Abstract
The Bordetella pertussis cyaA gene encodes a virulence factor which is a bifunctional protein exhibiting calmodulin-sensitive adenylate cyclase and hemolytic activities (P. Glaser, H. Sakamoto, J. Bellahov, A. Ullmann, and A. Danchin, EMBO J. 7:3997-4004, 1988). We characterized the hemolytic and toxin activities of the 200-kilodalton (kDa) bifunctional (CyaA) protein and showed that, whether cell associated or secreted, the 200-kDa CyaA protein carries hemolytic and toxin functions. The catalytically active 45-kDa form of adenylate cyclase released by proteolytic digestion of the 200-kDa CyaA protein displayed neither hemolytic nor toxin activities. We constructed in-phase deletions in the 3' region of the cyaA gene, which presumably carries the hemolytic determinant, and showed that the resulting proteins exhibited wild-type adenylate cyclase activity and were secreted without processing into culture supernatants. The hemolytic activities of these mutant CyaA proteins were severely reduced, and their toxin activities were abolished. These results suggest that the structural integrity of the 200-kDa CyaA protein is necessary for toxin activity and that distinct structural determinants within the CyaA protein are involved in secretion, pore formation, and entry into target cells.
Full text
PDF
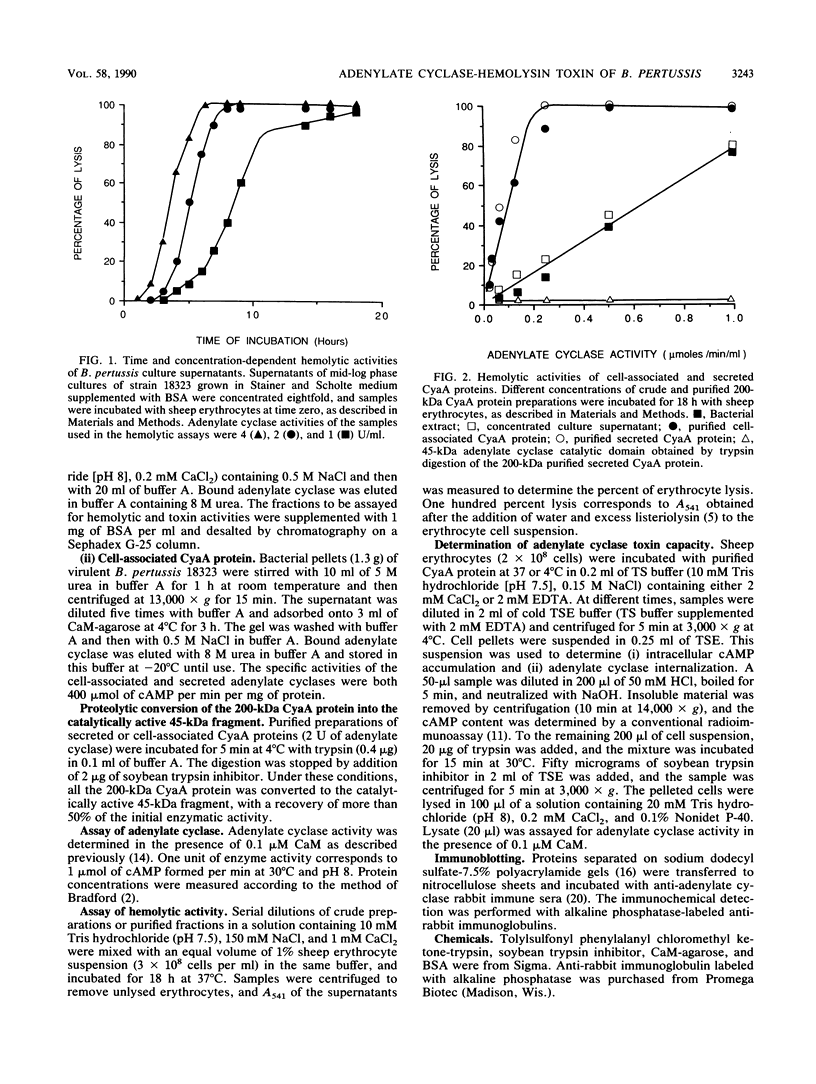
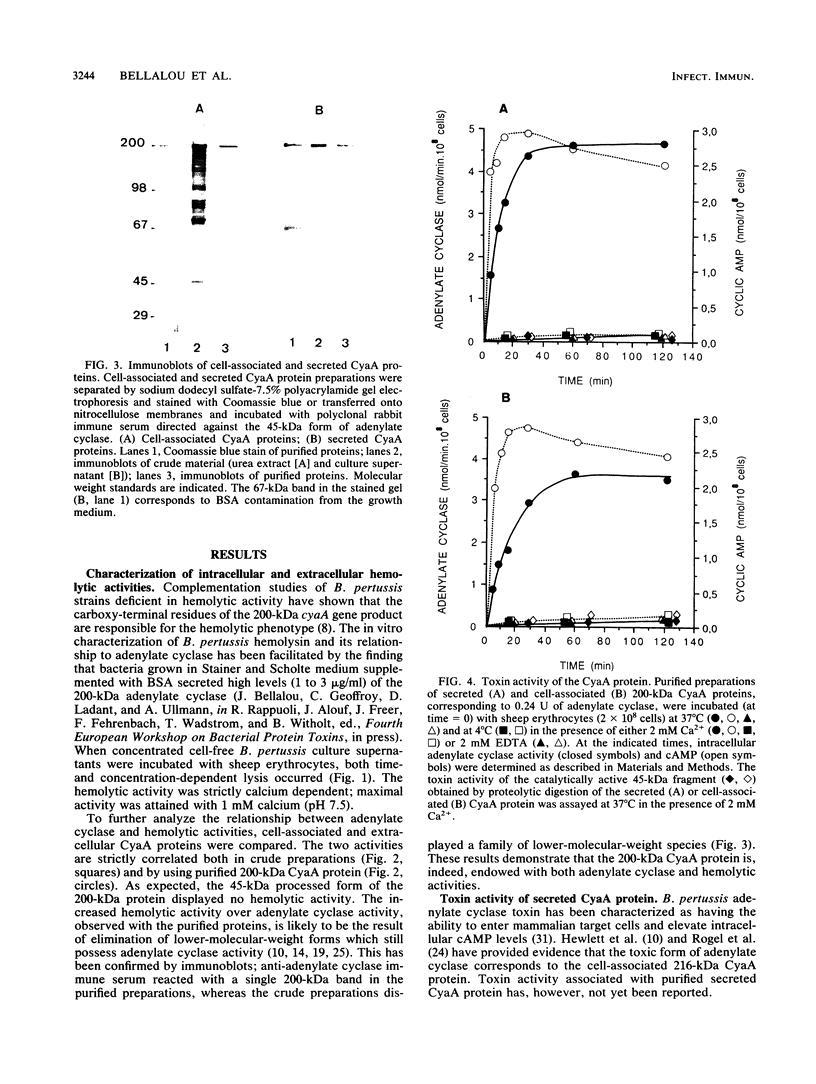



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellalou J., Ladant D., Sakamoto H. Synthesis and secretion of Bordetella pertussis adenylate cyclase as a 200-kilodalton protein. Infect Immun. 1990 May;58(5):1195–1200. doi: 10.1128/iai.58.5.1195-1200.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Donovan M. G., Masure H. R., Storm D. R. Isolation of a protein fraction from Bordetella pertussis that facilitates entry of the calmodulin-sensitive adenylate cyclase into animal cells. Biochemistry. 1989 Oct 3;28(20):8124–8129. doi: 10.1021/bi00446a024. [DOI] [PubMed] [Google Scholar]
- Frey J., Nicolet J. Regulation of hemolysin expression in Actinobacillus pleuropneumoniae serotype 1 by Ca2+. Infect Immun. 1988 Oct;56(10):2570–2575. doi: 10.1128/iai.56.10.2570-2575.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy C., Gaillard J. L., Alouf J. E., Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987 Jul;55(7):1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Elmaoglou-Lazaridou A., Krin E., Ladant D., Bârzu O., Danchin A. Identification of residues essential for catalysis and binding of calmodulin in Bordetella pertussis adenylate cyclase by site-directed mutagenesis. EMBO J. 1989 Mar;8(3):967–972. doi: 10.1002/j.1460-2075.1989.tb03459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988 Dec 1;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Farfel Z. Bordetella pertussis invasive adenylate cyclase. Partial resolution and properties of its cellular penetration. J Biol Chem. 1985 May 10;260(9):5526–5532. [PubMed] [Google Scholar]
- Hewlett E. L., Gordon V. M., McCaffery J. D., Sutherland W. M., Gray M. C. Adenylate cyclase toxin from Bordetella pertussis. Identification and purification of the holotoxin molecule. J Biol Chem. 1989 Nov 15;264(32):19379–19384. [PubMed] [Google Scholar]
- Joseph E., Bernsley C., Guiso N., Ullmann A. Multiple regulation of the activity of adenylate cyclase in Escherichia coli. Mol Gen Genet. 1982;185(2):262–268. doi: 10.1007/BF00330796. [DOI] [PubMed] [Google Scholar]
- Kessin R. H., Franke J. Secreted adenylate cyclase of Bordetella pertussis: calmodulin requirements and partial purification of two forms. J Bacteriol. 1986 Apr;166(1):290–296. doi: 10.1128/jb.166.1.290-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V., Cross M., Senior B., Koronakis E., Hughes C. The secreted hemolysins of Proteus mirabilis, Proteus vulgaris, and Morganella morganii are genetically related to each other and to the alpha-hemolysin of Escherichia coli. J Bacteriol. 1987 Apr;169(4):1509–1515. doi: 10.1128/jb.169.4.1509-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladant D., Brezin C., Alonso J. M., Crenon I., Guiso N. Bordetella pertussis adenylate cyclase. Purification, characterization, and radioimmunoassay. J Biol Chem. 1986 Dec 5;261(34):16264–16269. [PubMed] [Google Scholar]
- Ladant D. Interaction of Bordetella pertussis adenylate cyclase with calmodulin. Identification of two separated calmodulin-binding domains. J Biol Chem. 1988 Feb 25;263(6):2612–2618. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalonde G., McDonald T. V., Gardner P., O'Hanley P. D. Identification of a hemolysin from Actinobacillus pleuropneumoniae and characterization of its channel properties in planar phospholipid bilayers. J Biol Chem. 1989 Aug 15;264(23):13559–13564. [PubMed] [Google Scholar]
- Ludwig A., Jarchau T., Benz R., Goebel W. The repeat domain of Escherichia coli haemolysin (HlyA) is responsible for its Ca2+-dependent binding to erythrocytes. Mol Gen Genet. 1988 Nov;214(3):553–561. doi: 10.1007/BF00330494. [DOI] [PubMed] [Google Scholar]
- Masure H. R., Storm D. R. Characterization of the bacterial cell associated calmodulin-sensitive adenylate cyclase from Bordetella pertussis. Biochemistry. 1989 Jan 24;28(2):438–442. doi: 10.1021/bi00428a005. [DOI] [PubMed] [Google Scholar]
- Monneron A., Ladant D., d'Alayer J., Bellalou J., Bârzu O., Ullmann A. Immunological relatedness between Bordetella pertussis and rat brain adenylyl cyclases. Biochemistry. 1988 Jan 26;27(2):536–539. doi: 10.1021/bi00402a005. [DOI] [PubMed] [Google Scholar]
- Nicaud J. M., Mackman N., Gray L., Holland I. B. Characterisation of HlyC and mechanism of activation and secretion of haemolysin from E. coli 2001. FEBS Lett. 1985 Aug 5;187(2):339–344. doi: 10.1016/0014-5793(85)81272-2. [DOI] [PubMed] [Google Scholar]
- Rogel A., Farfel Z., Goldschmidt S., Shiloach J., Hanski E. Bordetella pertussis adenylate cyclase. Identification of multiple forms of the enzyme by antibodies. J Biol Chem. 1988 Sep 15;263(26):13310–13316. [PubMed] [Google Scholar]
- Rogel A., Schultz J. E., Brownlie R. M., Coote J. G., Parton R., Hanski E. Bordetella pertussis adenylate cyclase: purification and characterization of the toxic form of the enzyme. EMBO J. 1989 Sep;8(9):2755–2760. doi: 10.1002/j.1460-2075.1989.tb08417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiebel E., Braun V. Integration of the Serratia marcescens haemolysin into human erythrocyte membranes. Mol Microbiol. 1989 Mar;3(3):445–453. doi: 10.1111/j.1365-2958.1989.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Shattuck R. L., Oldenburg D. J., Storm D. R. Purification and characterization of a calmodulin-sensitive adenylate cyclase from Bordetella pertussis. Biochemistry. 1985 Nov 5;24(23):6356–6362. doi: 10.1021/bi00344a006. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Huggins M. B. The toxic role of alpha-haemolysin in the pathogenesis of experimental Escherichia coli infection in mice. J Gen Microbiol. 1985 Feb;131(2):395–403. doi: 10.1099/00221287-131-2-395. [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Black W., Falkow S. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene. 1986;50(1-3):133–140. doi: 10.1016/0378-1119(86)90318-5. [DOI] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989 Feb;171(2):916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Goodwin M. S. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect Immun. 1989 Dec;57(12):3757–3764. doi: 10.1128/iai.57.12.3757-3764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Zwadyk P., Jr, Snyder I. S. Purification and kinetic studies of the hemolysin from Escherichia coli. Can J Microbiol. 1971 Jun;17(6):741–745. doi: 10.1139/m71-118. [DOI] [PubMed] [Google Scholar]