Abstract
Puccinia striiformis f. sp. tritici (Pst) is an obligate biotrophic fungus that causes the destructive wheat stripe rust disease worldwide. Due to the lack of reliable transformation and gene disruption method, knowledge about the function of Pst genes involved in pathogenesis is limited. Mitogen-activated protein kinase (MAPK) genes have been shown in a number of plant pathogenic fungi to play critical roles in regulating various infection processes. In the present study, we identified and characterized the first MAPK gene PsMAPK1 in Pst. Phylogenetic analysis indicated that PsMAPK1 is a YERK1 MAP kinase belonging to the Fus3/Kss1 class. Single nucleotide polymerphisms (SNPs) and insertion/deletion were detected in the coding region of PsMAPK1 among six Pst isolates. Real-time RT-PCR analyses revealed that PsMAPK1 expression was induced at early infection stages and peaked during haustorium formation. When expressed in Fusarium graminearum, PsMAPK1 partially rescued the map1 mutant in vegetative growth and pathogenicity. It also partially complemented the defects of the Magnaporthe oryzae pmk1 mutant in appressorium formation and plant infection. These results suggest that F. graminearum and M. oryzae can be used as surrogate systems for functional analysis of well-conserved Pst genes and PsMAPK1 may play a role in the regulation of plant penetration and infectious growth in Pst.
Introduction
In a variety of eukaryotic organisms, a family of serine/threonine protein kinases known as the mitogen-activated protein kinases (MAPKs) play critical roles in the transduction of a variety of extracellular signals and regulation of various development and differentiation processes [1]. The MAPK cascades are conserved in eukaryotes and have been studied extensively in many organisms. In filamentous fungi, MAPKs mainly fall into three subgroups represented by Fus3/Kss1, Slt2, and Hog1 of Saccharomyces cerevisiae [2], [3], [4]. The Fus3/Kss1 homolog is more extensively studied than the other two MAPKs in fungal pathogens [1], [5], [6], [7]. In the rice blast fungus Magnaporthe oryzae, the PMK1 MAP kinase gene is essential for appressorium formation and invasive growth [8]. In the wheat scab fungus Fusarium graminearum, the map1 deletion mutants are female sterile, non-pathogenic, and reduced in conidiation and infectious growth [9], [10]. In Ustilago maydis, Kpp2 (Ubc3) and Kpp6 are two Fus3/Kss1 MAPKs with overlapping functions in mating and plant infection [11], [12]. The ubc3/kpp2 mutant is defective in pheromone responses and the formation of filamentous dikaryons and reduced in virulence. In contrast, Kpp6 plays a more critical role in appressorial penetration than Kpp2. The kpp6 mutant is reduced in virulence and defective in the penetration of plant cuticle [13]. The kpp2 kpp6 double mutants are abolished in mating and nonpathogenic on maize plants. The Fus3/Kss1 homologs also have been functionally characterized in several human pathogens. In Candida albicans, the Cek1 MAPK plays a critical role in pathogenesis [14]. In Cryptococcus neoformans, the CPK1 MAPK pathway is important for mating and haploid fruiting but dispensable for virulence [15].
Wheat stripe rust, caused by Puccinia striiformis f. sp. tritici (Pst), is one of the most important diseases of wheat worldwide. Pst is an obligate biotrophic fungus belonging to the Uredinales. The major phase of the stripe rust life cycle is urediniospores, which can germinate in water but germ tubes will die without host cells [16]. After successful adhesion to the wheat leaves, urediniospores produce germ tubes, which elongate along leaf veins until they encounter stomatal opening. After entering the substomatal space in wheat leaves, the fungus starts to successively differentiate other infection structures, e.g., substomatal vesicles, infection hypha, haustorial mother cell, and eventually haustoria, a structure to withdraw nutrients from host cells [17], [18], [19]. The majority of germ tubes penetrates stomata after 12 hours of germination, and formation of haustorial mother cells increases rapidly after 18 hours of inoculation [20]. During Pst infection, it is believed that the fungus recognizes various signals from the host plant at different stages and responds accordingly to establish a successful colonization. However, little is known about the role of signal transduction pathways in Pst and other rust fungi due to their obligate nature and the lack of an efficient and reliable transformation system. When expressed in Ustilago maydis, the PtMAPK1 MAPK gene of Puccinia triticina was able to partially complement the kpp2 kpp6 mutant for mating, virulence, and pathogenicity [21].
In a previous study [22], a MAPK gene, designated PsMAPK1, was identified in ESTs generated from a Pst cDNA library. Here we examined the expression profiles of PsMAPK1 and its activities in two ascomycetous pathogens. PsMAPK1 could partially complement the F. graminearum map1 and M. oryzae pmk1 mutants. Results from these studies indicate that F. graminearum or M. oryzae can be used as a heterologous expression system for functional studies with Pst genes and PsMAPK1 may play an important role in regulating penetration and infectious growth of the wheat stripe rust fungus.
Results
PsMAPK1 encodes a Fus3/Kss1 type MAP kinase
One of the expressed sequence tags (ESTs), 20C8, from a full-length cDNA library of Pst [22] is highly similar to PgMAPK from P. graminis f. sp. tritici (GenBank accession number EFP88010) and PtMAPK1 from P. triticina (GenBank accession number AAY89655). By further sequencing analysis with clone 20C8, we designed primers and obtained the full-length cDNA of this MAPK gene from Chinese Pst race CYR32, which was designated PsMAPK1 in this study (GenBank accession number HM535614). The open reading frame (ORF) of PsMAPK1 was predicted to encode a 408-amino acid protein with typical features of MAP kinases, including 11 protein kinase domains and the TEY dual phosphorylation site (Fig. S1).
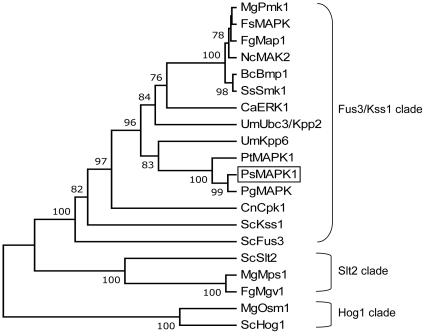
The level of conservation among the PsMAPK1 protein, PgMAPK from P. graminis f. sp. tricitici, PtMAPK1 from P. triticina, Pmk1 from M. oryzae, and F. graminearum Map1 is indicated in Figure S1. PsMAPK1 had the highest homology with PgMAPK (91% identity), followed by PtMAPK1 (87% identity), M. oryzae Pmk1 (75% identity) and F. graminearum Map1 (75% identity). Phylogenetic analysis revealed that PsMAPK1 is more closely related to M. oryzae Pmk1 and F. graminearum Map1 than to MAPKs belonging to the Slt2 and Hog1 (Fig. 1). In the Fus3/Kss1 clade, PsMAPK1 is more similar to MAPKs from basidiomycetous fungi than to those from ascomycetous fungi. Grouping of PsMAPK1 in the Fus3/Kss1 clade implies that it may play a role in mating, morphogenesis, or pathogenic development in Pst based on the functions of its orthologs in other plant pathogenic fungi [1], [8], [9], [10], [13].
Figure 1. Phylogenetic analysis with PsMAPK1 and selected fungal MAP kinases (GenBank accession numbers in parenthesis).
Botrytis cinerea, BcBmp1 (AAG23132); Candida albicans, CaERK1 (P28869); Cryptococcus neoformans, CnCpk1 (Q8NK05); Fusarium solani, FsMAPK (AAB72017); Fusarium graminearum, FgMap1 (AAL73403) and FgMgv1 (AAM13670); Magnaporthe oryzae, MgPmk1 (AAC49521), MgMps1 (AAC63682) and MgOsm1 (AAF09475); Neurospora crassa, NcMAK2 (AAK25816); Puccinia striiformis f. sp. tritici, PsMAPK1 (HM535614); Puccinia triticina, PtMAPK1 (AAY89655); Puccinia graminis f. sp. tritici, PgMAPK (EFP88010); Saccharomyces cerevisiae, ScFus3 (CAA49292), ScHog1 (CAA97680), ScKss1 (CAA97038) and ScSlt2 (CAA41954); Sclerotinia sclerotiorum, SsSmk1 (AAQ54908); Ustilago maydis, UmKpp6 (CAD43731) and UmUbc3/Kpp2 (AAF09452). The unrooted phylogram was constructed based on NJ analysis. Confidence of groupings was estimated by using 1,000 bootstrap replicates. Numbers next to the branching point indicate the percentage of replicates supporting each branch.
To identify coding single nucleotide polymorphism (cSNP) in PsMAPK1, we performed PCR amplifications with cDNA of Chinese Pst races CYR32, CYR23, CYR25, CYR29, CYR31, and CYR33 (Table 1). At least six positive amplicons amplified with primers PKO1 and PKO2 were cloned and sequenced. In comparison with the PsMAPK1 sequence from race CYR32, a total of 32 cSNPs, including 27 synonymous cSNPs, five non-synonymous cSNPs, and one amino acid insertion/deletion, were observed among Pst reference isolates (Table S1). Regarding five non-synonymous cSNPs, two were identified in CYR23 and three in CYR29. The one amino acid insertion/deletion was found in CYR29 (Fig. S2). These results indicate that although overall it is highly conserved, sequence variations do exist in the PsMAPK1 gene among different isolates of Pst [2].
Table 1. Primers and strains used in the study.
| Primers | Sequences (5′→3′) |
| FP1s | TTTTAGCCTGCCCATCCC |
| FP1as | CCCAGCACCTCCAGAATCA |
| FP2s | GGGACAGAGGGTATTGTTTTG |
| FP2as | ATCCTGGCCGAGATGCTT |
| PMKO1 | ATGGCAGCCGTTGTAGCTCT |
| PMKO2 | TTAAGCAGTGGAGTGGAAGCT |
| CFg1 | CCATCGATGGCAGCCGTTGTAGCTCTAC a |
| CFg2 | CGCGGATCCTTAAGCAGTGGAGTGGAA b |
| CMo1 | CAGATCTTGGCTTTCGTAGGAACCCAATCTTCAATGGCAGCCGTTGTAGCTC |
| CMo2 | CACCACCCCGGTGAACAGCTCCTCGCCCTTGCTCACAGCAGTGGAGTGGAAGCT |
| PKQF | CGCCTCTACTACTTCAACTACTGCC |
| PKQR | CCCTCGCCGATAACATCCATAAC |
| PsEFQF | TTCGCCGTCCGTGATATGAGACAA |
| PsEFQR | ATGCGTATCATGGTGGTGGAGTGA |
The ClaI and BamHI sites introduced in the primer sequences were underlined.
PsMAPK1 expression was increased during haustorium development
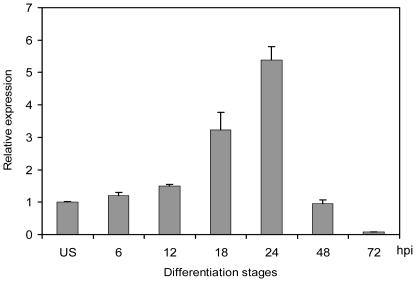
Histological observation with wheat plants infected by Pst [20] had showed that urediniospores germinated and began to produce the germ tube at 6 hours post-inoculation (hpi). A penetration hypha then entered the stomatal pore and formed a substomatal vesicle, which further differentiates into primary hyphae and haustorium mother cells at 12 hpi in infected wheat leaves. Primary haustoria appeared at 18 hpi and could be observed at most of the infection sites at 24 hpi. From 48 to 72 hpi, secondary hyphae were differentiated and grew rapidly in colonized plant tissues. In qRT-PCR assays with RNA isolated from different infection stages, we found that the transcript level of PsMAPK1 gradually increased at early infection stages from 6 to 24 hpi but decreased after 24 hpi (Fig. 2). During early infection stages, from 6 to 12 hpi, PsMAPK1 transcription was not significantly up-regulated. However, at 18 and 24 hpi, i.e., haustorial formation stage, the amount of PsMAPK1 mRNA was increased over 3- to 5- fold in comparison with that in urediniospores. After that, the transcription of PsMAPK1 was down-regulated during the secondary hypha development stage.
Figure 2. Assays for the transcript levels of PsMAPK1 during different infection stages.
RNA samples were isolated from urediniospores or leaves of wheat cultivar Suwon 11 inoculated with CYR32 and collected at the indicated time points. The expression level of PsMAPK1 was estimated by the comparative ΔΔCt method with the elongation factor gene of Pst as the endogenous reference for normalization. Relative quantification was computed with their expression levels in different stages in comparison to that in urediniospores. Means and standard errors were calculated from three biological replicates. US, urediniospores; hpi, hours post inoculation.
PsMAPK1 partially complements the F. graminearum map1 mutant
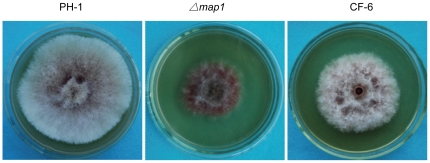
To determine its function in F. graminearum, the PsMAPK1 gene was cloned between the BamHI and ClaI sites of pHZ100-TCH and transformed into protoplasts of the F. graminearum map1 mutant [9], [10]. Ten resulting neomycin-resistant transformants were obtained and confirmed by PCR analysis to contain the transforming PsMAPK1 construct. These transformants had identical phenotypes although only data with transformant CF-6 were presented below. The F. graminearum map1 mutant has a reduced growth rate [10]. On PDA plates, colonies of transformant CF-6 were larger and more fluffy than those of the map1 mutant (Fig. 3). The growth rate of transformant CF-6 measured at different time points was significantly increased in comparison with that of the map1 mutant (P<0.05). However, CF-6 and other map1/PsMAPK1 transformants still grew slower than the wide-type strain PH-1 (Fig. 3). These results indicate that expression of the PsMAPK1 gene in F. graminearum partially complemented the defects of the map1 mutant in vegetative growth.
Figure 3. Colony morphology of Fusarium graminearum strains.
Colonies of the wild-type (PH-1), map1 deletion mutant, and complemented strain (CF-6) grown on PDA plates for 5 days.
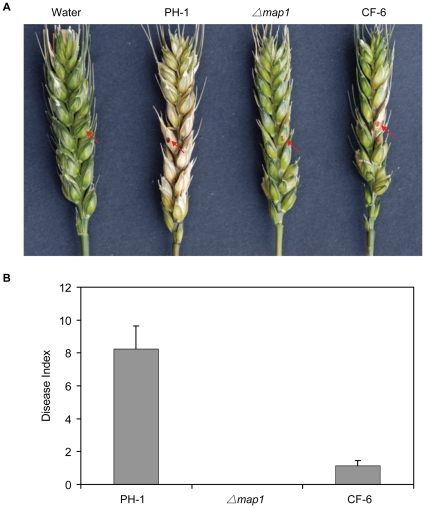
In F. graminearum, MAP1 is essential to cause the wheat scab or head blight disease [10]. To determine the virulence of complemented strain CF-6, flowering wheat heads were point-inoculated with conidia suspensions. On wheat heads inoculated with the wild-type strain PH-1, typical scab symptoms were observed in the inoculated and neighbouring spikelets 14 days post-inoculation (dpi). No scab symptoms could be detected on wheat heads inoculated with the map1 mutant (Fig. 4A). Under the same conditions, wheat heads inoculated with the complemented strain CF-6 developed scab symptoms at the inoculated spikelets (Fig. 4A). However, most of the spikelets adjacent to the inoculation sites remained healthy 14 dpi. Transformant CF-6 rarely (approximately 13%) spread from the inoculated spikelet and to neighbouring spikelets. On average, the complemented strain CF-6 had a disease index score of 1.1 (Fig. 4B), indicating that the defects of the F. graminearum map1 mutant in plant infection also was partially complemented by the PsMAPK1 gene. We repeated infection assays with two additional complementation strains CF-8 and CF-10 (Table 1) and obtained similar results (data not shown).
Figure 4. Infection assays with flowering wheat heads.
(A) Wheat heads were drop-inoculated with sterile water or conidia from the wild-type strain PH-1, map1 mutant, and complemented map1/PsMAPK1 transformant CF-6. The inoculation sites are marked with arrows. Typical heads were photographed 14 days after inoculation. (B) Disease index scores of PH-1, map1, and CF-6. Mean and standard error were calculated from three independent infection assays.
Complementation of the M. oryzae pmk1 deletion mutant by PsMAPK1
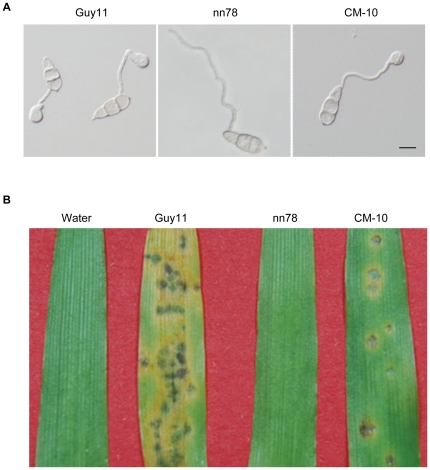
The PsMAPK1 protein shares 75% identity and 88% similarity with Pmk1 of M. oryzae. To test whether it can functionally complement the pmk1 mutant, we transform the PsMAPK1-eGFP fusion construct (under the control of RP27 promoter) into the pmk1 mutant nn78 [8]. Neomycin-resistant transformants were isolated and verified by PCR to confirm the introduction of PsMAPK1. One resulting pmk1/PsMAPK1 transformant CM-10 was assayed for appressorium formation and plant infection. While over 90% of the germ tubes formed appressoria by 24 h in Guy11, the original pmk1 mutant, nn78, failed to form appressoria under the same conditions (Fig. 5A). In transformant CM-10, approximately 25% of the germ tubes formed appressoria by 24 h (Fig. 5A). However, no GFP signals could be detected in appressoria formed by CM-10 (data not shown).
Figure 5. Complementation of the pmk1 mutant with the GFP-PsMAPK1 fusion construct.
(A) Appressorium formation assay. Germ tubes from the wild-type strain (Guy11) developed appressoria by 18 h, but no appressorium formation was observed in the pmk1 mutant (nn78). Under the same conditions, a transformant of nn78 expressing the GFP-PsMAPK1 fusion construct (CM-10) formed appressoria. Bar = 25 µm. (B) Barley infection assay. Left to right, barley leaves were sprayed with sterile and conidia of Guy11, nn78, or CM-10. Typical leaves were photographed at 6 days post inoculation.
To determine whether the GFP-PsMAPK1 construct could complement the defects of the pmk1 mutant in plant infection, eight-day-old barley seedlings of cultivar NB6 were sprayed with conidia of transformant CM-10. At 6 dpi, leaves inoculated with CM-10 or Guy11 developed typical blast lesions (Fig. 5B). No lesions were observed on leaves sprayed with water or conidia of nn78 (Fig. 5B). In comparison with Guy11, transformant CM-10 caused fewer and smaller lesions on barley leaves. These results indicated that PsMAPK1 could partially complement the pmk1 mutant in appressorium formation and plant infection.
Discussion
In this study we described the isolation and characterization of the first MAPK gene from the wheat stripe rust fungus Pst. Phylogenetic analysis revealed PsMAPK1 is more closely related to Fus/Kss1 orthologs from basidiomycetes than those from ascomycetes. In the corn smut fungus U. maydis, Ubc3/Kpp2 and Kpp6 are two MAP kinase genes belonging to the Fus3/Kss1 (YERK1) clade. Ubc3/Kpp2 and Kpp6 have overlapping functions in mating and plant infection but Kpp6 plays a more critical role in appressorial penetration than Kpp2 [11], [12]. Other basidiomycetes, including Cryptococcus neoformans and Phanerochaete chrysosporium [7], [13], also have two YERK1 MAPK genes. In the genome sequence of P. graminis f. sp. tritici, we also identified two Fus3/Kss1 MAPK homologs (GenBank accession numbers EFP88010 and EFP80661). Similar to PsMAPK1, EFP88010 of P. graminis f. sp. tritici has six introns. No introns were found in EFP80661. These observations were consistent with what has been reported in P. triticina and U. maydis MAPKs [13], [21]. Based on the sequence similarity and intron distribution pattern, we conclude that PsMAPK1 is more closely related to Kpp6 than to Ubc3/Kpp2 of U. maydis. It is likely that, similar to P. triticina and P. graminis f. sp. tritici, Pst has two YERK1 genes. As Kpp6 in U. maydis, PsMAPK1 may play a more important role in early stages of infection in Pst, such as haustorium formation, than the other Fus3/Kss1 homolog, which may be involved in stabilization and maintenance of the dikaryotic state [21].
Single nucleotide polymorphism markers are important tools for various studies, such as recombination, chromosomal dynamics, genome rearrangement, and genetic relatedness between individuals. SNPs can be located in the coding or intergenic regions [23], [24]. In coding regions, SNPs may result changes in protein structures and functions [25]. In this study, we identified SNPs in the PsMAPK1 coding region among different field isolates of Pst. In the human genome, SNPs are estimated to occur once every 1 kb [26]. In Candida albicans, the average SNP frequency set was 1 SNP per 83 bp [27]. Between the PsMAPK1 sequences from Pst isolates CYR29 and CYR32, the SNP frequency was about 1 SNP per 50 bp, indicating that significant genetic variations exist among different isolates of Pst. In a previous study, a higher genetic recombination rate was observed in strains originating from the Tianshui county, suggesting the existence of sexual reproduction in Pst [28]. A recent study also revealed that Berberis can serve as the alternate host for Pst. Therefore, we speculate that sexual recombination may account for the high gene variations in Pst. In addition, the SNPs identified from Pst reference isolates can be used as useful molecular markers to distinguish different races in the field.
In a number of plant pathogenic fungi, Fus3/Kss1 orthologs have been shown to regulate various plant infection processes, such as appressorium formation in M. oryzae [8], Colletotrichum lagenarium [29], and Cochliobolus heterostrophus [30]. In the barley powdery mildew fungus Blumeria graminis, a MAPK gene also has been implicated in the regulation of appressorium development by complementation assays [31]. In this study, we found that PsMAPK1 could partially complement the pmk1 mutant in appressorium formation and plant infection, indicating the functional conservation between PsMAPK1 and PMK1. Real-time RT-PCR assays revealed that PsMAPK1 has an increased expression level in early plant infection stages. Its expression peaked during haustorium formation, which is similar to the expression pattern of PtMAPK1 in P. triticina during plant infection [21]. These observations suggest that the development of highly specialized infection structures such as haustoria in rust fungi is regulated by a well conserved MAPK signaling cascade.
Expression of the PsMAPK1 gene also partially restored the defects of the F. graminearum map1 mutant in vegetative growth and plant infection. The fact that the YERK1 subfamily genes are highly conserved may explain for observed functional relatedness among pathogens with different plant infection mechanisms, such as F. graminearum, M. oryzae, and Pst. However, the phenotypes of the map1 and pmk1 mutants were only partially complemented, indicating that PsMAPK1 is not fully functional in ascomycetous fungi. Pst is a rust pathogen that has a distinct life style from M. oryzae and F. graminearum. During evolution, sequence and structural changes in PsMAPK1 may enable it to interact with other components of this MAPK pathway that are not conserved. These changes may reduce the efficiency of PsMAPK1 in signal transduction in ascomycetes and account for partial complementation. Complementation assays with the MAPK mutants of the basidiomycetous pathogen U. maydis may be better for functional analysis with PsMAPK1. However, the PtMAPK1 gene from P. triticina also only partially complemented the U. maydis kpp2 mutant [21]. Sequence alignment revealed that PsMAPK1 shares 77%, 74%, 75%, and 75% amino acid sequence identity with Kpp6 and Kpp2 of U. maydis, Pmk1 of M. oryzae, and Map1 of F. graminearum, respectively. Therefore, the overall homology of PsMAPK1 with its orthologs from U. maydis is not significantly higher than with its orthologs from two ascomycetes.
Although PsMAPK1 partially rescued the pmk1 mutant for appressorium formation, no GFP signals could be detected in appressoria formed by transformant CM-10. A similar observation has been reported by Yang and colleagues [32]. Although expression of a COM1-eGFP fusion construct complemented the com1 deletion mutant, GFP signals were not detectable in vegetative hyphae, conidia, germination tubes, appressoria, or infection hyphae of M. oryzae. The abundance of the PsMAPK1-eGFP fusion proteins may be too low to be detected by fluorescence microscopy in these transformants. However, it is more likely that the PsMAPK1-eGFP fusion proteins are not stable or lack fluorescent signals. Fusion with the PsMAPK1 protein may change the structure of GFP proteins.
In qRT-PCR assays, PsMAPK1 was highly expressed during the haustorium formation stage. However, its expression was not significantly up-regulated from 6 to 12 hpi, which corresponded to the appressorium formation stage. There are contradictory reports on the formation of appressoria by Pst in penetration of wheat stomata [20], [33]. When wheat cultivar Mingxian 169 was inoculated with twelve Pst isolates worldwide, eight of them formed appressoria on wheat leaves although the percentage of appressorium formation was less than 3.29% (unpublished data). Race CYR32 used for qRT-PCR assays in this study did not form appressoria. Therefore, appressorium formation appears to be dispensable for Pst infection. Our qRT-PCR data suggest that PsMAPK1 is not important for penetration through stomata by directional growth of germ tubes. Also, penetration of mesophyll cells by haustorium mother cell in Pst is a process that is more similar to appressorium penetration in M. oryzae. It is likely that PsMAPK1 plays a critical role in the regulation of penetration peg formation by the haustorium mother cell and differentiation of haustoria in plant cells in Pst. In addition, similar to symbiosis of Tuber borchii, Pst may use this MAPK pathway for its biotrophic growth in planta [34].
Materials and Methods
Strains and culture conditions
Pst strains CYR32, CYR23, CYR25, CYR29, CYR31 and CYR33 were inoculated and propagated on wheat cultivar Huixianhong as described previously [35]. Fresh urediniospores were harvested from infected wheat plants. For RNA isolation, wheat leaves of susceptible cultivar Suwon 11 inoculated with CYR32 urediniospores were harvested at 6, 12, 18, 24, 48, and 72 hpi.
The F. graminearum and M. oryzae strains used in this study (Table 1) were maintained at 25°C as described [8], [36]. Protoplast preparation and transformation of F. graminearum were performed as described [36], [37]. Complete medium (CM) with 250 µg/ml hygromycin B (Calbiochem) and 250 µg/ml geneticin (Sigma, St. Louis, MO) was used for selection of transformants. Transformation of the M. oryzae pmk1 mutant was performed as described [8], [38]. Appressorium formation assays and plant infection were conducted as previous described [39], [40].
Nucleic acid manipulations
Standard molecular biology procedures were performed as previously described [41]. Genomic DNA was isolated from urediniospores of Pst strain CYR32 [42]. Total RNA was isolated from urediniospores and infected wheat leaves according to established procedures [35]. DNaseI treatment was used to remove genomic DNA. First-strand cDNA was synthesized using the SMART™ reverse transcription Kit (Clontech) with pd(N)6 random primer (Takara) in the presence of recombinant RNasin ribonuclease inhibitor (Promega) according to the manufacturer's instruction. To assay coding region single nucleotide polymorphisms of PsMAPK1, PsMAPK1 ORF was amplified from cDNA of different Pst isolates with the Pfu proofreading polymerase (Promega) with primers PKO1 and PKO2 (Table 1).
Isolation and sequence analysis of PsMAPK1
The sequence of clone 20C8 of a full-length Pst cDNA library [22] was highly similar to several fungal MAPKs. To obtain the full-length cDNA of PsMAPK1 from Chinese Pst race CYR32, two primer pairs FP1 (FP1s and FP1as) and FP2 (FP2s and FP2as) were designed (Table 1). The genomic region of the PsMAPK1 gene was amplified with primers PKO1 and PKO2 (Table 1). DNA sequencing was performed with an ABI3130 Genetic Analyzer (Applied Biosystems, CA, USA) using the BigDye Terminator Cycle Sequence chemistry (Applied Biosystems, CA, USA). DNA sequences were analyzed with the DNASTAR (http://www.dnastar.com), BLAST (http://www.ncbi.nlm.nih.gov/blast/), and ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) programs. ClustalW 1.83 [43] and DNAMAN6.0 (Lynnon BioSoft, USA) were used for sequence alignment analyses. MEGA4 [44] was used for phylogenetic analysis using the Neighbor-Joining (NJ) method. The PsMAPK1 gene sequence has been deposited in GenBank (GenBank accession number HM535614).
Quantitative RT-PCR (qRT-PCR)
To analyze the expression levels of PsMAPK1, relative quantification of gene expression was performed by using SYBR Green qRT-PCR mixtures in an ABI prism 7500 sequence detection system (Applied Biosystems, USA). PCR was performed with the program of 95°C for 1 min, and 40 cycles of 10 s at 95°C, 20 s at 60°C and 40 s at 72°C. The transcript level of PsMAPK1 was calculated by the 2−ΔΔCT method [45] with the Pst elongation factor 1 (EF1) gene [22] as the endogenous reference for normalization (Table 1). Transcript abundance was assessed with three independent biological replicates.
Complementation of the F. graminearum map1 mutant with PsMAPK1
For F. graminearum complementation assays, the vector pFgPsMAPK1 was constructed as follows. First, the SpeI-SalI fragment containing the PTrpC-hph-TTrpC cassette from pTFCM [46] was cloned into pHZ100 [47] as pHZ100-TCH. The open reading frame (ORF) of PsMAPK1 was amplified with primers CFg1 and CFg2 (Table 1) and cloned between the BamHI and ClaI sites of pHZ100-TCH to obtain the complementation construct pFgPsMAPK1. Plasmid pFgPsMAPK1 was transformed into the map1 mutant as described [48]. The resulting neomycin-resistant transformants were confirmed by PCR analysis to contain the transforming vector pFgPsMAPK1.
Six-week-old plants of wheat cv. Xiaoyan 22 were used in infection assays with conidia collected from 5-day-old CMC cultures as previously described [36]. The third spikelet from the base of the inflorescence was injected with 10 µl of the conidium suspension (106 conidia/ml). The inoculated wheat heads were covered with a small plastic bag for 2 days. Symptomatic spikelets in each head were counted 14 days after inoculation and disease index scores were calculated as described [49], [50] with results from three independent replicates. The raw data were subjected to an analysis of variance (ANOVA) followed by determining differences between treatment means by Duncan's multiple range test.
Complementation of the M. oryzae pmk1 mutant with PsMAPK1
For complementation assays with the M. oryzae pmk1 mutant, the 1, 295 bp fragment of the PsMAPK1 gene amplified with primers CMo1 and CMo2 (Table 1) was co-transformed with XhoI-digested pFL2 into S. cerevisiae strain XK1-25 [39]. Plasmid pMoPsMAPK1 containing the PsMAPK1-eGFP construct was transformed into protoplasts of the pmk1 deletion mutant nn78 [8]. Zeocin-resistant transformants were isolated and verified by PCR with primers CMo1 and CMo2 to contain the PsMAPK1 gene integrated in the M. oryzae genome. Appressorium formation and GFP signals were assayed as described [5], [51]. For plant infection assays, conidia were resuspended to 105 conidia/ml in sterile distilled water. Eight-day-old barley seedlings of cultivar NB6 were used for spray infection assays as described previously [52], [53]. Lesion formation was examined 6 days after inoculation.
Supporting Information
Sequence alignment of PsMAPK1 with Puccinia triticina PtMAPK1, Puccinia graminis f. sp. tritici PgMAPK, Magnaporthe oryzae Pmk1 and Fusarium graminearum Map1. Identical and similar residues are shaded in black and light grey, respectively. The 11 protein kinase subdomains are labeled with roman numerals on the top (Hanks et al. 1988). The tyrosine and threonine residues, two putative phosphorylation sites for MAP kinase, are indicated by asterisks.
(TIF)
Amino acid polymorphisms in the PsMAPK1 gene of Pst isolates CYR32, CYR23 and CYR29. The arrow indicates a serine insertion in PsMAPK1 from CYR29. The five non-synonymous amino acid substitutions are shaded and marked with asterisks.
(TIF)
Overview of the nucleotide variation in PsMAPK1 from six Pst reference isolates.
(DOC)
Acknowledgments
We thank Professor Xianming Chen from Washington State University, for providing EST clones for sequencing.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Hangye Program from the Ministry of Agriculture of China (200903035-02), the 111 Project from Ministry of Education of China (No. B07049), the Key Project of Chinese Ministry of Education (No. 107104), and the Program for Excellent Young Scholars in Northwest A&F University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zhao XH, Mehrabi R, Xu JR. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot Cell. 2007;6:1701–1714. doi: 10.1128/EC.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kultz D. Phylogenetic and functional classification of mitogen- and stress-activated protein kinases. J Mol Evol. 1998;46:571–588. doi: 10.1007/pl00006338. [DOI] [PubMed] [Google Scholar]
- 3.Saito H, Tatebayashi K. Regulation of the osmoregulatory HOG MAPK cascade in yeast. J Biochem. 2004;136:267–272. doi: 10.1093/jb/mvh135. [DOI] [PubMed] [Google Scholar]
- 4.Xu JR. MAP kinases in fungal pathogens. Fungal Genet Biol. 2000;31:137–152. doi: 10.1006/fgbi.2000.1237. [DOI] [PubMed] [Google Scholar]
- 5.Liu WD, Zhou XY, Li GT, Li L, Kong LG, et al. Multiple plant surface signals are sensed by different mechanisms in the rice blast fungus for appressorium formation. PLoS Pathog. 2011;7:e1001261. doi: 10.1371/journal.ppat.1001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Good M, Tang G, Singleton J, Remenyi A, Lim WA. The Ste5 scaffold directs mating signaling by catalytically unlocking the Fus3 MAP kinase for activation. Cell. 2009;136:1085–1097. doi: 10.1016/j.cell.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rispail N, Soanes DM, Ant C, Czajkowski R, Grunler A, et al. Comparative genomics of MAP kinase and calcium-calcineurin signalling components in plant and human pathogenic fungi. Fungal Genet Biol. 2009;46:287–298. doi: 10.1016/j.fgb.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Xu JR, Hamer JE. MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev. 1996;10:2696–2706. doi: 10.1101/gad.10.21.2696. [DOI] [PubMed] [Google Scholar]
- 9.Jenczmionka NJ, Maier FJ, Losch AP, Schafer W. Mating, conidiation and pathogenicity of Fusarium graminearum, the main causal agent of the head-blight disease of wheat, are regulated by the MAP kinase GPMK1. Curr Genet. 2003;43:87–95. doi: 10.1007/s00294-003-0379-2. [DOI] [PubMed] [Google Scholar]
- 10.Urban M, Mott E, Farley T, Hammond-Kosack K. The Fusarium graminearum MAP1 gene is essential for pathogenicity and development of perithecia. Mol Plant Path. 2003;4:347–359. doi: 10.1046/j.1364-3703.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 11.Basse CW, Steinberg G. Ustilago maydis, model system for analysis of the molecular basis of fungal pathogenicity. Mol Plant Patho. 2004;5:83–92. doi: 10.1111/j.1364-3703.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 12.Kahmann R, Kamper J. Ustilago maydis: how its biology relates to pathogenic development. New Phytol. 2004;164:31–42. doi: 10.1111/j.1469-8137.2004.01156.x. [DOI] [PubMed] [Google Scholar]
- 13.Brachmann A, Schirawski J, Muller P, Kahmann R. An unusual MAP kinase is required for efficient penetration of the plant surface by Ustilago maydis. EMBO J. 2003;22:2199–2210. doi: 10.1093/emboj/cdg198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monge RA, Roman E, Nombela C, Pla J. The MAP kinase signal transduction network in Candida albicans. Microbiology. 2006;152:905–912. doi: 10.1099/mic.0.28616-0. [DOI] [PubMed] [Google Scholar]
- 15.Davidson RC, Nicholls CB, Cox GM, Perfect JR, Heitman J. A MAP kinase cascade composed of cell type specific and non-specific elements controls mating and differentiation of the fungal pathogen Cryptococcus neoformans. Mol Microbio. 2003;49:469–485. doi: 10.1046/j.1365-2958.2003.03563.x. [DOI] [PubMed] [Google Scholar]
- 16.Petersen RH. The rust fungus life cycle. Bot Rev. 1974;40:453–513. [Google Scholar]
- 17.Szabo LJ, Bushnell WR. Hidden robbers: the role of fungal haustoria in parasitism of plants. Proc Natl Acad Sci U S A. 2001;98:7654–7655. doi: 10.1073/pnas.151262398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voegele RT, Mendgen K. Rust haustoria: nutrient uptake and beyond. New Phytol. 2003;159:93–100. doi: 10.1046/j.1469-8137.2003.00761.x. [DOI] [PubMed] [Google Scholar]
- 19.Voegele RT, Struck C, Hahn M, Mendgen K. The role of haustoria in sugar supply during infection of broad bean by the rust fungus Uromyces fabae. Proc Natl Acad Sci U S A. 2001;98:8133–8138. doi: 10.1073/pnas.131186798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang CF, Huang LL, Buchenauer H, Han QM, Zhang HC, et al. Histochemical studies on the accumulation of reactive oxygen species (O2 − and H2O2) in the incompatible and compatible interaction of wheat-Puccinia striiformis f. sp. tritici. Physiol Mol Plant Pathol. 2007;71:230–239. [Google Scholar]
- 21.Hu GG, Kamp A, Linning R, Naik S, Bakkeren G. Complementation of Ustilago maydis MAPK mutants by a wheat leaf rust, Puccinia triticina homolog: potential for functional analyses of rust genes. Mol Plant Microbe Interact. 2007;20:637–647. doi: 10.1094/MPMI-20-6-0637. [DOI] [PubMed] [Google Scholar]
- 22.Ling P, Wang MN, Chen XM, Campbell KG. Construction and characterization of a full-length cDNA library for the wheat stripe rust pathogen (Puccinia striiformis f. sp. tritici). BMC Genomics. 2007;8:145. doi: 10.1186/1471-2164-8-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chasman D, Adams RM. Predicting the functional consequences of non-synonymous single nucleotide polymorphisms: structure-based assessment of amino acid variation. J Mol Biol. 2001;307:683–706. doi: 10.1006/jmbi.2001.4510. [DOI] [PubMed] [Google Scholar]
- 24.Koch R, van Luenen HGAM, van der Horst M, Thijssen KL, Plasterk RHA. Single nucleotide polymorphisms in wild isolates of Caenorhabditis elegans. Genome Res. 2000;10:1690–1696. doi: 10.1101/gr.gr-1471r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakaeda T, Nakamura T, Okumura K. Pharmacogenetics of drug transporters and its impact on the pharmacotherapy. Curr Top Med Chem. 2004;4:1385–1398. doi: 10.2174/1568026043387692. [DOI] [PubMed] [Google Scholar]
- 26.Su ZG, Zhang SZ, Hou YP, Li T, Nebert DW, et al. Single-nucleotide polymorphisms in the lipoprotein lipase gene associated with coronary heart disease in Chinese. Eur J Clin Pharmacol. 2002;454:9–18. doi: 10.1016/s0014-2999(02)02427-5. [DOI] [PubMed] [Google Scholar]
- 27.Forche A, Magee PT, Magee BB, May G. Genome-wide single-nucleotide polymorphism map for Candida albicans. Eukaryot Cell. 2004;3:705–714. doi: 10.1128/EC.3.3.705-714.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mboup M, Leconte M, Gautier A, Wan AM, Chen W, et al. Evidence of genetic recombination in wheat yellow rust populations of a Chinese oversummering area. Fungal Genet Biol. 2009;46:299–307. doi: 10.1016/j.fgb.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Takano Y, Kikuchi T, Kubo Y, Hamer JE, Mise K, et al. The Colletotrichum lagenarium MAP kinase gene CMK1 regulates diverse aspects of fungal pathogenesis. Mol Plant Microbe Interact. 2000;13:374–383. doi: 10.1094/MPMI.2000.13.4.374. [DOI] [PubMed] [Google Scholar]
- 30.Lev S, Sharon A, Hadar R, Ma H, Horwitz BA. A mitogen-activated protein kinase of the corn leaf pathogen Cochliobolus heterostrophus is involved in conidiation, appressorium formation, and pathogenicity: diverse roles for mitogen-activated protein kinase homologs in foliar pathogens. Proc Natl Acad Sci U S A. 1999;96:13542–13547. doi: 10.1073/pnas.96.23.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinane J, Oliver RP. Evidence that the appressorial development in barley powdery mildew is controlled by MAP kinase activity in conjunction with the cAMP pathway. Fungal Genet Biol. 2003;39:94–102. doi: 10.1016/s1087-1845(02)00587-x. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Zhao XY, Sun J, Kang ZS, Ding SL, et al. A novel protein Com1 is required for normal conidium morphology and full virulence in Magnaporthe oryzae. Mol Plant Microbe Interact. 2010;23:112–123. doi: 10.1094/MPMI-23-1-0112. [DOI] [PubMed] [Google Scholar]
- 33.Moldenhauer J, Moerschbacher BM, Van der Westhuizen AJ. Histological investigation of stripe rust (Puccinia striiformis f. sp. tritici) development in resistant and susceptible wheat cultivars. Plant Pathol. 2006;55:469–474. [Google Scholar]
- 34.Menotta M, Pierleoni R, Amicucci A, Sisti D, Cerasi A, et al. Characterization and complementation of a Fus3/Kss1 type MAPK from Tuber borchii, TBMK. Mol Gen Genet. 2006;276:126–134. doi: 10.1007/s00438-006-0128-6. [DOI] [PubMed] [Google Scholar]
- 35.Zhang YH, Qu ZP, Zheng WM, Liu B, Wang XJ, et al. Stage-specific gene expression during urediniospore germination in Puccinia striiformis f. sp. tritici. BMC Genomics. 2008;9:203. doi: 10.1186/1471-2164-9-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou ZM, Xue CY, Peng YL, Katan T, Kistler HC, et al. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol Plant Microbe Interact. 2002;15:1119–1127. doi: 10.1094/MPMI.2002.15.11.1119. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Liu WD, Hou ZM, Wang CF, Zhou XY, et al. A novel transcriptional factor important for pathogenesis and ascosporogenesis in Fusarium graminearum. Mol Plant Microbe Interact. 2011;24:118–128. doi: 10.1094/MPMI-06-10-0129. [DOI] [PubMed] [Google Scholar]
- 38.Zhao XH, Xu JR. A highly conserved MAPK-docking site in Mst7 is essential for Pmk1 activation in Magnaporthe grisea. Mol Microbio. 2007;63:881–894. doi: 10.1111/j.1365-2958.2006.05548.x. [DOI] [PubMed] [Google Scholar]
- 39.Bruno KS, Tenjo F, Li L, Hamer JE, Xu JR. Cellular localization and role of kinase activity of PMK1 in Magnaporthe grisea. Eukaryot Cell. 2004;3:1525–1532. doi: 10.1128/EC.3.6.1525-1532.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehrabi R, Ding S, Xu JR. MADS-box transcription factor Mig1 is required for infectious growth in Magnaporthe grisea. Eukaryot Cell. 2008;7:791–799. doi: 10.1128/EC.00009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsh EF, Maniatis T. Molecular cloning: A laboratory Manual. Plainview, NY: Cold Spring Harbor Laoratory Press; 1989. [Google Scholar]
- 42.Wang BT, Hu XP, Li Q, Hao BJ, Zhang B, et al. Development of race-specific SCAR markers for detection of Chinese races CYR32 and CYR33 of Puccinia striiformis f. sp. tritici. Plant Dis. 2010;94:221–228. doi: 10.1094/PDIS-94-2-0221. [DOI] [PubMed] [Google Scholar]
- 43.Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, et al. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Gong XY, Fu YP, Jiang DH, Li GQ, Yi XH, et al. L-arginine is essential for conidiation in the filamentous fungus Coniothyrium minitans. Fungal Genet Biol. 2007;44:1368–1379. doi: 10.1016/j.fgb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Bluhm BH, Zhao X, Flaherty JE, Xu JR, Dunkle LD. RAS2 regulates growth and pathogenesis in Fusarium graminearum. Mol Plant Microbe Interact. 2007;20:627–636. doi: 10.1094/MPMI-20-6-0627. [DOI] [PubMed] [Google Scholar]
- 48.Proctor RH, Hohn TM, Mccormick SP. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant Microbe Interact. 1995;8:593–601. doi: 10.1094/mpmi-8-0593. [DOI] [PubMed] [Google Scholar]
- 49.Gale LR, Chen LF, Hernick CA, Takamura K, Kistler HC. Population analysis of Fusarium graminearum from wheat fields in eastern China. Phytopathology. 2002;92:1315–1322. doi: 10.1094/PHYTO.2002.92.12.1315. [DOI] [PubMed] [Google Scholar]
- 50.Seong K, Li L, Hou ZM, Tracy M, Kistler HC, et al. Cryptic promoter activity in the coding region of the HMG-CoA rediactase gene in Fusarium graminearum. Fungal Genet Biol. 2006;43:34–41. doi: 10.1016/j.fgb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Ding SL, Liu WD, Iliuk A, Ribot C, Vallet J, et al. The Tig1 histone deacetylase complex regulates infectious growth in the rice blast fungus Magnaporthe oryzae. Plant Cell. 2010;22:2495–2508. doi: 10.1105/tpc.110.074302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu JR, Urban M, Sweigard JA, Hamer JE. The CPKA gene of Magnaporthe grisea is essential for appressorial penetration. Mol Plant Microbe Interact. 1997;10:187–194. [Google Scholar]
- 53.Xu JR, Staiger CJ, Hamer JE. Inactivation of the mitogen-activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc Natl Acad Sci U S A. 1998;95:12713–12718. doi: 10.1073/pnas.95.21.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence alignment of PsMAPK1 with Puccinia triticina PtMAPK1, Puccinia graminis f. sp. tritici PgMAPK, Magnaporthe oryzae Pmk1 and Fusarium graminearum Map1. Identical and similar residues are shaded in black and light grey, respectively. The 11 protein kinase subdomains are labeled with roman numerals on the top (Hanks et al. 1988). The tyrosine and threonine residues, two putative phosphorylation sites for MAP kinase, are indicated by asterisks.
(TIF)
Amino acid polymorphisms in the PsMAPK1 gene of Pst isolates CYR32, CYR23 and CYR29. The arrow indicates a serine insertion in PsMAPK1 from CYR29. The five non-synonymous amino acid substitutions are shaded and marked with asterisks.
(TIF)
Overview of the nucleotide variation in PsMAPK1 from six Pst reference isolates.
(DOC)