Abstract
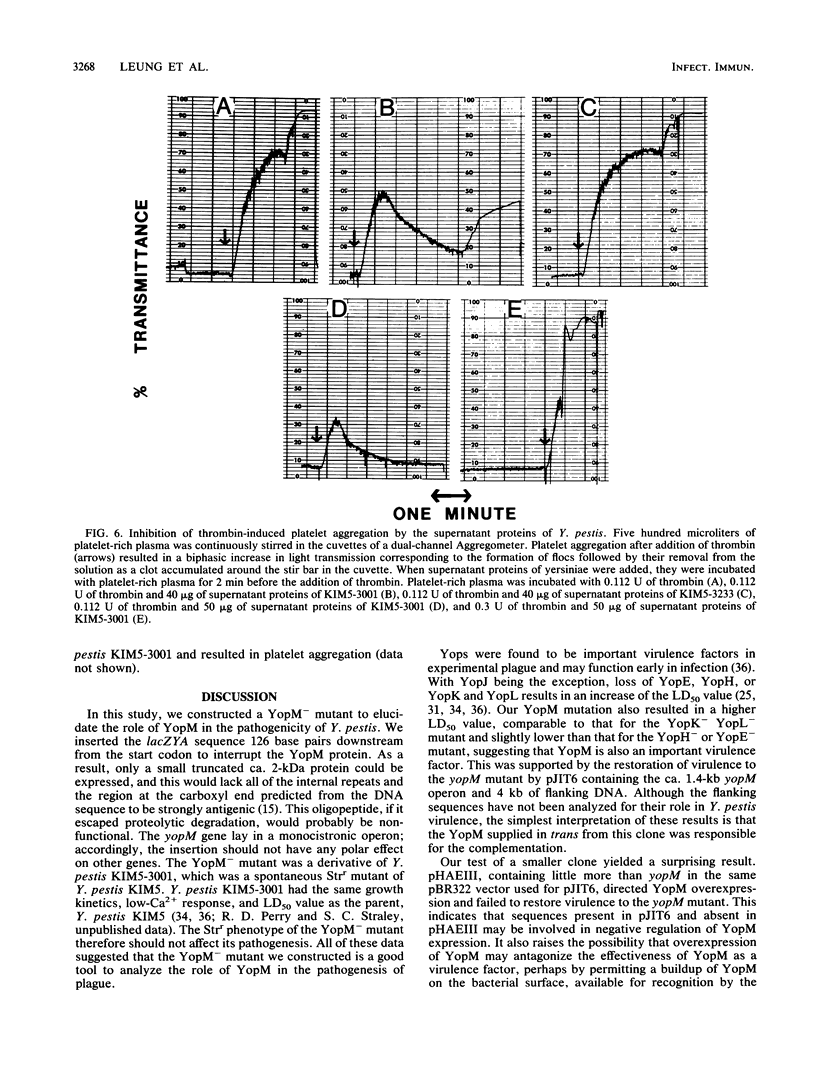
In Yersinia pestis KIM there are 11 Yops (yersinial outer membrane proteins) encoded by the low-Ca2+ response virulence plasmid pCD1. Only YopM and YopN are found in easily detectable amounts in the culture medium. In our previous work, we characterized the yopM gene. In the present study, we constructed a YopM- mutant to elucidate the role of YopM in the virulence of Y. pestis. A lacZYA sequence was inserted 126 base pairs downstream from the start codon of the yopM gene in pCD1. The YopM- mutant had the same growth properties as the parent, Y. pestis KIM5-3001. The inserted lacZ gene was regulated by the promoter of the yopM gene. Accordingly, it was expressed strongly at 37 degrees C in the absence of Ca2+ and was decreased in expression when Ca2+ was present. Northern blot (RNA blot) analysis revealed that the yopM gene was in a monocistronic operon, suggesting that the yopM insertion mutation was unlikely to have polar effects on other genes. The YopM- mutant had strongly decreased virulence in mice, with a 50% lethal dose of 3.4 x 10(5) CFU. Virulence was restored by the cloned yopM-containing 5.5-kilobase HindIII F fragment of pCD1. However, supplying a cloned 1.57-kilobase fragment containing little more than the yopM structural gene caused the yopM mutant to significantly overexpress YopM and failed to restore virulence. The infection kinetics of the YopM- mutant revealed growth in both spleens and livers from days 2 to 4 after infection, followed by a precipitous clearance of the bacteria. YopM-containing supernatant proteins of Y. pestis inhibited thrombin- or ristocetin-induced platelet aggregation, whereas there was no inhibition by supernatant proteins from the YopM- Y. pestis mutant. Accordingly, YopM may prevent platelet-mediated events and serve as an important strategy for the yersiniae in the initial stages of a plague infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhous K. M., Graham J. E., Cooper H. A., Allain J. P., Wagner R. H. Assay of von Willebrand factor in von Willebrand's disease and hemophilia: use of a macroscopic platelet aggregation test. Thromb Res. 1975 Mar;6(3):267–272. doi: 10.1016/0049-3848(75)90074-2. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Mutation rate to nonpigmentation in Pasteurella pestis. J Bacteriol. 1969 Jun;98(3):1404–1406. doi: 10.1128/jb.98.3.1404-1406.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R., Sample A. K., Yu D. Z., Zahorchak R. J., Hu P. C., Fowler J. M. Proteolysis of V antigen from Yersinia pestis. Microb Pathog. 1987 Jan;2(1):49–62. doi: 10.1016/0882-4010(87)90114-8. [DOI] [PubMed] [Google Scholar]
- Bölin I., Portnoy D. A., Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985 Apr;48(1):234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Wolf-Watz H. The plasmid-encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol Microbiol. 1988 Mar;2(2):237–245. doi: 10.1111/j.1365-2958.1988.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Caen J. P., Nurden A. T., Jeanneau C., Michel H., Tobelem G., Levy-Toledano S., Sultan Y., Valensi F., Bernard J. Bernard-Soulier syndrome: a new platelet glycoprotein abnormality. Its relationship with platelet adhesion to subendothelium and with the factor VIII von Willebrand protein. J Lab Clin Med. 1976 Apr;87(4):586–596. [PubMed] [Google Scholar]
- Coller B. S., Gralnick H. R. Studies on the mechanism of ristocetin-induced platelet agglutination. Effects of structural modification of ristocetin and vancomycin. J Clin Invest. 1977 Aug;60(2):302–312. doi: 10.1172/JCI108778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P. J., Chavin S. I., Schroeder D., Young F. E., Marder V. J. Purification and characterization of a highly purified human factor VIII consisting of a single type of polypeptide chain. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7200–7204. doi: 10.1073/pnas.79.23.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goguen J. D., Yother J., Straley S. C. Genetic analysis of the low calcium response in Yersinia pestis mu d1(Ap lac) insertion mutants. J Bacteriol. 1984 Dec;160(3):842–848. doi: 10.1128/jb.160.3.842-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung K. Y., Straley S. C. The yopM gene of Yersinia pestis encodes a released protein having homology with the human platelet surface protein GPIb alpha. J Bacteriol. 1989 Sep;171(9):4623–4632. doi: 10.1128/jb.171.9.4623-4632.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J. A., Chung D. W., Fujikawa K., Hagen F. S., Papayannopoulou T., Roth G. J. Cloning of the alpha chain of human platelet glycoprotein Ib: a transmembrane protein with homology to leucine-rich alpha 2-glycoprotein. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5615–5619. doi: 10.1073/pnas.84.16.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Perry R. D., Haddix P., Atkins E. B., Soughers T. K., Straley S. C. Regulation of expression of V antigen and outer membrane proteins in Yersinia pestis. Contrib Microbiol Immunol. 1987;9:173–178. [PubMed] [Google Scholar]
- Perry R. D., Harmon P. A., Bowmer W. S., Straley S. C. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect Immun. 1986 Nov;54(2):428–434. doi: 10.1128/iai.54.2.428-434.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Bölin I., Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988 Aug;56(8):2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., De Marco L., Gatti L., Bader R., Montgomery R. R. Platelets have more than one binding site for von Willebrand factor. J Clin Invest. 1983 Jul;72(1):1–12. doi: 10.1172/JCI110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample A. K., Brubaker R. R. Post-translational regulation of Lcr plasmid-mediated peptides in pesticinogenic Yersinia pestis. Microb Pathog. 1987 Oct;3(4):239–248. doi: 10.1016/0882-4010(87)90057-x. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Sodeinde O. A., Goguen J. D. Genetic analysis of the 9.5-kilobase virulence plasmid of Yersinia pestis. Infect Immun. 1988 Oct;56(10):2743–2748. doi: 10.1128/iai.56.10.2743-2748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeinde O. A., Sample A. K., Brubaker R. R., Goguen J. D. Plasminogen activator/coagulase gene of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect Immun. 1988 Oct;56(10):2749–2752. doi: 10.1128/iai.56.10.2749-2752.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Bowmer W. S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986 Feb;51(2):445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Brubaker R. R. Cytoplasmic and membrane proteins of yersiniae cultivated under conditions simulating mammalian intracellular environment. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1224–1228. doi: 10.1073/pnas.78.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Cibull M. L. Differential clearance and host-pathogen interactions of YopE- and YopK- YopL- Yersinia pestis in BALB/c mice. Infect Immun. 1989 Apr;57(4):1200–1210. doi: 10.1128/iai.57.4.1200-1210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Harmon P. A. Growth in mouse peritoneal macrophages of Yersinia pestis lacking established virulence determinants. Infect Immun. 1984 Sep;45(3):649–654. doi: 10.1128/iai.45.3.649-654.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C. The plasmid-encoded outer-membrane proteins of Yersinia pestis. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S323–S326. doi: 10.1093/cid/10.supplement_2.s323. [DOI] [PubMed] [Google Scholar]
- Titani K., Takio K., Handa M., Ruggeri Z. M. Amino acid sequence of the von Willebrand factor-binding domain of platelet membrane glycoprotein Ib. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5610–5614. doi: 10.1073/pnas.84.16.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T., Brubaker R. R. In vivo comparison of avirulent Vwa- and Pgm- or Pstr phenotypes of yersiniae. Infect Immun. 1984 Mar;43(3):895–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T., Nakajima R., Brubaker R. R. Roles of V antigen in promoting virulence in Yersiniae. Contrib Microbiol Immunol. 1987;9:179–185. [PubMed] [Google Scholar]
- Vicente V., Houghten R. A., Ruggeri Z. M. Identification of a site in the alpha chain of platelet glycoprotein Ib that participates in von Willebrand factor binding. J Biol Chem. 1990 Jan 5;265(1):274–280. [PubMed] [Google Scholar]
- Weiss H. J. Platelet physiology and abnormalities of platelet function (first of two parts). N Engl J Med. 1975 Sep 11;293(11):531–541. doi: 10.1056/NEJM197509112931105. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Tschopp T. B., Baumgartner H. R., Sussman I. I., Johnson M. M., Egan J. J. Decreased adhesion of giant (Bernard-Soulier) platelets to subendothelium. Further implications on the role of the von Willebrand factor in hemostasis. Am J Med. 1974 Dec;57(6):920–925. doi: 10.1016/0002-9343(74)90170-3. [DOI] [PubMed] [Google Scholar]
- Wicki A. N., Clemetson K. J. Structure and function of platelet membrane glycoproteins Ib and V. Effects of leukocyte elastase and other proteases on platelets response to von Willebrand factor and thrombin. Eur J Biochem. 1985 Nov 15;153(1):1–11. doi: 10.1111/j.1432-1033.1985.tb09259.x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]