Abstract
Baccharis dracunculifolia is the source of Brazilian green propolis (BGP). Considering the broad spectrum of biological activities attributed to green proplis, B. dracunculifolia has a great potential for the development of new cosmetic and pharmaceutical products. In this work, the cultivation of 10 different populations of native B. dracunculifolia had been undertaken aiming to determine the role of seasonality on its phenolic compounds. For this purpose, fruits of this plant were collected from populations of 10 different regions, and 100 individuals of each population were cultivated in an experimental area of 1800 m2. With respect to cultivation, the yields of dry plant, essential oil and crude extract were measured monthly resulting in mean values of 399 ± 80 g, 0.6 ± 0.1% and 20 ± 4%, respectively. The HPLC analysis allowed detecting seven phenolic compounds: caffeic acid, ferulic acid, aromadendrin-4′-methyl ether (AME), isosakuranetin, artepillin C, baccharin and 2-dimethyl-6-carboxyethenyl-2H-1-benzopyran acid, which were the major ones throughout the 1-year monthly analysis. Caffeic acid was detected in all cultivated populations with mean of 4.0%. AME displayed the wide variation in relation to other compounds showing means values of 0.65 ± 0.13% at last quarter. Isosakuranetin and artepillin C showed increasing concentrations with values between 0% and 1.4% and 0% and 1.09%, respectively. The obtained results allow suggesting that the best time for harvesting this plant, in order to obtain good qualitative and quantitative results for these phenolic compounds, is between December and April.
1. Introduction
Baccharis dracunculifolia D. C. (Asteraceae) is a native plant from Brazil commonly known as “Alecrim do campo” and “Vassoura”. This plant is well known for its interaction with insects, mainly Apis mellifera L., and for bearing a wide range of secondary metabolites. Its leaves are punctuated with secretory thricomes that are rich in secondary metabolites, as well as secretory ducts that produce and store essential oils and phenolic compounds. Baccharis dracunculifolia secondary metabolites are collected by A. mellifera to produce Brazilian green propolis (BGP) [1], which is of great importance for food and pharmaceutical industries [2] as it displays anticancer [3], antibacterial [4], anti-inflammatory [5] and antiulcer [6] properties among others. Lemos et al. [7] described the gastric protective effect of the hydroalcoholic extract of B. dracunculifolia aerial parts. Fukuda et al. [8] reported the cytotoxic activity of B. dracunculifolia constituents. Da Silva Filho et al. [9] showed the presence of flavonoids [isosakuranetin, aromadendrin-4′-methyl ether (AME)] and cinnamic acid derivatives (caffeic acid, p-coumaric acid, ferulic acid) with trypanocidal activity. Munari et al. [10] reported the antimutagenic activity of the hydroalcoholic extract of the leaves of this plant. Akao et al. [11] showed that prenylated p-coumaric acid derivatives (artepillin C, drupanin and baccharin) exhibited antitumor properties. Missima et al. [12] identified diterpenes and triterpenes with immunomodulatory activity. Leitão et al. [13] reported that B. dracunculifolia displays anticariogenic activity. Klopell et al. [14] found that (E)-nerolidol, the major constituent of the volatile fraction, stood out for antiulcer activity.
It is important to point out that honey is another major bee product, and recently there were three reported works: the inhibition of lipid peroxidation in biological systems [15], the antiseptic agent in wound care [16] and the enhancement of immune function and antitumor activity [17]. In addition, seasonal variation, chemical composition and antioxidant activity of Brazilian propolis samples were reported as well [18]. Forty phenolic substances were identified, in different concentrations, from Brazilian propolis extracts produced in three distinct regions. It is well known that B. dracunculifolia is the main botanical source of BGP. Therefore, considering that the majority of reported works with B. dracunculifolia were undertaken with native plants and that this plant has a great potential for the development of new products, the aim of this work was to evaluate the seasonality role in the phenols chemical profile of 10 different populations of B. dracunculifolia cultivated during 1 year. It would not only allow the selection of the B. dracunculifolia population bearing higher production of the phenolic compounds, but also to determine the best timing of plant harvesting.
2. Methods
2.1. Reagents and Solvents
Organic solvents for HPLC analyses were purchased from Mallinckrodt Co. (Xalostoc, Mexico) and filtered through 0.45-μm cellulose membranes prior to use. Water was purified using Milli-Q-plus filter systems (Millipore, Bedford, MA, USA). Caffeic acid and ferulic acid were bought from Acros Organics (Morris Plains, NJ, USA). The internal standard (IS), 3,4-dimethoxybenzaldehyde (veratraldehyde) was purchased from Merck (Darmstadt, Germany). The phenolic AME, isosakuranetin, artepillin C, baccharin and 2,2-dimethyl-6-carboxyethenyl-2H-1-benzopyran acid (DCBEN) were isolated and identified in our laboratory from either B. dracunculifolia or propolis samples [19, 20]. The purity of each standard was determined by both HPLC and 13C NMR to be higher than 96%.
2.2. Cultivation and Sampling
Initially, the fruits of B. dracunculifolia were collected from populations of 10 different regions of Brazil in their natural habitat (Table 1). Professor Nelson Ivo Matzenbacher authenticated the plant material. The fruits were first germinated in a nursery and then propagated under glasshouse conditions for 30 days. The obtained seedlings were transplanted to the experimental field area of Chemical, Biological and Agricultural Pluridisciplinary Research Center (CPQBA), University of Campinas, São Paulo, in January 2004. The field was divided into four replications. Each replication was composed of 10 blocks. Each block was composed of 25 plants, totalling 1000 cultivated plants in the area. Voucher specimens of each replication were deposited in the herbarium at CPQBA-UNICAMP (No. 1298). The cultivation experiment was carried out in an experimental area of 1800 m2 (22˚48′S, 47˚03′W, altitude 669 m), at the University of Campinas—CPQBA, Brazil. Baccharis dracunculifolia was cultivated according to the Good Agriculture Practices (GAPs) and the cultivated area was kept free of weeds by manual procedures and no herbicides were used.
Table 1.
Collection sites of the fruits of B. dracunculifolia and their coordinates.
| State | Regions | Latitude | Longitude | Altitude |
|---|---|---|---|---|
| (S) | (W) | (m) | ||
| São Paulo | Franca 1 | 20˚ 32′ 19′′ | 47˚ 24′ 03′′ | 996 |
| Cajurú | 21˚ 16′ 31′′ | 47˚ 18′ 15′′ | 775 | |
| Franca 2 | 20˚ 15′ 25′′ | 47˚ 28′ 36′′ | 1035 | |
| Ribeirão Preto | 21˚ 10′ 39′′ | 47˚ 48′ 37′′ | 546 | |
| Campinas | 22˚ 54′ 20′′ | 47˚ 03′ 39′′ | 854 | |
|
| ||||
| Minas Gerais | Alfenas | 21˚ 25′ 45′′ | 45˚ 56′ 50′′ | 881 |
| Paraguaçu | 21˚ 32′ 50′′ | 45˚ 44′ 15′′ | 826 | |
| Ouro Fino | 22˚ 16′ 59′′ | 46˚ 22′ 08′′ | 908 | |
|
| ||||
| Paraná | Colombo 1 | 25˚ 17′ 30′′ | 49˚ 13′ 27′′ | 1027 |
| Colombo 2 | 25˚ 19′ 29′′ | 49˚ 18′ 36′′ | 945 | |
The sampling of the leaves started 4 months later and it was undertaken between May 2004 and April 2005. Ten randomized samples consisting of branches measuring 20 cm each, containing both buds (30%) and adult leaves (70%) from individual plants, representing each replication, were collected as homogeneous samples from each population. The homogenous samples were harvested monthly to determine both chemical profile and seasonal role on the major phenolic constituents.
2.3. Sample Preparation and Chromatographic Analysis
The sample preparation was undertaken following the analytical method previously developed [21]. The buds and adult leaves from 10 dried collected branches of B. dracunculifolia were removed and powdered using a knife mill. To a homogeneous sample of 500 mg, 20 ml of 90% ethanol containing 300 μg/ml of the internal standard in 125 ml Erlenmeyer flasks was added. The solution was stirred at 170 r.p.m. and 40˚C on a shaker (Innova 4300, New Brunswick Scientific, Edison, NJ, USA). After 2 h of extraction, the flasks were cooled to room temperature and filtered through analytical filter papers. A 1.0 ml aliquot of the extracts was then filtered through a Millex-LCR-PTFE (Millipore, Bedford, MA, USA 0.45 μm × 13 mm i.d.) and transferred to an appropriate vial for automatic injection, and a 15-μl aliquot was injected into the HPLC system, which was described by Sousa et al. [21].
Veratraldehyde was used as internal standard, and it was added to the extracting solvent prior to extraction. The spectral data from the photodiode array detector were collected within 60 min over the 265–320 nm range of the absorption spectrum, and the chromatograms were plotted at 280 nm. Peaks were assigned according to their retention times and by co-elution with authentic standards, as well as based on UV spectra for both the standards and samples under the same chromatographic conditions.
2.4. Quantitative Analysis and Statistical Studies
The calibration curves were prepared in the concentration range expected of each compound in B. dracunculifolia sample, ranging from 25 to 1200 μg/ml. The linearity was investigated by calculation of the regression plots by the least squares and it was expressed by the determination coefficient (R 2) showing values ranging from 0.9982 to 0.9998. Absolute concentrations of four compounds (acid caffeic, AME, isosakuranetin and artepillin C) in the B. dracunculifolia samples were calculated based on the phenolic area/IS area. Regarding the chromatographic profile of the hydroalcoholic extracts, the relative percentages of each peak of interest were obtained monthly, taking into account the area percentage.
After checking for normality (Kolmogorov-Smirnov test) and homogeneity of the variances (Bartlett's test), the inter-group variation of different parameter was estimated by the analysis of variance (ANOVA). These ANOVA analyses were then completed by Tukey's multiple range tests, in order to locate the differences [22]. Thus, qualitative treatments were compared by Tukey test, with a probability of 95% and significance levels of 5% (P < .05) for comparative studies among populations. It was also considered the probability of 99% and significance levels of 1% (P < .01) for comparison among months and considering each population as well. Statistics calculations, as well as graphic representation were prepared by using GraphPad Prim® (v. 4.0) and additional calculations were carried out with aid of Microsoft® Excel 2003.
3. Results
3.1. Agronomy Aspects
Baccharis dracunculifolia was cultivated rapidly and developed from the production of seedlings in nursery, showing excessive and intense growth in an interval of 2 months. At the fourth month it was about 0.8 m in height, achieving 2.5–3.0 m in 1 year, despite of no use of chemical fertilizers during the plant development. The productive potential of the species expressed as amount of dry biomass per plant was variable depending on the population. According to the obtained results, the mean of yielding of plant dry biomass, after 16 months of seeding and considering 10 populations, was 399 ± 80 g. The Paraguaçu-MG (302 ± 34 g) and Colombo 1-PR (584 ± 75 g) populations displayed the lowest and the highest yield of dry biomass, respectively. Additionally, the essential oil of the dry leaves of each cultivated population was studied as well [23].
3.2. Chemical Composition
The HPLC method used allowed the analysis of seven major phenolic compounds along 1 year in B. dracunculifolia samples: caffeic acid, ferulic acid, AME, isosakuranetin, artepillin C, baccharin and DCBEN. Chemical structures for these seven phenolics are displayed in Figure 1.
Figure 1.
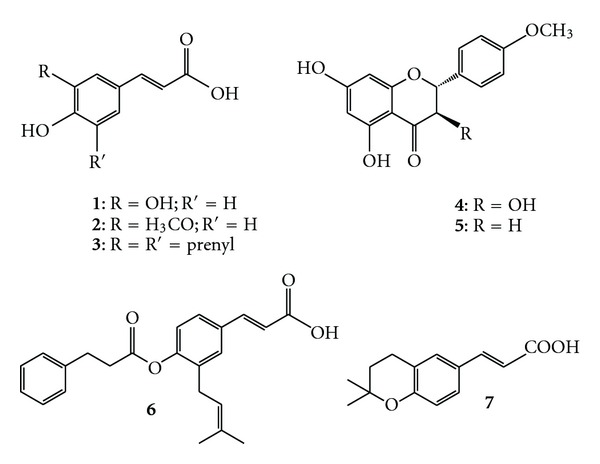
Chemical structures of main phenolics identified in extracts from the leaves of cultivated specimens of B. dracunculifolia. 1: caffeic acid; 2: ferulic acid; 3: artepillin C; 4: AME; 5: isosakuranetin; 6: baccharin and 7: DCBEN.
3.3. Seasonality and Phenolic Contents
The quantification of caffeic acid, AME, isosakuranetin and artepillin C were undertaken for all cultivated plants from 10 distinct regions. Tables 2–5 display the variation on the concentration of these four phenolic compounds between May 2004 and April 2005. Considering the mean values calculated monthly for B. dracunculifolia from these regions, the relative percentages of these phenolics were obtained by taking their percentages in the ethanolic extracts. The sum of these four compounds correspond to ∼30% of the total extract, caffeic acid (23.7 ± 2.4%) being the major one, followed by AME (2.9 ± 1.1%), isosakuranetin (2.1 ± 1.2%) and artepillin C (1.3 ± 0.8%), respectively.
Table 2.
Effect of seasonality on caffeic acid content (%) of leaves of B. dracunculifolia along 1 year.
| Month/ | São Paulo state | Minas Gerais state | Paraná state | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| regions | Franca 1 | Cajurú | Franca 2 | Ribeirão Preto | Campinas | Paraguaçu | Alfenas | Ouro Fino | Colombo 1 | Colombo 2 |
| May/04(A) | 3.70 ± 0.20(a) | 3.41 ± 0.21(a) | 3.57 ± 0.25(a) | 3.50 ± 0.28 (a) | 3.15 ± 0.08(a) | 3.45 ± 0.18(a) | 3.70 ± 0.23(a) | 3.45 ± 0.14(a) | 4.09 ± 0.41(a) | 3.51 ± 0.37(a) |
| June/04(A) | 4.32 ± 0.18(a) | 4.14 ± 0.13(a) | 3.96 ± 0.24(a) | 3.95 ± 0.21 (a) | 4.29 ± 0.11(a) | 4.68 ± 0.49(b) | 3.99 ± 0.11(a) | 4.07 ± 0.38(b) | 3.99 ± 0.11(a) | 4.14 ± 0.10(b) |
| July/04(A) | 5.07 ± 0.56(b) | 4.09 ± 0.69(a) | 4.42 ± 0.30(a) | 3.58 ± 0.77(a) | 4.01 ± 0.42(a) | 4.41 ± 0.15(a) | 4.66 ± 0.80(b) | 3.53 ± 0.59(a) | 4.90 ± 0.16(b) | 4.67 ± 0.15(b) |
| August/04(A) | 4.37 ± 0.38(a) | 3.40 ± 0.65(a) | 4.10 ± 0.11(a) | 4.09 ± 0.14(a) | 4.24 ± 0.40(a) | 3.74 ± 0.35(a) | 4.24 ± 0.49(a) | 3.54 ± 0.36(a) | 4.57 ± 0.87(a) | 3.34 ± 0.78(a) |
| Sept/04(A) | 3.40 ± 0.35(a) | 3.12 ± 0.20(a) | 3.25 ± 0.68(a) | 3.03 ± 0.48(a) | 3.99 ± 0.15(a) | 3.58 ± 0.45(a) | 2.94 ± 0.16(a) | 2.71 ± 0.45(a) | 3.81 ± 0.91(a) | 2.53 ± 0.81(a) |
| Oct/04(B) | 4.29 ± 0.76(a) | 4.35 ± 0.10(b) | 5.57 ± 0.83(b) | 4.82 ± 0.88(b) | 6.00 ± 0.62(b) | 5.68 ± 1.11(b) | 4.11 ± 0.31(a) | 4.55 ± 0.81(b) | 6.50 ± 1.97(b) | 3.71 ± 1.70(a) |
| Nov/04(A) | 4.18 ± 0.34(a) | 4.05 ± 0.09(a) | 4.49 ± 0.47(b) | 3.58 ± 0.36(a) | 4.24 ± 0.46(a) | 4.41 ± 0.28(a) | 4.02 ± 0.21(a) | 4.32 ± 0.20(b) | 5.14 ± 1.28(b) | 3.33 ± 1.15(a) |
| Dec/04(A) | 3.86 ± 0.18(a) | 3.47 ± 0.28(a) | 3.44 ± 0.13(a) | 3.41 ± 0.23(a) | 3.6 ± 0.55(a) | 4.02 ± 0.04(a) | 3.96 ± 0.20(a) | 3.73 ± 0.16(a) | 3.70 ± 0.25(a) | 3.34 ± 0.23(b) |
| Jan/05(B) | 5.35 ± 0.46(b) | 4.70 ± 0.46(b) | 5.24 ± 0.72(b) | 4.85 ± 0.51(b) | 5.87 ± 0.28(b) | 4.31 ± 0.61(a) | 5.17 ± 0.17(b) | 5.00 ± 0.46(b) | 4.76 ± 0.52(b) | 4.02 ± 0.47(b) |
| Feb/05(A) | 4.65 ± 0.90(b) | 3.76 ± 0.63(a) | 3.32 ± 0.75(a) | 4.60 ± 0.60(b) | 5.66 ± 0.65(b) | 4.57 ± 1.00(a) | 5.30 ± 0.59(b) | 4.47 ± 1.50(b) | 3.42 ± 1.00(a) | 4.06 ± 0.90(b) |
| March/05(B) | 5.58 ± 0.33 (b) | 5.06 ± 0.37(b) | 5.58 ± 0.51(b) | 5.09 ± 0.37(b) | 5.81 ± 0.29(b) | 4.87 ± 0.53(b) | 5.62 ± 0.42(b) | 5.03 ± 0.40(b) | 3.97 ± 0.77(a) | 5.06 ± 0.70(b) |
| April/05(B) | 4.23 ± 0.47 (a) | 4.34 ± 0.08(b) | 4.36 ± 0.17(b) | 5.03 ± 0.54(b) | 5.27 ± 0.39(b) | 4.07 ± 0.23(a) | 4.39 ± 0.13(b) | 4.20 ± 0.22(b) | 3.67 ± 0.10(a) | 3.78 ± 0.11(b) |
Capital and small letters denote significant differences at P < .01. (A) and (B) refer to the mean values among population for each month; (a) and (b) correspond to the significant differences for each population in time frame studied.
Table 5.
Effect of seasonality on artepillin C content (%) of leaves of B. dracunculifolia along 1 year.
| Month/ | São Paulo state | Minas Gerais state | Paraná state | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| regions | Franca 1(I) | Cajurú(I) | Franca 2(I) | Ribeirão Preto(I) | Campinas(I) | Paraguaçu(I) | Alfenas(I) | Ouro Fino(I) | Colombo 1(II) | Colombo 2(I) |
| May/04(a) | 0.04 ± 0.01(a) | 0.04 ± 0.01(a) | 0.03 ± 0.02(a) | 0.00(a) | 0.04 ± 0.02(a) | 0.04 ± 0.01(a) | 0.03 ± 0.01(a) | 0.04 ± 0.01(a) | 0.00(a) | 0.00(a) |
| June/04(a) | 0.11 ± 0.02(a) | 0.08 ± 0.06(a) | 0.15 ± 0.03(b) | 0.08 ± 0.03(a) | 0.10 ± 0.01(a) | 0.11 ± 0.02(a) | 0.14 ± 0.02(a) | 0.10 ± 0.03(a) | 0.00(a) | 0.03 ± 0.01(a) |
| July/04(a) | 0.15 ± 0.07(b) | 0.05 ± 0.01(a) | 0.15 ± 0.05(b) | 0.10 ± 0.05(a) | 0.16 ± 0.04(b) | 0.12 ± 0.01(a) | 0.11 ± 0.01(a) | 0.10 ± 0.01(a) | 0.00(a) | 0.06 ± 0.02(a) |
| August/04(a) | 0.10 ± 0.01(a) | 0.09 ± 0.02(a) | 0.10 ± 0.01(a) | 0.11 ± 0.02(a) | 0.13 ± 0.01(a) | 0.15 ± 0.04(b) | 0.10 ± 0.03(a) | 0.09 ± 0.01(a) | 0.00(a) | 0.03 ± 0.01(a) |
| Sept/04(a) | 0.10 ± 0.01(a) | 0.11 ± 0.07(a) | 0.13 ± 0.02(a) | 0.09 ± 0.02(a) | 0.14 ± 0.04(a) | 0.16 ± 0.01(b) | 0.17 ± 0.02(b) | 0.19 ± 0.02(b) | 0.00(a) | 0.04 ± 0.01(a) |
| Oct/04(a) | 0.13 ± 0.04(a) | 0.19 ± 0.10(b) | 0.26 ± 0.05(b) | 0.21 ± 0.05(b) | 0.20 ± 0.01(b) | 0.28 ± 0.04(b) | 0.23 ± 0.03(b) | 0.22 ± 0.03(b) | 0.00(a) | 0.13 ± 0.05(b) |
| Nov/04(b) | 0.26 ± 0.04(b) | 0.31 ± 0.05(b) | 0.45 ± 0.11(b) | 0.20 ± 0.09(b) | 0.31 ± 0.08(b) | 0.35 ± 0.04(b) | 0.29 ± 0.03(b) | 0.30 ± 0.01(b) | 0.06 ± 0.01(b) | 0.10 ± 0.02(b) |
| Dec/04(b) | 0.41 ± 0.06(b) | 0.32 ± 0.02(b) | 0.38 ± 0.08(b) | 0.23 ± 0.09(b) | 0.22 ± 0.01(b) | 0.36 ± 0.01(b) | 0.37 ± 0.06(b) | 0.26 ± 0.08(b) | 0.05 ± 0.02(b) | 0.15 ± 0.04(b) |
| Jan/05(b) | 0.46 ± 0.02(b) | 0.49 ± 0.12(b) | 0.50 ± 0.15(b) | 0.18 ± 0.14(b) | 0.29 ± 0.08(b) | 0.36 ± 0.01(b) | 0.38 ± 0.04(b) | 0.31 ± 0.05(b) | 0.05 ± 0.02(b) | 0.18 ± 0.05(b) |
| Feb/05(b) | 0.53 ± 0.04(b) | 0.47 ± 0.05(b) | 0.71 ± 0.12(b) | 0.44 ± 0.11(b) | 0.58 ± 0.10(b) | 0.50 ± 0.15(b) | 0.71 ± 0.13(b) | 0.46 ± 0.18(b) | 0.08 ± 0.03(b) | 0.26 ± 0.06(b) |
| March/05(b) | 0.46 ± 0.01(b) | 0.47 ± 0.09(b) | 0.55 ± 0.04(b) | 0.51 ± 0.05(b) | 0.59 ± 0.06(b) | 0.53 ± 0.01(b) | 0.55 ± 0.05(b) | 0.45 ± 0.07(b) | 0.10 ± 0.05(b) | 0.37 ± 0.10(b) |
| April/05(b) | 0.72 ± 0.01(b) | 0.71 ± 0.07(b) | 0.87 ± 0.08(b) | 0.73 ± 0.16(b) | 1.09 ± 0.25(b) | 0.77 ± 0.13(b) | 0.96 ± 0.16(b) | 0.65 ± 0.22(b) | 0.16 ± 0.05(b) | 0.45 ± 0.10(b) |
Capital and small letters denote significant differences at P < .01. (A) and (B) refer to the mean values among population for each month; (a) and (b) correspond to the significant differences for each population in time frame studied. (I) and (II) denote significant differences at P < .05 among populations in time frame studied.
Caffeic acid and AME were detected in all the studied populations during the entire year (Tables 2 and 3). Artepillin C was found in most of the studied populations, with exception of the one from Colombo 1 (Table 5). Isosakuranetin was the phenolic that displayed larger qualitative variation, since it was detected mainly in the last 6 months, between November and April of 2005 (Table 4). Ferulic acid, baccharin and DCBEN, were found in almost every period of analysis, but in concentrations lower than the limit of quantification previously established [21].
Table 3.
Effect of seasonality on AME content (%) of leaves of B. dracunculifolia along 1 year.
| Month/ | São Paulo state | Minas Gerais state | Paraná state | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| regions | Franca 1(I) | Cajurú(I) | Franca 2(I) | Ribeirão Preto(I) | Campinas(I) | Paraguaçu(I) | Alfenas(I) | Ouro Fino(I) | Colombo 1(II) | Colombo 2(I) |
| May/04(B) | 0.64 ± 0.06(b) | 0.73 ± 0.07(b) | 0.78 ± 0.08(b) | 0.60 ± 0.07(b) | 0.72 ± 0.08(b) | 0.49 ± 0.22(b) | 0.80 ± 0.02(b) | 0.83 ± 0.19(b) | 0.79 ± 0.23(b) | 0.47 ± 0.11(b) |
| June/04(A) | 0.29 ± 0.01(a) | 0.30 ± 0.06(a) | 0.40 ± 0.05(a) | 0.29 ± 0.05(a) | 0.30 ± 0.01(a) | 0.29 ± 0.07(a) | 0.39 ± 0.12(a) | 0.56 ± 0.14(b) | 0.45 ± 0.13(a) | 0.27 ± 0.06(a) |
| July/04(A) | 0.32 ± 0.02(a) | 0.29 ± 0.02(a) | 0.29 ± 0.02(a) | 0.26 ± 0.03(a) | 0.25 ± 0.01(a) | 0.20 ± 0.08(a) | 0.31 ± 0.04(a) | 0.32 ± 0.07(a) | 0.56 ± 0.21(a) | 0.27 ± 0.10(a) |
| August/04(A) | 0.39 ± 0.01(a) | 0.38 ± 0.02(a) | 0.42 ± 0.03(a) | 0.34 ± 0.09(a) | 0.20 ± 0.10(a) | 0.18 ± 0.14(a) | 0.38 ± 0.01(a) | 0.37 ± 0.11(a) | 0.72 ± 0.16(b) | 0.50 ± 0.08(b) |
| Sept/04(B) | 0.91 ± 0.16(b) | 0.69 ± 0.11(b) | 0.79 ± 0.13(b) | 0.62 ± 0.13(b) | 0.59 ± 0.02(b) | 0.37 ± 0.27(b) | 0.76 ± 0.09(b) | 0.89 ± 0.28(b) | 1.11 ± 0.22(b) | 0.80 ± 0.11(b) |
| Oct/04(B) | 0.51 ± 0.16(b) | 0.29 ± 0.16(a) | 0.59 ± 0.13(b) | 0.43 ± 0.11(b) | 0.45 ± 0.01(b) | 0.43 ± 0.02(b) | 0.46 ± 0.01(b) | 0.45 ± 0.02(b) | 0.92 ± 0.21(b) | 0.63 ± 0.10(b) |
| Nov/04(A) | 0.38 ± 0.04(a) | 0.32 ± 0.03(a) | 0.32 ± 0.05(a) | 0.27 ± 0.04(a) | 0.32 ± 0.04(a) | 0.24 ± 0.04(a) | 0.30 ± 0.04(a) | 0.25 ± 0.03(a) | 0.90 ± 0.29(b) | 0.49 ± 0.14(b) |
| Dec/04(A) | 0.22 ± 0.04(a) | 0.27 ± 0.06(a) | 0.33 ± 0.06(a) | 0.20 ± 0.08(a) | 0.39 ± 0.13(a) | 0.23 ± 0.05(a) | 0.24 ± 0.02(a) | 0.21 ± 0.02(a) | 0.88 ± 0.23(b) | 0.56 ± 0.11(b) |
| Jan/05(A) | 0.20 ± 0.11(a) | 0.35 ± 0.13(a) | 0.45 ± 0.11(a) | 0.29 ± 0.09(a) | 0.32 ± 0.02(a) | 0.13 ± 0.01(a) | 0.15 ± 0.01(a) | 0.14 ± 0.01(a) | 0.92 ± 0.34(b) | 0.44 ± 0.17(b) |
| Feb/05(B) | 0.81 ± 0.12(b) | 0.64 ± 0.10(b) | 0.81 ± 0.15(b) | 0.49 ± 0.13(b) | 0.67 ± 0.13(b) | 0.81 ± 0.03(b) | 0.85 ± 0.13(b) | 0.67 ± 0.09(b) | 0.91 ± 0.30(b) | 0.06 ± 0.30(a) |
| March/05(B) | 0.61 ± 0.05(b) | 0.54 ± 0.06(b) | 0.66 ± 0.06(b) | 0.55 ± 0.05(b) | 0.56 ± 0.01(b) | 0.66 ± 0.06(b) | 0.58 ± 0.09(b) | 0.71 ± 0.07(b) | 0.86 ± 0.35(b) | 0.37 ± 0.17(b) |
| April/05(B) | 0.67 ± 0.16(b) | 0.45 ± 0.17(b) | 0.79 ± 0.14(b) | 0.57 ± 0.13(b) | 0.56 ± 0.03(b) | 0.72 ± 0.02(b) | 0.75 ± 0.06(b) | 0.67 ± 0.04(b) | 0.90 ± 0.29(b) | 0.47 ± 0.15(b) |
Capital and small letters denote significant differences at P < .01. (A) and (B) refer to the mean values among population for each month; (a) and (b) correspond to the significant differences for each population in time frame studied. (I) and (II) denote significant differences at P < .05 among populations in time frame studied.
Table 4.
Effect of seasonality on isosakuranetin content (%) of leaves of B. dracunculifolia along 1 year.
| Month/ | São Paulo state | Minas Gerais state | Paraná state | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| regions | Franca 1(I) | Cajurú(I) | Franca 2(I) | Ribeirão Preto(I) | Campinas(I) | Paraguaçu(II) | Alfenas(I) | Ouro Fino(I) | Colombo 1(II) | Colombo 2(I) |
| May/04(A) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) |
| June/04(A) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) |
| July/04(A) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) | 0.00(a) |
| August/04(A) | 0.49 ± 0.17(b) | 0.00(a) | 0.00(a) | 0.37 ± 0.05(b) | 0.00(a) | 0.71 ± 0.16(b) | 0.34 ± 0.01(b) | 0.35 ± 0.11(b) | 0.00(a) | 0.00(a) |
| Sept/04(A) | 0.53 ± 0.20(b) | 0.00(a) | 0.37 ± 0.08(b) | 0.50 ± 0.06(b) | 0.45 ± 0.06(b) | 1.01 ± 0.23(b) | 0.69 ± 0.05(b) | 0.62 ± 0.21(b) | 0.00(a) | 0.00(a) |
| Oct/04(A) | 0.00(a) | 0.00(a) | 0.34 ± 0.24(b) | 0.48 ± 0.03(b) | 0.40 ± 0.04(b) | 0.86 ± 0.17(b) | 0.48 ± 0.02(b) | 0.45 ± 0.23(b) | 0.00(a) | 0.26 ± 0.03(b) |
| Nov/04(B) | 0.61 ± 0.08(b) | 0.50 ± 0.05(b) | 0.56 ± 0.24(b) | 0.56 ± 0.01(b) | 0.57 ± 0.02(b) | 1.19 ± 0.26(b) | 0.68 ± 0.02(b) | 0.65 ± 0.30(b) | 0.00(a) | 0.26 ± 0.02(b) |
| Dec/04(B) | 0.92 ± 0.26(b) | 0.55 ± 0.04(b) | 0.49 ± 0.05(b) | 0.59 ± 0.04(b) | 0.61 ± 0.01(b) | 1.18 ± 0.28(b) | 0.79 ± 0.10(b) | 0.65 ± 0.27(b) | 0.00(a) | 0.25 ± 0.10(b) |
| Jan/05(B) | 0.74 ± 0.16(b) | 0.52 ± 0.01(b) | 0.50 ± 0.19(b) | 0.47 ± 0.07(b) | 0.53 ± 0.04(b) | 0.78 ± 0.16(b) | 0.55 ± 0.02(b) | 0.58 ± 0.13(b) | 0.00(a) | 0.26 ± 0.02(b) |
| Feb/05(B) | 1.11 ± 0.25(b) | 0.75 ± 0.04(b) | 0.81 ± 0.12(b) | 0.81 ± 0.02(b) | 0.86 ± 0.12(b) | 1.27 ± 0.08(b) | 1.16 ± 0.27(b) | 0.78 ± 0.26(b) | 0.00(a) | 0.35 ± 0.05(b) |
| March/05(B) | 0.89 ± 0.06(b) | 0.80 ± 0.08(b) | 0.69 ± 0.16(b) | 0.86 ± 0.26(b) | 0.83 ± 0.10(b) | 1.32 ± 0.32(b) | 0.87 ± 0.07(b) | 0.77 ± 0.29(b) | 0.00(a) | 0.36 ± 0.07(b) |
| April/05(B) | 0.16 ± 0.09(a) | 0.67 ± 0.05(b) | 0.60 ± 0.20(b) | 0.75 ± 0.03(b) | 0.79 ± 0.05(b) | 1.40 ± 0.28(b) | 0.86 ± 0.08(b) | 0.74 ± 0.25(b) | 0.00(a) | 0.47 ± 0.08(b) |
Capital and small letters denote significant differences at P < .01. (A) and (B) refer to the mean values among population for each month; (a) and (b) correspond to the significant differences for each population in time frame studied. (I) and (II) denote significant differences at P < .05 among populations in time frame studied.
Taking into account the agronomic aspect, chemical composition, seasonality role and phenolic contents, the results of this work are summarized in Figure 2 showing the potential of B. dracunculifolia for both pharmaceutical and cosmetic industries in the production not only of BGP, but also the standardized extracts and essential oil.
Figure 2.
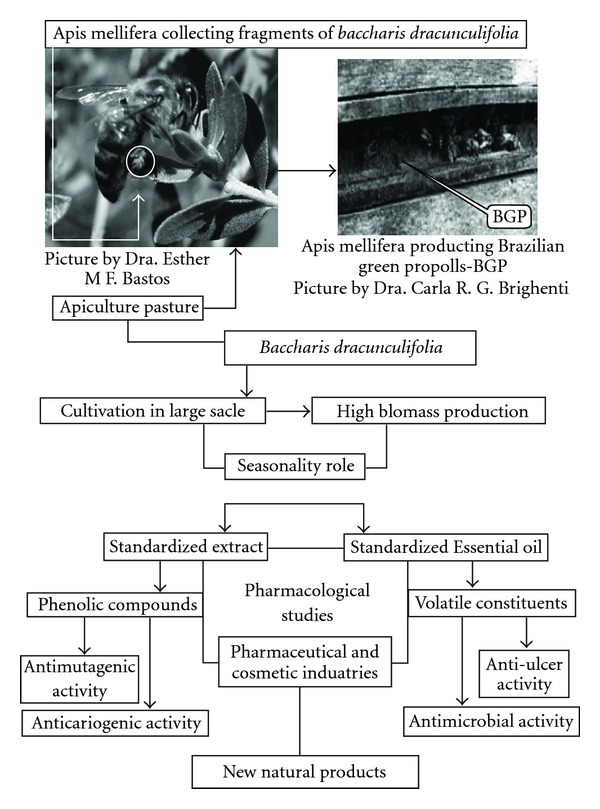
Diagram demonstrating the potential of B. dracunculifolia for both pharmaceutical and cosmetic industries in the production of BGP, standardized extracts and essential oil.
4. Discussion
4.1. Cultivation of B. dracunculifolia
Seasonality is an important factor to be considered in the cultivation of B. dracunculifolia because rainfall availability, humidity, temperature, nutrients as well as herbivory or attack of pathogens are basic factors that along with environment can influence biosynthesis of plant secondary metabolites. Baccharis dracunculifolia have drawn great attention among agronomists, chemists and pharmacists, aiming to develop the agro technological knowledge to allow both the acclimatization and the selection of a high productive population. In this regard, the knowledge about the best cultivation techniques is one of the first steps to develop commercial scale production.
The developed cultivation technique demonstrated to be feasible of cultivating 1000 plants in an area of 1800 m2. The yields of dry plant, essential oil and crude extract were measured monthly resulting in mean values of 399 g, 0.6 ± 0.1% and 20 ± 4%, respectively. Hence, the cultivation of B. dracunculifolia in large scale using an area of 10 000 m2, which is equivalent to 1 hectare, would allow to cultivate 5556 individuals furnishing, after 12 months of cultivation, about 2200 kg of dry plant, from which it could be obtained 13 kg of essential oil or 440 kg of crude extract. Therefore, it is viable to cultivate this plant in large scale for commercial use, since industries of medium and large productivity, specializing in plants, have the ability to grow at least 5 hectares of the plant of interest.
Considering B. dracunculifolia plant, this is the first time that cultivation studies, involving chemical composition analysis and seasonality role, have been reported. Thus, the cultivation of this species can provide biomass for phytochemical and pharmacological studies, as well as for the continuous supply of botanical raw material. Moreover, the selection of a good population for cultivation could enhance the production of desired compounds.
4.2. Standardized Extract and Phenolic Compounds
To obtain standardized extracts it is necessary to produce biomass with excellent quality. For that, the development of an analytical-validated method is mandatory to determine the concentration of each metabolite of interest in the plant biomass. Moreover, the analytical method is an important tool to study the influence of seasonality, to select a good population for cultivation, to determine the best time for harvesting, to develop the extraction and formulation process, to analyze the final products, to run the pre-clinical and clinical assays, among others.
Most of the works with B. dracunculifolia report its secondary metabolites as main source for the production of BGP. Because of that, there are researches reporting comparative studies about the chemical composition of this plant and its relationship with green propolis [24, 25]. So far, at least 100 substances in native B. dracunculifolia have been identified including: cinnamic acid derivatives, anthracene derivatives, phenolics, prenylated phenylpropanoids, sesquiterpenes, diterpenes, and triterpenes, among others, for which different biology activities have been found [9, 13, 20, 26].
Intake of the phenolic compounds present in both BGP and B. dracunculifolia as health promoter has been linked to reduced risk of colon cancer and gastrointestinal disorders [27, 28]. Caffeic, ferulic and p-coumaric acids are trans-cinnamic acids that occur naturally in their free forms, and as a family of mono or diesters with (–)-quinic acid, collectively known as chlorogenic acids (CGAs). CGAs are antioxidant components produced by plants in response to environmental stress conditions such as infection by microbial pathogens, mechanical wounding as well as excessive UV or visible light levels [29].
An important phenolic acid, present in both Brazilian propolis and B. dracunculifolia, is 3,5-diprenyl-4-hydroxycinnamic acid (DHCA), which is known as artepillin C. Studies have shown that DHCA inhibits lipid peroxidation and the development of pulmonary cancer in mice prevents colon cancer through the induction of cell-cycle arrest, and displays chemopreventive action in colon carcinogenesis, as well as anti-leukemic effect with low inhibitory effect on normal lymphocytes [30]. Isosakuranetin and AME, along with other flavonoids have been widely investigated [9, 13, 31], and their intake may have beneficial effects such as: increase vitamin absorption and action, help wound-healing processes, act as antioxidant, antimicrobial and immunomodulatory [32]. With respect to derivatives of p-coumaric and caffeolyquinic acids, a positive association of the biological activity against Staphylococcus aureus, S. pneumoniae and Trypanosoma cruzi was found [26]. DHCA and DCBEN, previously characterized in both BGP and B. dracunculifolia, were active against T. cruzi and S. aureus [33]. In addition, Barros et al. [28] demonstrated that caffeic, ferulic, p-coumaric and cinnamic acids possess gastro-protective activity. Therefore, the knowledge of the chemical variations of phenolic compounds in B. dracunculifolia becomes essential to provide raw materials of high quality for the development of new products. In this regard, the developed protocols allowed to undertake the analysis of 480 samples of B. dracunculifolia collected monthly during 1 year. Also, based on these results AME along with caffeic acid could be considered good chemical markers for the analysis of cultivated B. dracunculifolia, considering that both were found in all the samples throughout the studied year.
4.3. Seasonal Variation
The phenolic compounds can be considered as a chemical interface between B. dracunculifolia and surrounding environment, and their biosynthesis can be amended by environmental conditions. Thus, Weather and Climate Applied to Agricultural Research Center CEPAGRI-UNICAMP monitored rainfall availability, humidity and temperature of the cultivation site. The mean of temperature, considering the 12 months of the experiment, was 22.5˚C. The lowest mean temperature (19˚C) was detected between May and July and the highest (25˚C) was detected between January and March. The average rainfall for the year was 120 mm. The lowest average of rain (42 mm) occurred from May to July and the highest amount of rain (218 mm) occurred from January to March. The humidity did not vary significantly along the year, resulting in mean values of 60%. It is important to point out that the flowering period of B. dracunculifolia in this experiment occurred from May to July of 2004, which was the period that displayed both lower temperature and less amount of rain. During the flowering period wide variation in the concentration of phenolics was observed (Tables 2–5).
It is mandatory to know the role of seasonality on the chemical profile and the content of each compound of interest in a cultivated plant for pharmaceutical use, aiming to obtain either standardized extract or pure compounds, which can define the potential of individual components and give its potency on the synergistic effect, considering the major metabolites as a whole.
According to statistic studies (one-way ANOVA), the caffeic acid did not vary significantly during the period of study in both individuals and among all populations, with mean value of 4.0% (Table 2). The population from Colombo 1-Paraná displayed significant variations for artepillin C (P < .05) (Table 5) and AME (P < .05) (Table 3). On one hand, the concentrations of AME, comparing population from Colombo 1 with other populations were higher ranging between 0.45% and 1.11%, and on the other hand the concentrations of artepillin C were lower (0–0.16%). The isosakuranetin (Table 4) showed statistic difference for the populations from Paraguaçu-Minas Gerais and Colombo 1. The concentration levels for this flavonoid in Paraguaçu region were higher (0–1%; P < .05) in comparison with other populations, while for the population from Colombo 1, isosakuranetin was not detected.
The statistical analysis of the data, considering each population and the mean values, which were obtained among the population for the different months of the experiment, are shown in Tables 2–5 with P < .01. In general, the significant values found for each population were similar to the ones obtained by the calculation of the mean values given by each month. Thus, taking into account the monthly mean values it is possible inferring that caffeic acid content was higher in the months of October (4.96 ± 0.92%), January (4.93 ± 0.53%), March (5.17 ± 0.52%) and April (4.33 ± 0.50%). Isosakuranetin and artepillin C displayed higher concentrations in the months of November, December and from January through April. Likewise, the means concentration for isosakuranetin ranged from 0.49 ± 0.20 to 0.79 ± 0.32%, and for the prenylated p-coumaric acid derivate, it ranged from 0.25 ± 0.10 to 0.71 ± 0.21%. Regarding AME, its concentrations behaved differently from the other phenolics. The mean yield for this phenolic was about 0.7 ± 0.13% in May, decreasing to 0.4 ± 0.15% until August. In September there was an increase again to 0.75 ± 0.20%, which decreased to 0.34 ± 0.24% in January, and increased to 0.65 ± 0.13% in February, which was maintained until April. Statistically, AME content was higher in the months of May, September and February through April. Moreover, during this same period other compounds, such as ferulic acid, baccharin and DCBEN were detected.
Considering the interaction between B. dracunculifolia and A. mellifera in the production of green propolis, it is interesting to note that, according to Lima [34], the optimum time of the year for the highest yield of BGP production was from December to April. Sousa et al. [23] demonstrate that the yield of essential oil from B. dracunculifolia leaves is higher from February to April. It is important to point out that the relationship between period of leafbud growing and contents of physiological compounds was confirmed by this experiment, once the period of leafbud growth was coincident with the period of higher content of secondary metabolites, which corresponds to rainfall season. Therefore, the optimum time for both green propolis and B. dracunculifolia essential oil production was the same for phenolic compounds production as well. Hence, it is suggested that the best time to obtain good qualitative and quantitative results, considering phenolic compounds, essential oil and green propolis production is mainly between December and April, which matches with the summer time. All populations were cultivated, and the population from Colombo 1 produced the highest yield of dry biomass and good concentration of AME, but it produced the lowest amounts of other important compounds, such as artepillin C and isosakuranetin.
The cultivation of B. dracunculifolia is economically viable, and it can be scaled up for commercial production, since the biomass production, mean yields of crude extract and essential oils, as well as phenolic compounds were excellent.
Funding
FAPESP (Grants, No. 04/13005-1; 06/59893-0; 01/14219-7); and CAPES (Grant. PDEE/BEX 0387/04-5), Brazil.
Acknowledgment
The cultivation of B. dracunculifolia is economically viable, and it can be scaled up for commercial production, since the biomass production, mean yields of crude extract and essential oils, as well as phenolic compounds were excellent.
References
- 1.Teixeira ÉW, Negri G, Meira RMSA, Message D, Salatino A. Plant origin of green propolis: bee behavior, plant anatomy and chemistry. Evidence-Based Complementary and Alternative Medicine. 2005;2(1):85–92. doi: 10.1093/ecam/neh055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sousa JPB, Furtado NAJC, Jorge R, Soares AEE, Bastos JK. Perfis físico-químico e cromatográfico de amostras de própolis produzidas nas microregiões de Franca (SP) e Passos (MG) Brazilian Journal of Pharmacognosy. 2007;17:85–93. [Google Scholar]
- 3.Bazo AP, Rodrigues MAM, Sforcin JM, De Camargo JLV, Ribeiro LR, Salvadori DMF. Protective action of propolis on the rat colon carcinogenesis. Teratogenesis Carcinogenesis and Mutagenesis. 2002;22(3):183–194. doi: 10.1002/tcm.10011. [DOI] [PubMed] [Google Scholar]
- 4.Sforcin JM, Fernandes A, Jr., Lopes CAM, Bankova V, Funari SRC. Seasonal effect on Brazilian propolis antibacterial activity. Journal of Ethnopharmacology. 2000;73(1-2):243–249. doi: 10.1016/s0378-8741(00)00320-2. [DOI] [PubMed] [Google Scholar]
- 5.Reis CMF, Carvalho JCT, Caputo LRG, Patrício KCM, Barbosa MVJ, Chieff AL, et al. Atividade antiinflamatória, antiúlcera gástrica e toxicidade subcrôncia do extrato etanólico de própolis. Brazilian Journal of Pharmacognosy. 2000;10:43–52. [Google Scholar]
- 6.de Barros MP, Sousa JPB, Bastos JK, de Andrade SF. Effect of Brazilian green propolis on experimental gastric ulcers in rats. Journal of Ethnopharmacology. 2007;110(3):567–571. doi: 10.1016/j.jep.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Lemos M, De Barros MP, Sousa JPB, Da Silva Filho AA, Bastos JK, De Andrade SF. Baccharis dracunculifolia the main botanical source of Brazilian green propolis, displays antiulcer activity. Journal of Pharmacy and Pharmacology. 2007;59(4):603–608. doi: 10.1211/jpp.59.4.0017. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M, Ohkoshi E, Makino M, Fujimoto Y. Constituents of the Leaves of Baccharis dracunculifolia (Asteraceae) and their cytotoxic activity. Chemical and Pharmaceutical Bulletin. 2006;54:1465–1468. doi: 10.1248/cpb.54.1465. [DOI] [PubMed] [Google Scholar]
- 9.Da Silva Filho AA, Bueno PCP, Gregório LE, Silva MLA, Albuquerque S, Bastos JK. In vitro trypanocidal evaluation of crude extract and isolated compouns from Baccharis dracunculifolia D. C. (Asteraceae) Journal of Pharmacy and Pharmacology. 2004;56:1195–1199. doi: 10.1211/0022357044067. [DOI] [PubMed] [Google Scholar]
- 10.Munari CC, Resende FA, Alves JM, De Sousa JPB, Bastos JK, Tavares DC. Mutagenicity and antimutagenicity of Baccharis dracunculifolia extract in chromosomal aberration assays in Chinese hamster ovary cells. Planta Medica. 2008;74(11):1363–1367. doi: 10.1055/s-2008-1081306. [DOI] [PubMed] [Google Scholar]
- 11.Akao Y, Maruyama H, Matsumoto K, et al. Cell growth inhibitory effect of cinnamic acid derivatives from propolis on human tumor cell lines. Biological and Pharmaceutical Bulletin. 2003;26(7):1057–1059. doi: 10.1248/bpb.26.1057. [DOI] [PubMed] [Google Scholar]
- 12.Missima F, Da Silva Filho AA, Nunes GA, et al. Effect of Baccharis dracunculifolia D.C. (Asteraceae) extracts and its isolated compounds on macrophage activation. Journal of Pharmacy and Pharmacology. 2007;59(3):463–468. doi: 10.1211/jpp.59.3.0017. [DOI] [PubMed] [Google Scholar]
- 13.Leitão DPS, Da Silva Filho AA, Polizello ACM, Bastos JK, Spadaro ACC. Comparative evaluation of in vitro effects of Brazilian green propolis and Baccharis dracunculifolia extracts on cariogenic factors of Streptococcus mutans . Biological and Pharmaceutical Bulletin. 2004;27:1834–1839. doi: 10.1248/bpb.27.1834. [DOI] [PubMed] [Google Scholar]
- 14.Klopell FC, Lemos M, Sousa JPB, et al. Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae) Zeitschrift fur Naturforschung. Section C. 2007;62(7-8):537–542. doi: 10.1515/znc-2007-7-812. [DOI] [PubMed] [Google Scholar]
- 15.Hegazi AG, Abd El-Hady FK. Influence of honey on the suppression of human low density lipoprotein (LDL) peroxidation (in vitro) Evidence-Based Complementary and Alternative Medicine. 2009;6(1):113–121. doi: 10.1093/ecam/nem071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon A, Traynor K, Santos K, Blaser G, Bode U, Molan P. Medical honey for wound carestill the latest resort. Evidence-Based Complementary and Alternative Medicine. 2009;6(2):165–173. doi: 10.1093/ecam/nem175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuda M, Kobayashi K, Hirono Y, Miyagawa M, Ishida T, Ejiogu EC, et al. Jungle honey enhances immune function and antitumor activity. doi: 10.1093/ecam/nen086. Evidence-Based Complementary and Alternative Medicine. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teixeira EW, Message D, Negri G, Salatino A, Stringheta PC. Seasonal variation, chemical composition and antioxidant activity of Brazilian Propolis samples. doi: 10.1093/ecam/nem177. Evidence-Based Complementary and Alternative Medicine. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Sousa JPB, Bueno PCP, Gregório LE, et al. A reliable quantitative method for the analysis of phenolic compounds in Brazilian propolis by reverse phase high performance liquid chromatography. Journal of Separation Science. 2007;30(16):2656–2665. doi: 10.1002/jssc.200700228. [DOI] [PubMed] [Google Scholar]
- 20.Da Silva Filho AA, De Sousa JPB, Soares S, et al. Antimicrobial activity of the extract and isolated compounds from Baccharis dracunculifolia D. C. (Asteraceae) Zeitschrift fur Naturforschung. Section C. 2008;63(1-2):40–46. doi: 10.1515/znc-2008-1-208. [DOI] [PubMed] [Google Scholar]
- 21.de Sousa JPB, da Silva Filho AA, Bueno PCP, et al. A validated reverse-phase HPLC analytical method for the quantification of phenolic compounds in Baccharis dracunculifolia . Phytochemical Analysis. 2009;20(1):24–32. doi: 10.1002/pca.1087. [DOI] [PubMed] [Google Scholar]
- 22.Zar JH. Biostatistical Analysis. 3rd edition. Englewood Cliffs, NJ, USA: Prentice-Hall; 1999. [Google Scholar]
- 23.Sousa JPB, Jorge RF, Da Silva Filho AA, Furtado NAJC, Leite MF, Queiroga CL, et al. Seasonal variation of the (E)-nerolidol and other volatile compounds within ten different cultivated populations of Baccharis dracunculifolia D.C. (Asteraceae) Journal of Essential Oil Research. 2009;21:308–314. [Google Scholar]
- 24.Park YK, Paredes-Guzman JF, Aguiar CL, Alencar SM, Fujiwara FY. Chemical constituents in Baccharis dracunculifolia as the main botanical origin of southeastern Brazilian propolis. Journal of Agricultural and Food Chemistry. 2004;52(5):1100–1103. doi: 10.1021/jf021060m. [DOI] [PubMed] [Google Scholar]
- 25.Salatino A, Teixeira ÉW, Negri G, Message D. Origin and chemical variation of Brazilian propolis. Evidence-Based Complementary and Alternative Medicine. 2005;2(1):33–38. doi: 10.1093/ecam/neh060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salomão K, Pereira PRS, Campos LC, et al. Brazilian propolis: correlation between chemical composition and antimicrobial activity. Evidence-Based Complementary and Alternative Medicine. 2008;5(3):317–324. doi: 10.1093/ecam/nem058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulino N, Abreu SRL, Uto Y, et al. Anti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolis. European Journal of Pharmacology. 2008;587(1–3):296–301. doi: 10.1016/j.ejphar.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 28.Barros MP, Lemos M, Maistro EL, Leite MF, Sousa JPB, Bastos JK, et al. Evaluation of antiulcer activity of the main phenolic acids found in Brazilian Green Própolis. Journal of Ethnopharmacology. 2008;120:372–377. doi: 10.1016/j.jep.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Farah A, Donangelo CM. Phenolic compounds in coffee. Brazilian Journal of Plant Physiology. 2006;18(1):23–36. [Google Scholar]
- 30.Piantino CR, Aquino FWB, Follegatti-Romero LA, Cabral FA. Supercritical CO2 extraction of phenolic compounds from Baccharis dracunculifolia . The Journal of Supercritical Fluids. 2008;47:209–214. [Google Scholar]
- 31.Volpi N, Bergonzini G. Analysis of flavonoids from propolis by on-line HPLC-electrospray mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2006;42(3):354–361. doi: 10.1016/j.jpba.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Sawaya ACHF, Tomazela DM, Cunha IBS, et al. Electrospray ionization mass spectrometry fingerprinting of propolis. Analyst. 2004;129(8):739–744. doi: 10.1039/b403873h. [DOI] [PubMed] [Google Scholar]
- 33.Marcucci MC, Ferreres F, García-Viguera C, et al. Phenolic compounds from Brazilian propolis with pharmacological activities. Journal of Ethnopharmacology. 2001;74(2):105–112. doi: 10.1016/s0378-8741(00)00326-3. [DOI] [PubMed] [Google Scholar]
- 34.Lima MG. Efeito de variáveis ambientais, rainhas selecionadas e sistemas coletores na produção de própolis por abelhas africanizadas Apis mellifera (Hymenoptera, Apoidea) Instituto de Biociências, UNESP, Rio Claro, SP, Brazil: Tese de Doutorado; 2005. p. 73. [Google Scholar]


