks at the same site of both sister chromatids are defined as isochromatid breaks, while breaks at either sister chromatid are chromatid breaks.
(EPS)
Supplementary materials and methods.
(DOC)
Acknowledgments
We thank Drs. E. Sonoda and T. Kawamoto for their technical assistance.
Footnotes
The authors have declared that no competing interests exist.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas and a Grant-in-Aid from the Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, and Science of Japan and by grants from The Uehara Memorial Foundation and The Naito Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sonoda E, Sasaki MS, Buerstedde JM, Bezzubova O, Shinohara A, et al. Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J. 1998;17:598–608. doi: 10.1093/emboj/17.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamazoe M, Sonoda E, Hochegger H, Takeda S. Reverse genetic studies of the DNA damage response in the chicken B lymphocyte line DT40. DNA Repair (Amst) 2004;3:1175–1185. doi: 10.1016/j.dnarep.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Venkitaraman AR. Linking the cellular functions of BRCA genes to cancer pathogenesis and treatment. Annu Rev Pathol. 2009;4:461–487. doi: 10.1146/annurev.pathol.3.121806.151422. [DOI] [PubMed] [Google Scholar]
- 4.Branzei D, Vanoli F, Foiani M. SUMOylation regulates Rad18-mediated template switch. Nature. 2008;456:915–920. doi: 10.1038/nature07587. [DOI] [PubMed] [Google Scholar]
- 5.Caldecott KW. Single-strand break repair and genetic disease. Nat Rev Genet. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 6.Ashworth A. Drug resistance caused by reversion mutation. Cancer Res. 2008;68:10021–10023. doi: 10.1158/0008-5472.CAN-08-2287. [DOI] [PubMed] [Google Scholar]
- 7.Takata M, Sasaki MS, Sonoda E, Morrison C, Hashimoto M, et al. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J. 1998;17:5497–5508. doi: 10.1093/emboj/17.18.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nojima K, Hochegger H, Saberi A, Fukushima T, Kikuchi K, et al. Multiple repair pathways mediate tolerance to chemotherapeutic cross-linking agents in vertebrate cells. Cancer Res. 2005;65:11704–11711. doi: 10.1158/0008-5472.CAN-05-1214. [DOI] [PubMed] [Google Scholar]
- 9.Adachi N, So S, Koyama H. Loss of nonhomologous end joining confers camptothecin resistance in DT40 cells. Implications for the repair of topoisomerase I-mediated DNA damage. J Biol Chem. 2004;279:37343–37348. doi: 10.1074/jbc.M313910200. [DOI] [PubMed] [Google Scholar]
- 10.Paques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda S, Nakamura K, Taniguchi Y, Paull TT. Ctp1/CtIP and the MRN complex collaborate in the initial steps of homologous recombination. Mol Cell. 2007;28:351–352. doi: 10.1016/j.molcel.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Sartori AA, Lukas C, Coates J, Mistrik M, Fu S, et al. Human CtIP promotes DNA end resection. Nature. 2007;450:509–514. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernstein KA, Rothstein R. At loose ends: resecting a double-strand break. Cell. 2009;137:807–810. doi: 10.1016/j.cell.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yun MH, Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009;459:460–463. doi: 10.1038/nature07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura K, Kogame T, Oshiumi H, Shinohara A, Sumitomo Y, et al. Collaborative action of Brca1 and CtIP in elimination of covalent modifications from double-strand breaks to facilitate subsequent break repair. PLoS Genet. 2010;6:e1000828. doi: 10.1371/journal.pgen.1000828. doi: 10.1371/journal.pgen.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nimonkar AV, Genschel J, Kinoshita E, Polaczek P, Campbell JL, et al. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 2011;25:350–362. doi: 10.1101/gad.2003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mimitou EP, Symington LS. DNA end resection–unraveling the tail. DNA Repair (Amst) 2011;10:344–348. doi: 10.1016/j.dnarep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawamoto T, Araki K, Sonoda E, Yamashita YM, Harada K, et al. Dual roles for DNA polymerase eta in homologous DNA recombination and translesion DNA synthesis. Mol Cell. 2005;20:793–799. doi: 10.1016/j.molcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 19.McIlwraith MJ, Vaisman A, Liu Y, Fanning E, Woodgate R, et al. Human DNA polymerase eta promotes DNA synthesis from strand invasion intermediates of homologous recombination. Mol Cell. 2005;20:783–792. doi: 10.1016/j.molcel.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Wooster R, Bignell G, Lancaster J, Swift S, Seal S, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 21.Miki Y, Katagiri T, Kasumi F, Yoshimoto T, Nakamura Y. Mutation analysis in the BRCA2 gene in primary breast cancers. Nat Genet. 1996;13:245–247. doi: 10.1038/ng0696-245. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 23.Bishop DK, Ear U, Bhattacharyya A, Calderone C, Beckett M, et al. Xrcc3 is required for assembly of Rad51 complexes in vivo. J Biol Chem. 1998;273:21482–21488. doi: 10.1074/jbc.273.34.21482. [DOI] [PubMed] [Google Scholar]
- 24.Fujimori A, Tachiiri S, Sonoda E, Thompson LH, Dhar PK, et al. Rad52 partially substitutes for the Rad51 paralog XRCC3 in maintaining chromosomal integrity in vertebrate cells. EMBO J. 2001;20:5513–5520. doi: 10.1093/emboj/20.19.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akamatsu Y, Tsutsui Y, Morishita T, Siddique MS, Kurokawa Y, et al. Fission yeast Swi5/Sfr1 and Rhp55/Rhp57 differentially regulate Rhp51-dependent recombination outcomes. EMBO J. 2007;26:1352–1362. doi: 10.1038/sj.emboj.7601582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin V, Chahwan C, Gao H, Blais V, Wohlschlegel J, et al. Sws1 is a conserved regulator of homologous recombination in eukaryotic cells. EMBO J. 2006;25:2564–2574. doi: 10.1038/sj.emboj.7601141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, et al. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell. 2006;22:719–729. doi: 10.1016/j.molcel.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Thorslund T, West SC. BRCA2: a universal recombinase regulator. Oncogene. 2007;26:7720–7730. doi: 10.1038/sj.onc.1210870. [DOI] [PubMed] [Google Scholar]
- 29.Forget AL, Kowalczykowski SC. Single-molecule imaging brings Rad51 nucleoprotein filaments into focus. Trends Cell Biol. 2010;20:269–276. doi: 10.1016/j.tcb.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shivji MK, Davies OR, Savill JM, Bates DL, Pellegrini L, et al. A region of human BRCA2 containing multiple BRC repeats promotes RAD51-mediated strand exchange. Nucleic Acids Res. 2006;34:4000–4011. doi: 10.1093/nar/gkl505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carreira A, Hilario J, Amitani I, Baskin RJ, Shivji MK, et al. The BRC repeats of BRCA2 modulate the DNA-binding selectivity of RAD51. Cell. 2009;136:1032–1043. doi: 10.1016/j.cell.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connor F, Bertwistle D, Mee PJ, Ross GM, Swift S, et al. Tumorigenesis and a DNA repair defect in mice with a truncating Brca2 mutation. Nat Genet. 1997;17:423–430. doi: 10.1038/ng1297-423. [DOI] [PubMed] [Google Scholar]
- 33.Hatanaka A, Yamazoe M, Sale JE, Takata M, Yamamoto K, et al. Similar effects of Brca2 truncation and Rad51 paralog deficiency on immunoglobulin V gene diversification in DT40 cells support an early role for Rad51 paralogs in homologous recombination. Mol Cell Biol. 2005;25:1124–1134. doi: 10.1128/MCB.25.3.1124-1134.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao GY, Sonoda E, Barber LJ, Oka H, Murakawa Y, et al. A critical role for the ubiquitin-conjugating enzyme Ubc13 in initiating homologous recombination. Mol Cell. 2007;25:663–675. doi: 10.1016/j.molcel.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 35.Takata M, Sasaki MS, Sonoda E, Fukushima T, Morrison C, et al. The Rad51 paralog Rad51B promotes homologous recombinational repair. Mol Cell Biol. 2000;20:6476–6482. doi: 10.1128/mcb.20.17.6476-6482.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yonetani Y, Hochegger H, Sonoda E, Shinya S, Yoshikawa H, et al. Differential and collaborative actions of Rad51 paralog proteins in cellular response to DNA damage. Nucleic Acids Res. 2005;33:4544–4552. doi: 10.1093/nar/gki766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murayama Y, Kurokawa Y, Mayanagi K, Iwasaki H. Formation and branch migration of Holliday junctions mediated by eukaryotic recombinases. Nature. 2008;451:1018–1021. doi: 10.1038/nature06609. [DOI] [PubMed] [Google Scholar]
- 38.Kurokawa Y, Murayama Y, Haruta-Takahashi N, Urabe I, Iwasaki H. Reconstitution of DNA strand exchange mediated by Rhp51 recombinase and two mediators. PLoS Biol. 2008;6:e88. doi: 10.1371/journal.pbio.0060088. doi: 10.1371/journal.pbio.0060088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akamatsu Y, Jasin M. Role for the mammalian Swi5-Sfr1 complex in DNA strand break repair through homologous recombination. PLoS Genet. 2010;6:e1001160. doi: 10.1371/journal.pgen.1001160. doi: 10.1371/journal.pgen.1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Doty T, Gibson B, Heyer WD. Human BRCA2 protein promotes RAD51 filament formation on RPA-covered single-stranded DNA. Nat Struct Mol Biol. 2010;17:1260–1262. doi: 10.1038/nsmb.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorslund T, McIlwraith MJ, Compton SA, Lekomtsev S, Petronczki M, et al. The breast cancer tumor suppressor BRCA2 promotes the specific targeting of RAD51 to single-stranded DNA. Nat Struct Mol Biol. 2010;17:1263–1265. doi: 10.1038/nsmb.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen RB, Carreira A, Kowalczykowski SC. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature. 2010;467:678–683. doi: 10.1038/nature09399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buerstedde JM, Takeda S. Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell. 1991;67:179–188. doi: 10.1016/0092-8674(91)90581-i. [DOI] [PubMed] [Google Scholar]
- 44.Fukushima T, Takata M, Morrison C, Araki R, Fujimori A, et al. Genetic analysis of the DNA-dependent protein kinase reveals an inhibitory role of Ku in late S-G2 phase DNA double-strand break repair. J Biol Chem. 2001;276:44413–44418. doi: 10.1074/jbc.M106295200. [DOI] [PubMed] [Google Scholar]
- 45.Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–272. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 46.Martin RW, Orelli BJ, Yamazoe M, Minn AJ, Takeda S, et al. RAD51 up-regulation bypasses BRCA1 function and is a common feature of BRCA1-deficient breast tumors. Cancer Res. 2007;67:9658–9665. doi: 10.1158/0008-5472.CAN-07-0290. [DOI] [PubMed] [Google Scholar]
- 47.Mazin AV, Mazina OM, Bugreev DV, Rossi MJ. Rad54, the motor of homologous recombination. DNA Repair (Amst) 2010;9:286–302. doi: 10.1016/j.dnarep.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wechsler T, Newman S, West SC. Aberrant chromosome morphology in human cells defective for Holliday junction resolution. Nature. 2011;471:642–646. doi: 10.1038/nature09790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng Z, Scott SP, Bussen W, Sharma GG, Guo G, et al. Rad52 inactivation is synthetically lethal with BRCA2 deficiency. Proc Natl Acad Sci U S A. 2011;108:686–691. doi: 10.1073/pnas.1010959107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kojic M, Kostrub CF, Buchman AR, Holloman WK. BRCA2 homolog required for proficiency in DNA repair, recombination, and genome stability in Ustilago maydis. Mol Cell. 2002;10:683–691. doi: 10.1016/s1097-2765(02)00632-9. [DOI] [PubMed] [Google Scholar]
- 51.Siaud N, Dray E, Gy I, Gerard E, Takvorian N, et al. Brca2 is involved in meiosis in Arabidopsis thaliana as suggested by its interaction with Dmc1. EMBO J. 2004;23:1392–1401. doi: 10.1038/sj.emboj.7600146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lim DS, Hasty P. A mutation in mouse rad51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol Cell Biol. 1996;16:7133–7143. doi: 10.1128/mcb.16.12.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ludwig T, Chapman DL, Papaioannou VE, Efstratiadis A. Targeted mutations of breast cancer susceptibility gene homologs in mice: lethal phenotypes of Brca1, Brca2, Brca1/Brca2, Brca1/p53, and Brca2/p53 nullizygous embryos. Genes Dev. 1997;11:1226–1241. doi: 10.1101/gad.11.10.1226. [DOI] [PubMed] [Google Scholar]
- 54.Ludwig T, Fisher P, Murty V, Efstratiadis A. Development of mammary adenocarcinomas by tissue-specific knockout of Brca2 in mice. Oncogene. 2001;20:3937–3948. doi: 10.1038/sj.onc.1204512. [DOI] [PubMed] [Google Scholar]
- 55.Cheung AM, Elia A, Tsao MS, Done S, Wagner KU, et al. Brca2 deficiency does not impair mammary epithelium development but promotes mammary adenocarcinoma formation in p53(+/-) mutant mice. Cancer Res. 2004;64:1959–1965. doi: 10.1158/0008-5472.can-03-2270. [DOI] [PubMed] [Google Scholar]
- 56.D'Andrea AD. Susceptibility pathways in Fanconi's anemia and breast cancer. N Engl J Med. 2010;362:1909–1919. doi: 10.1056/NEJMra0809889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huen MS, Sy SM, Chen J. BRCA1 and its toolbox for the maintenance of genome integrity. Nat Rev Mol Cell Biol. 2010;11:138–148. doi: 10.1038/nrm2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sy SM, Huen MS, Chen J. PALB2 is an integral component of the BRCA complex required for homologous recombination repair. Proc Natl Acad Sci U S A. 2009;106:7155–7160. doi: 10.1073/pnas.0811159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bunting SF, Callen E, Wong N, Chen HT, Polato F, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spies M, Kowalczykowski SC. The RecA binding locus of RecBCD is a general domain for recruitment of DNA strand exchange proteins. Mol Cell. 2006;21:573–580. doi: 10.1016/j.molcel.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Yang H, Li Q, Fan J, Holloman WK, Pavletich NP. The BRCA2 homologue Brh2 nucleates RAD51 filament formation at a dsDNA-ssDNA junction. Nature. 2005;433:653–657. doi: 10.1038/nature03234. [DOI] [PubMed] [Google Scholar]
- 62.Kohzaki M, Nishihara K, Hirota K, Sonoda E, Yoshimura M, et al. DNA polymerases nu and theta are required for efficient immunoglobulin V gene diversification in chicken. J Cell Biol. 2010;189:1117–1127. doi: 10.1083/jcb.200912012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takao N, Kato H, Mori R, Morrison C, Sonada E, et al. Disruption of ATM in p53-null cells causes multiple functional abnormalities in cellular response to ionizing radiation. Oncogene. 1999;18:7002–7009. doi: 10.1038/sj.onc.1203172. [DOI] [PubMed] [Google Scholar]
- 64.Yoshimura M, Kohzaki M, Nakamura J, Asagoshi K, Sonoda E, et al. Vertebrate POLQ and POLbeta cooperate in base excision repair of oxidative DNA damage. Mol Cell. 2006;24:115–125. doi: 10.1016/j.molcel.2006.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iiizumi S, Nomura Y, So S, Uegaki K, Aoki K, et al. Simple one-week method to construct gene-targeting vectors: application to production of human knockout cell lines. Biotechniques. 2006;41:311–316. doi: 10.2144/000112233. [DOI] [PubMed] [Google Scholar]
- 66.Takata M, Tachiiri S, Fujimori A, Thompson LH, Miki Y, et al. Conserved domains in the chicken homologue of BRCA2. Oncogene. 2002;21:1130–1134. doi: 10.1038/sj.onc.1205168. [DOI] [PubMed] [Google Scholar]
- 67.Ji K, Kogame T, Choi K, Wang X, Lee J, et al. A novel approach using DNA-repair-deficient chicken DT40 cell lines for screening and characterizing the genotoxicity of environmental contaminants. Environ Health Perspect. 2009;117:1737–1744. doi: 10.1289/ehp.0900842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sonoda E, Okada T, Zhao GY, Tateishi S, Araki K, et al. Multiple roles of Rev3, the catalytic subunit of polzeta in maintaining genome stability in vertebrates. EMBO J. 2003;22:3188–3197. doi: 10.1093/emboj/cdg308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamaguchi-Iwai Y, Sonoda E, Buerstedde JM, Bezzubova O, Morrison C, et al. Homologous recombination, but not DNA repair, is reduced in vertebrate cells deficient in RAD52. Mol Cell Biol. 1998;18:6430–6435. doi: 10.1128/mcb.18.11.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bezzubova O, Silbergleit A, Yamaguchi-Iwai Y, Takeda S, Buerstedde JM. Reduced X-ray resistance and homologous recombination frequencies in a RAD54-/- mutant of the chicken DT40 cell line. Cell. 1997;89:185–193. doi: 10.1016/s0092-8674(00)80198-1. [DOI] [PubMed] [Google Scholar]
- 71.Takata M, Sasaki MS, Tachiiri S, Fukushima T, Sonoda E, et al. Chromosome instability and defective recombinational repair in knockout mutants of the five Rad51 paralogs. Mol Cell Biol. 2001;21:2858–2866. doi: 10.1128/MCB.21.8.2858-2866.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generation of BRCA2−/− (ver.2) cells. (A) Experimental methods to generate BRCA2−/− (ver.2) cells. (B) Schematic representation of the BRCA2 conditional-null allele-2. The conditional-null allele-2 (con2) was generated by targeting the his selection-marker gene flanked by two loxP signals (ploxP-his) in intron 12 of the BRCA2 conditional allele 1. Treatment of the BRCA2−/con2 cells causes deletion from the promoter to exon 11, encoding all BRC motifs. The relevant XhoI sites in the conditional-null allele-1 and the position of the probe used in the Southern-blot analysis are indicated. The solid boxes and arrowheads represent the exons and loxP signals, respectively.
(EPS)
Proliferation and spontaneous chromosomal aberrations of brca2/rad52/xrcc3 triple knockout cells. (A) Growth kinetics of the indicated cell cultures in the absence (right) and presence (left) of TAM. Each value represents the averaged results from two separate clones. (B) Cell viability was assessed by flow cytometric analysis of PI uptake and forward scatter (FSC) representing the cell size after tamoxifen (TAM) treatment 4 days. A fixed number of plastic beads was added before flow cytometric analysis to calibrate cell number. Cells falling in the R1 and R2 gates identify dead and viable cells, respectively, and numbers given show their percentages. (C) Spontaneous chromosomal aberrations in cells with indicated genotype after tamoxifen (TAM) treatment 6 days. The data shown in the histogram indicate the types and numbers of chromosomal breaks in 50 analyzed mitotic cells. Two breaks at the same site of both sister chromatids are defined as isochromatid breaks, while breaks at either sister chromatid are chromatid breaks.
(EPS)
Supplementary materials and methods.
(DOC)