Abstract
This article studies a contemporary treatment approach toward both diabetes and depression management by vanadium-enriched Cordyceps sinensis (VECS). Streptozotocin-induced hyperglycemic rats were used in the study. After the rats were administered with VECS, a significant reduction in blood glucose levels was seen (P < .05) and the levels of serum insulin increased significantly (P < .05). At the same time, the study revealed a significant decrease in immobility with a corresponding increase in the swimming and climbing behavior in hyperglycemic rats following VECS treatment. The results described herein demonstrate that VECS is a contemporary treatment approach that advocates an aggressive stance toward both diabetes and depression management.
1. Introduction
Diabetes mellitus is accompanied by hormonal and neurochemical changes that can be associated with anxiety and depression [1, 2]. The prevalence of depression is ∼18% higher in diabetic patients than in the general population, and only 33% of depression cases among diabetic patients are diagnosed and treated [3, 4].These associations may be related to increased risk of depressive symptoms in individuals with diabetes, increased risk of type 2 diabetes in individuals with depressive symptoms or both. Growing evidence from clinical studies indicates that diabetic patients with major depression demonstrate poor adherence to antidiabetic regimens, have poor glycemic control and are at increased risk of retinopathy [5] and macrovascular complications [6].
The two processes of diabetes and depression negatively interact, in that depression leads to poor metabolic control whereas hyperglycemia exacerbates depression. A contemporary treatment approach advocates an aggressive stance toward both diabetes and depression management to optimize global outcome. To our knowledge, however, an algorithm incorporating the management of both has not been discovered in the literature. Thus, the present study was carried out to investigate the possible role of vanadium-enriched Cordyceps sinensis (VECS) in preventing depression in diabetes and influencing the longer-term course of glycemic control. One novel vanadium complex of VECS is designed and evaluated in this article.
As a potential therapeutic agent, vanadium has been attracting increasing attention. Vanadium compounds have the ability to imitate the action of insulin [7], and oral administration of inorganic vanadium salts has shown anti-diabetic activity in vitro [8], in vivo [9] and even in patients [10]. However, the toxicity associated with vanadium limits its role as a therapeutic agent for diabetic treatment [11]. Typical clinical manifestations are diarrhea, vomiting, abdominal cramps, green tongue, bronchospasm and irreversible renal excretion damage [12]. Using trace elements at lower doses, in combination with fungus, has been ascribed as one potent way to reduce toxicity associated with trace elements and maintain their effect [13, 14].
Mushrooms and primarily basidiomycetous fungi are popular and valuable foods that are low in calories and high in minerals, essential amino acids, vitamins and fibers [15, 16]. With no starch and low sugars, mushrooms might be considered the “delight of diabetics” [17]. Some of them produce substances with potential medical effects, and are called medicinal mushrooms [18–21].
Cordyceps sinensis is a fungus known as a traditional medicine in China. Many studies have shown that C. sinensis possesses hypoglycemic [22, 23] and vasorelaxant properties [24]. Another important property of fungus is the ability to take up and accumulate trace metals such as cadmium, lead, arsenic, copper, nickel, silver, chromium and mercury in the body or mycelium of the fungus [25–27]. The purpose of this study was to investigate the effect of fermented fungus of C. sinensis that is rich in vanadium on depression in diabetes and its influence on the course of glycemic control.
2. Methods
2.1. Chemicals
Streptozotocin (analytical grade) was purchased from Sigma. Sodium vanadate (SV; analytical grade) was purchased from Beijing Chemical Factory, China.
2.2. VECS
The seed of C. sinensis was purchased from the Agricultural Culture Collection of China. Firstly, the seed was grown at 28°C for 5 days on PDA slants (1000 mL 20% potato extract liquid, 20.0 g dextrose and 20.0 g agar). Mycelia of C. sinensis (five to six pieces) were transferred from a slant into 250 mL Erlenmeyer flasks containing 100 mL liquid medium (20% potato extract liquid, 2.0% dextrose, 0.1% KH2PO4 and 0.05% MgSO4). The culture was incubated at 27°C on a rotary shaker at 180 rmp for 4 days.
A 96-h-old liquid culture was homogenized using a sterilized blender and then inoculated to 500 mL Erlenmeyer flasks containing 300 mL of fermented culture medium (20% potato extract liquid, 2.0% dextrose, 0.1% KH2PO4, 0.05% MgSO4 and 0.9% NaVO3). The volume of inoculum was 15 mL, which was then cultivated under the same condition. The 96-h-old fermented liquid culture constituted the VECS. An ampule was filled with 4 mL of VECS stirred by a homogenizer and then was sterilized in microwave oven for 3 min. The concentration of vanadate in VECS was 0.074 M.
2.3. Fermented Mushroom of C. sinensis
The fermented mushroom of C. sinensis (FMCS) was produced using the same method to produce VECS except that there was no NaVO3 in the fermented culture medium.
2.4. SV Solution
SV (0.9 g) was dissolved in 100 mL of normal saline. An ampule was filled with 4 mL of SV and then was sterilized in a microwave oven for 3 min. The concentration of vanadate in SV was 0.074 M.
2.5. Animals
This study was performed in accordance with the Guidelines for Ethical Conduct in the Care and Use of Animals developed by the American Psychological Association (APA). Care was taken to minimize discomfort, distress and pain to the animals. Wistar rats of either sex weighing 150–200 g were housed in polypropylene cages (six animals/cage) under controlled temperature (27 ± 2°C) and in a natural light/dark cycle. They were fed with standard laboratory pellets. Food and water were provided ad libitum.
2.6. Drugs and Treatment
Healthy rats were made diabetic by intraperitoneal injection of streptozotocin (55 mg kg−1). Serum glucose levels were measured 7 days after the injection. A total of 24 animals showing hyperglycemia were selected as diabetic rats. They were randomly divided into four groups. From then on, the four groups of rats were treated daily (by oral gavage) with 4 mL of saline, VECS, FMCS and SV, respectively. The other six normal rats were treated daily (by oral gavage) with 4 mL of saline and used as the control group. At the end of the treatment (4 weeks later), the rats were fasted for 12 h, blood samples were collected from the tail vein and serum was separated. The blood glucose was analyzed with a Glucometer-4 (Bayer). Serum insulin level was determined with an enzyme-linked immunosorbant assay (ELISA) kit (Biosource, Europe).
Five weeks after diabetes induction, the rats were submitted to the forced swimming test [28]. Briefly, the rats were forced to swim individually in a cylinder (40 cm height, 15 cm diameter) containing fresh water (temperature 22 ± 2°C) up to a height of 30 cm for 15 min. This constituted the “pre-test” swim. Twenty-four hours later, each rat was re-exposed to the swimming condition in a similar environment in a 6-min “test session”. The total duration of climbing, swimming and immobility in the last 5 min of the 6-min test session was recorded for each animal. The animals were treated with 4 mL of saline, VECS, FMCS and SV, respectively, 30 min prior to the test session.
Climbing behavior consisted of upward directed movements of the forepaws along the side of the swim chamber. Swimming behavior was defined as movement (usually horizontal) throughout the swim chamber, and immobility was assigned when no additional activity was observed other than that required to keep the rat's head above water.
2.7. Statistical Analysis
The data were expressed as mean ± standard error of mean (SEM) and results were analyzed by ANOVA followed by Dunnett's t test. The P-value of <.05 was considered significant.
3. Results
3.1. Serum Glucose Levels in Rats
A significant reduction in blood glucose levels was seen when VECS (4 mL of VECS per day) was given to diabetic rats. The same doses of FMCS and SV did not significantly alter the glucose levels alone (Table 1).
Table 1.
Effect of VECS on and other treatments on serum glucose levels in streptozotocin-hyperglycemic rats.
| Different groups | Serum glucose (mg dl−1) |
|---|---|
| Streptozotocin treated | 231.9 ± 36.7a |
| Streptozotocin and SV treated | 178.8 ± 10.6a |
| Streptozotocin and FMCS treated | 183.2 ± 11.4a |
| Streptozotocin and VECS treated | 95.8 ± 8.9b |
| Control group | 100.9 ± 6.8b |
Values are mean ± SEM. Number of animals = 6. Superscript letters in the same column indicate a statistical difference of P < .05.
3.2. Serum Insulin Levels in Rats
Table 2 depicts the serum insulin levels. Streptozotocin significantly reduced the insulin concentration. Treatment with SV had no effect on streptozotocin-induced reduction. However, the levels of serum insulin significantly increased after administration of VECS (16.69 ± 3.3 IU mL−1; P < .05) and FMCS (15.57 ± 2.1 IU mL−1; P < .05).
Table 2.
Effect of VECS on serum insulin level in streptozotocin-induced diabetic rats.
| Different groups | Serum insulin (IU mL−1) |
|---|---|
| Streptozotocin treated | 12.9 ± 1.7a |
| Streptozotocin and SV treated | 11.4 ± 1.6a |
| Streptozotocin and FMCS treated | 15.57 ± 2.1b |
| Streptozotocin and VECS treated | 16.69 ± 3.3b |
| Saline treated | 19.26 ± 2.4b |
Values are mean ± SEM. Number of animals = 6. Superscript letters in the same column indicate a statistical difference of P < .05.
3.3. Modified Forced Swimming Test in Rats
There was a significant decrease in climbing behavior in diabetic rats. On the contrary, VECS increased climbing duration to levels similar to those of non-diabetic animals (Figure 1). No significant change in climbing behavior was observed with the treatments of FMCS and SV alone. At the same time, VECS increased swimming in diabetic rats significantly. FMCS also increased swimming. However, the same result did not occur in the SV-treated group (Figure 2). There was a subsequent reduction in immobility time with VECS treatment, whereas same doses of SV did not reduce immobility significantly. Figure 3 shows that VECS and FMCS significantly decreased the immobility of diabetic rats in comparison with diabetic rats and the SV-treated group. However, only VECS decreased immobility to the same level seen in non-diabetic rats (P < .01).
Figure 1.
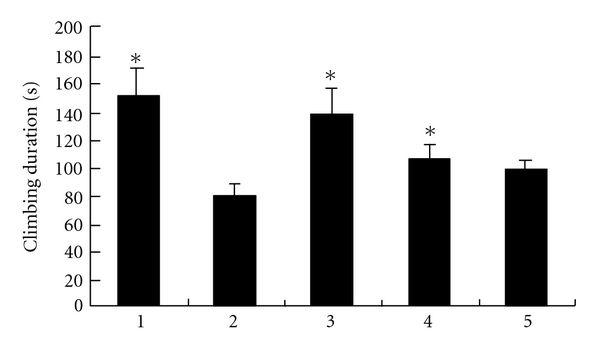
Effect of VECS on climbing time in modified forced swimming test. All bars represent mean values with vertical lines indicating SEM. Number of animals = 6. *P < .05 versus group 2. (1, Saline-treated group; 2, Steptozotocin-treated group; 3, VECS-trested group; 4, FMCS-treated group; and 5, SV-treated group).
Figure 2.
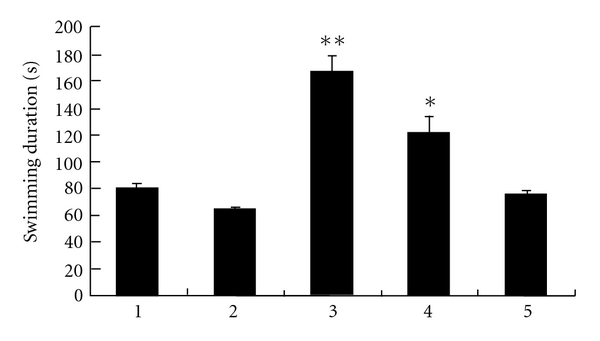
Effect of VECS on swimming time in modified forced swimming test. All bars represent mean values with vertical lines indicating SEM. Number of animals = 6. *P < .05 and **P < .01 versus group 2. (1, Saline-treated group; 2, Steptozotocin-treated group; 3, VECS-trested group; 4, FMCS-treated group; and 5, SV-treated group).
Figure 3.
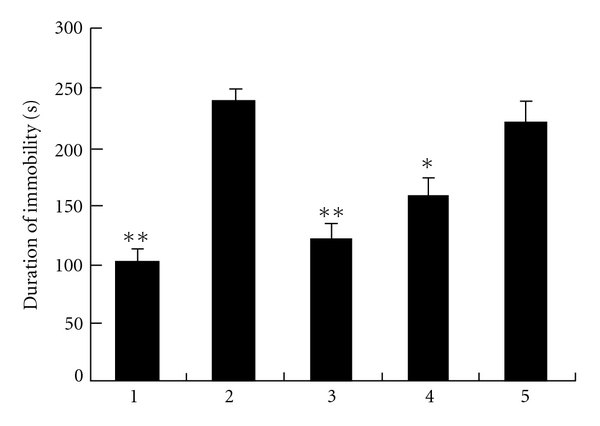
Effect of VECS on duration of immobility. All bars represent mean values with vertical lines indicating SEM. Number of animals = 6. *P < .05 and **P < .001 versus group 2. (1, Saline-treated group; 2, Steptozotocin-treated group; 3, VECS-trested group; 4, FMCS-treated group; and 5, SV-treated group).
4. Discussion and Conclusion
The presence of clinical depression and elevated depressive symptoms are higher among persons with diabetes compared with the general population. These associations may be related to increased risk of depressive symptoms in individuals with diabetes, increased risk of type 2 diabetes in individuals with depressive symptoms, or both [29, 30]. The mechanisms involved in depression are still a matter of extensive debate. Diabetes-associated depression could be related to the changes in the quality of life imposed by the chronic illness and/or its treatment, or may be a consequence of neurochemical changes induced by the disease. Studies have shown that streptozotocin-induced diabetes unbalances γ-aminobutyric acid, noradrenaline, serotonin and monoamine metabolite concentration in the ventromedial hypothalamus [31].
The present study characterizes the effect of a fermented fungus of C. sinensis rich in vanadium in a modified forced swimming test model of depression using streptozotocin-induced diabetic rats. Due to its chemical properties, in particular its greater stability, streptozotocin is the agent of choice for reproducible induction of a diabetic metabolic state in experimental animals. Streptozotocin inhibits insulin secretion and causes a state of insulin-dependent diabetes mellitus [32]. Streptozotocin-induced diabetic rats prematurely and repeatedly present more intense immobility in the forced swimming test, demonstrating their susceptibility to behavioral alterations in this animal model [33].
This is the first study reporting the antidepressant effects of fermented C. sinensis rich in vanadium on a modified forced swimming test, which is accepted as an animal model of depression due to its very good face and predictive validity.
The hypoglycemic effect of the fermented C. sinensis rich in vanadium is in agreement with other similar reports [34]. It implies that the hypoglycemic effect on the hyperglycemic rats was caused by the co-effect of C. sinensis and vanadium (Figure 4). Vanadium is known to act as a potent insulin mimetic agent by increasing glucose transport and metabolism in skeletal muscle, liver and adipose tissue [35]. Fermented C. sinensis improved the diabetes-induced decrease in serum insulin concentration and attenuated the diabetes-induced increases in blood glucose concentrations [36].
Figure 4.
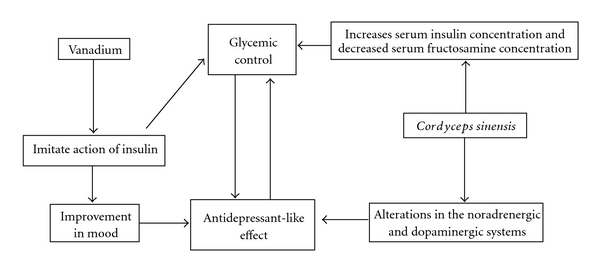
Diagram illustrating processing scheme of contemporary treatement approach of Vanadium and Cordyceps sinensis toward both diabetes and depression.
Streptozotocin-induced diabetic rats prematurely and repeatedly present more intense immobility in the forced swimming test, demonstrating their susceptibility to behavioral alterations in this animal model. The results described herein demonstrate a significant decrease in immobility with a corresponding increase in the swimming and climbing behavior following VECS treatment (Figures 1, 2, and 3).
It is likely that the mechanism of the antidepressant effect of VECS is related to the co-effect of C. sinensis and vanadium (Figure 4). Poor glycemic control may adversely affect mood and thereby reinforce the relationship between diabetes and depression [37]. Some studies have shown the hypoglycemic functions of vanadium by insulin mimicry [38, 39]. The improved metabolic control can improve mood and that insulin mimicry may have further, favorable effects on treatment satisfaction and mood [40]. Cordyceps sinensis has an antidepressant-like activity and some of its constituents might act as adrenoceptor and dopamine D2 receptor agonists or noradrenaline/dopamine reuptake inhibitors [41]. Both hypoglycemic activity and antidepressant effect were caused by the co-effect of C. sinensis and vanadium. Neither C. sinensis nor vanadium could have antidepressant effect when given to the hyperglycemic rats singly.
In summary, this study has demonstrated that C. sinensis rich in vanadium has an antidepressant-like activity in streptozotocin-induced diabetic rats. It is a contemporary treatment approach that advocates an aggressive stance toward both diabetes and depression management.
Funding
This work was supported by projects for Young Scientist from the Institute of Psychology, Chinese Academy of Sciences (08CX043004), National Natural Science Foundation of China (30800301), the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-YW-R-254) and China Postdoctoral Science Foundation (20090450546). This research was also supported by Key Laboratory of Mental Health, Chinese Academy of Sciences.
References
- 1.Bellush LL, Rowland NE. Stress and behavior in streptozotocin diabetic rats: biochemical correlates of passive avoidance learning. Behavioral Neuroscience. 1989;103(1):144–150. doi: 10.1037//0735-7044.103.1.144. [DOI] [PubMed] [Google Scholar]
- 2.Lustman PJ, Amado H, Wetzel RD. Depression in diabetics: a critical appraisal. Comprehensive Psychiatry. 1983;24(1):65–74. doi: 10.1016/0010-440x(83)90051-2. [DOI] [PubMed] [Google Scholar]
- 3.Gavard JA, Lustman PJ, Clouse RE. Prevalence of depression in adults with diabetes: an epidemiological evaluation. Diabetes Care. 1993;16(8):1167–1178. doi: 10.2337/diacare.16.8.1167. [DOI] [PubMed] [Google Scholar]
- 4.Lustman PJ, Griffith LS, Gavard JA, Clouse RE. Depression in adults with diabetes. Diabetes Care. 1992;15(11):1631–1639. doi: 10.2337/diacare.15.11.1631. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs M, Mukerji P, Drash A, Iyengar S. Biomedical and psychiatric risk factors for retinopathy among children with IDDM. Diabetes Care. 1995;18(12):1592–1599. doi: 10.2337/diacare.18.12.1592. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd CE, Matthews KA, Wing RR, Orchard TJ. Psychosocial factors and complications of IDDM. The Pittsburgh Epidemiology of Diabetes Complications Study. VIII. Diabetes Care. 1992;15(2):166–172. doi: 10.2337/diacare.15.2.166. [DOI] [PubMed] [Google Scholar]
- 7.Zorzano A, Palacín M, Marti L, García-Vicente S. Arylalkylamine vanadium salts as new anti-diabetic compounds. Journal of Inorganic Biochemistry. 2009;103(4):559–566. doi: 10.1016/j.jinorgbio.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Tolman EL, Barris E, Burns M, Pansini A, Partridge R. Effects of vanadium on glucose metabolism in vitro . Life Sciences. 1979;25:1159–1164. doi: 10.1016/0024-3205(79)90138-3. [DOI] [PubMed] [Google Scholar]
- 9.Meeks MJ, Landolt RR, Kessler WV, Born GS. Effect of vanadium on metabolism of glucose in the rat. Journal of Pharmaceutical Sciences. 1971;60(3):482–483. doi: 10.1002/jps.2600600336. [DOI] [PubMed] [Google Scholar]
- 10.Goldfine AB, Simonson DC, Folli F, Patti M-E, Kahn CR. In vivo and in vitro studies of vanadate in human and rodent diabetes mellitus. Molecular and Cellular Biochemistry. 1995;153(1-2):217–231. doi: 10.1007/BF01075941. [DOI] [PubMed] [Google Scholar]
- 11.Domingo JL. Vanadium and tungsten derivatives as antidiabetic agents: a review of their toxic effects. Biological Trace Element Research. 2002;88:97–112. doi: 10.1385/BTER:88:2:097. [DOI] [PubMed] [Google Scholar]
- 12.Scior T, Guevara-García A, Bernard P, Do Q-T, Domeyer D, Laufer S. Are vanadium compounds drugable? Structures and effects of antidiabetic vanadium compounds: a critical review. Mini-Reviews in Medicinal Chemistry. 2005;5(11):995–1008. doi: 10.2174/138955705774575264. [DOI] [PubMed] [Google Scholar]
- 13.Han C, Cui B, Wang Y. Vanadium uptake by biomass of Coprinus comatus and their effect on hyperglycemic mice. Biological Trace Element Research. 2008;124:35–39. doi: 10.1007/s12011-008-8120-0. [DOI] [PubMed] [Google Scholar]
- 14.Han C, Liu T. A comparison of hypoglycemic activity of three species of basidiomycetes rich in vanadium. Biological Trace Element Research. 2009;127(2):177–182. doi: 10.1007/s12011-008-8231-7. [DOI] [PubMed] [Google Scholar]
- 15.Firenzuoli F, Gori L, Lombardo G. The medicinal mushroom Agaricus blazei murrill: review of literature and pharmaco-toxicological problems. Evidence-Based Complementary and Alternative Medicine. 2008;5(1):3–15. doi: 10.1093/ecam/nem007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindequist U, Niedermeyer THJ, Jülich W-D. The pharmacological potential of mushrooms. Evidence-Based Complementary and Alternative Medicine. 2005;2(3):285–299. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper EL. Ayurveda and eCAM: a closer connection. Evidence-Based Complementary and Alternative Medicine. 2008;5(2):121–122. doi: 10.1093/ecam/nen035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper EL. The immune system and complementary and alternative medicine. Evidence-Based Complementary and Alternative Medicine. 2007;4(1):5–8. doi: 10.1093/ecam/nem093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madamanchi G, Tzeng Y-M. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evidence-Based Complementary and Alternative Medicine. doi: 10.1093/ecam/nep108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu JW, Huang HC, Chen ST, Wong CH, Juan HF. Ganoderma lucidum polysaccharides induce macrophage-like differentiation in human leukemia THP-1 cells via caspase and p53 activation. Evidence-Based Complementary and Alternative Medicine. doi: 10.1093/ecam/nep107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Fatimi MAA, Jülich W-D, Jansen R, Lindequist U. Bioactive components of the traditionally used mushroom Podaxis pistillaris. Evidence-Based Complementary and Alternative Medicine. 2006;3(1):87–92. doi: 10.1093/ecam/nek008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Sun H, Qin F, Pan Y, Sun C. Effect of various extracts and a polysaccharide from the edible mycelia of Cordyceps sinensis on cellular and humoral immune response against ovalbumin in mice. Phytotherapy Research. 2006;20(8):646–652. doi: 10.1002/ptr.1921. [DOI] [PubMed] [Google Scholar]
- 23.Zhang G, Huang Y, Bian Y, Wong JH, Ng TB, Wang H. Hypoglycemic activity of the fungi Cordyceps militaries, Cordyceps sinensis, Tricholoma mongolicum, and Omphalia lapidescens in streptozottocin-induced diabetic rats. Applied Microbiology and Biotechnology. 2006;72:1152–1156. doi: 10.1007/s00253-006-0411-9. [DOI] [PubMed] [Google Scholar]
- 24.Balon TW, Jasman AP, Zhu J-S. A fermentation product of Cordyceps sinensis increases whole-body insulin sensitivity in rats. Journal of Alternative and Complementary Medicine. 2002;8(3):315–323. doi: 10.1089/10755530260128005. [DOI] [PubMed] [Google Scholar]
- 25.Kalac P, Niznanská M, Bevilaqua D, Stasková I. Concentrations of mercury, copper, cadmium and lead in fruiting bodies of edible mushrooms in the vicinity of a mercury smelter and a copper smelter. Science of the Total Environment. 1996;177:251–258. doi: 10.1016/0048-9697(95)04850-2. [DOI] [PubMed] [Google Scholar]
- 26.Kalač P, Svoboda L. A review of trace element concentrations in edible mushrooms. Food Chemistryistry. 2000;69(3):273–281. [Google Scholar]
- 27.Malinowska E, Szefer P, Falandaysz J. Metals bioaccumulation by Bay bolete, Xerocomus badius, from selected sites in Poland. Food Chemistry. 2004;84:405–416. [Google Scholar]
- 28.Cryan JF, Page ME, Lucki I. Noradrenergic lesions differentially alter the antidepressant-like effects of reboxetine in a modified forced swimming test. European Journal of Pharmacology. 2002;436:197–205. doi: 10.1016/s0014-2999(01)01628-4. [DOI] [PubMed] [Google Scholar]
- 29.Talbot F, Nouwen A. A review of the relationship between depression and diabetes in adults: is there a link? Diabetes Care. 2000;23:1556–1562. doi: 10.2337/diacare.23.10.1556. [DOI] [PubMed] [Google Scholar]
- 30.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 31.Ohtani N, Ohta M, Sugano T. Microdialysis study of modification of hypothalamic neurotransmitters in streptozotocin-diabetic rats. Journal of Neurochemistry. 1997;69(4):1622–1628. doi: 10.1046/j.1471-4159.1997.69041622.x. [DOI] [PubMed] [Google Scholar]
- 32.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 33.Gomez R, Barros HMT. Ethopharmacology of the antidepressant effect of clonazepam in diabetic rats. Pharmacology Biochemistry and Behavior. 2000;66(2):329–335. doi: 10.1016/s0091-3057(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 34.Han C, Yuan J, Wang Y, Li L. Hypoglycemic activity of fermented mushroom of Coprinus comatus rich in vanadium. Journal of Trace Elements in Medicine and Biology. 2006;20(3):191–196. doi: 10.1016/j.jtemb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Shechter Y. Insulin-mimetic effects of vanadate. Possible implications for future treatment of diabetes. Diabetes. 1990;39(1):1–5. doi: 10.2337/diacare.39.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Lo HC, Hsu TH, Tu ST, Lin KC. Anti-hyperglycemic activity of natural and fermented Cordyceps sinensis in rats with diabetes induced by nicotinamide and streptozotocin. The American Journal of Chinese Medicine. 2006;34:819–832. doi: 10.1142/S0192415X06004314. [DOI] [PubMed] [Google Scholar]
- 37.Lustman P, Carney R, Amado H. Acute stress and metabolism in diabetes. Diabetes Care. 1981;4(6):658–659. doi: 10.2337/diacare.4.6.658. [DOI] [PubMed] [Google Scholar]
- 38.Lu B, Ennis D, Lai R, et al. Enhanced sensitivity of insulin-resistant adipocytes to vanadate is associated with oxidative stress and decreased reduction of vanadate (+5) to vanadyl (+4) Journal of Biological Chemistry. 2001;276(38):35589–35598. doi: 10.1074/jbc.M106783200. [DOI] [PubMed] [Google Scholar]
- 39.Semiz S, Orvig C, McNeill JH. Effects of diabetes, vanadium, and insulin on glycogen synthase activation in Wistar rats. Molecular and Cellular Biochemistry. 2002;231(1-2):23–35. doi: 10.1023/a:1014437019586. [DOI] [PubMed] [Google Scholar]
- 40.Reza M, Taylor CD, Towse K, Ward JD, Hendra TJ. Insulin improves well-being for selected elderly type 2 diabetic subjects. Diabetes Research and Clinical Practice. 2002;55(3):201–207. doi: 10.1016/s0168-8227(01)00327-8. [DOI] [PubMed] [Google Scholar]
- 41.Nishizawa K, Torii K, Kawasaki A, Katada M, Ito M, Terashita K. Antidepressant-like effect of Cordyceps sinensis in the mouse tail suspension test biol. Biological and Pharmaceutical Bulletin. 2007;30:1758–1762. doi: 10.1248/bpb.30.1758. [DOI] [PubMed] [Google Scholar]


