Abstract
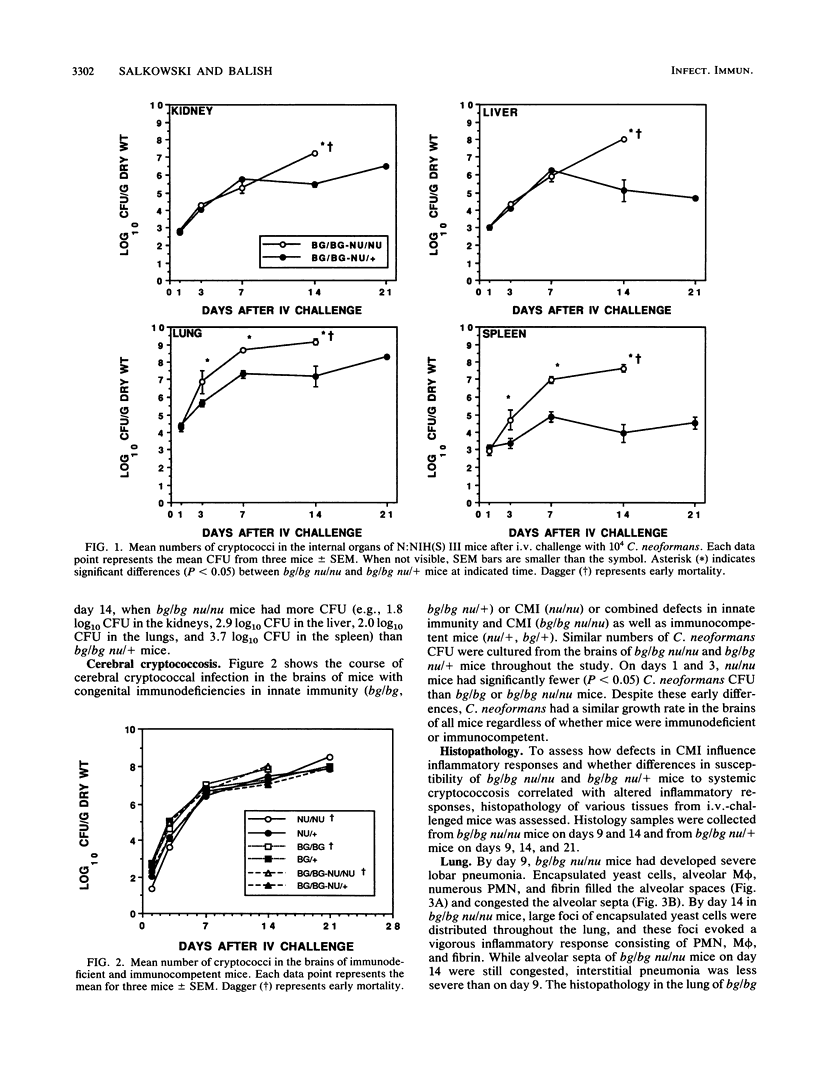
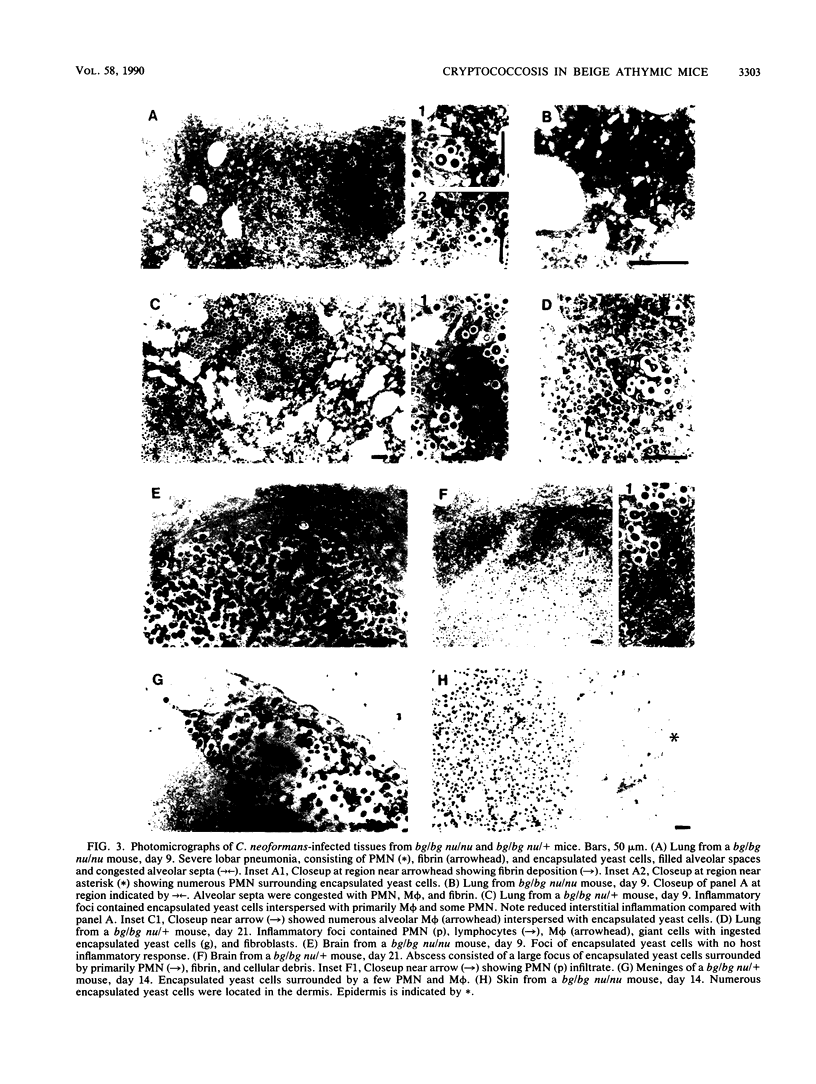
Mortality after intravenous challenge with 10(4) Cryptococcus neoformans demonstrated that doubly immunodeficient beige athymic (bg/bg nu/nu) mice were more susceptible to systemic cryptococcosis than either bg/bg or nu/nu mice. Infected bg/bg nu/nu mice also had a shortened lifespan compared with their bg/bg nu/+ littermates. Beige athymic (bg/bg nu/nu) but not bg/bg nu/+mice developed cryptococcal lesions in the skin, demonstrating that C. neoformans is dermatotropic in a T-cell-deficient host. Higher numbers of C. neoformans were isolated from the lungs and spleen of infected bg/bg nu/nu than bg/bg nu/+ mice as early as day 3 after challenge, indicating that in lymphoid-rich organs, T cells can alter the course of systemic cryptococcosis early in the infection. Despite extensive abscess formation in the brains of bg/bg nu/+ mice, dissemination and growth rate of C. neoformans in the brain was similar in both genotypes. The primary histopathological feature in tissues from bg/bg nu/nu mice infected with C. neoformans consisted of foci of encapsulated yeast cells with minimal to no inflammatory response. In contrast to bg/bg nu/nu mice, bg/bg nu/+ mice mounted a vigorous inflammatory response to C. neoformans that progressed from acute to chronic inflammation. Beige athymic mice are a new animal model that will be useful in clarifying the innate and acquired immune factors important in resistance to cryptococcosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azar H. A., Hansen C. T., Costa J. N:NIH(S)-nu/nu mice with combined immunodeficiency: a new model for human tumor heterotransplantation. J Natl Cancer Inst. 1980 Aug;65(2):421–430. [PubMed] [Google Scholar]
- Balish E., Filutowicz H., Oberley T. D. Correlates of cell-mediated immunity in Candida albicans-colonized gnotobiotic mice. Infect Immun. 1990 Jan;58(1):107–113. doi: 10.1128/iai.58.1.107-113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartizal K. F., Salkowski C., Pleasants J. R., Balish E. The effect of microbial flora, diet, and age on the tumoricidal activity of natural killer cells. J Leukoc Biol. 1984 Dec;36(6):739–750. doi: 10.1002/jlb.36.6.739. [DOI] [PubMed] [Google Scholar]
- Blackstock R., Hall N. K. Non-specific immunosuppression by Cryptococcus neoformans infection. Mycopathologia. 1984 Apr 30;86(1):35–43. doi: 10.1007/BF00437227. [DOI] [PubMed] [Google Scholar]
- Borton L. K., Wintroub B. U. Disseminated cryptococcosis presenting as herpetiform lesions in a homosexual man with acquired immunodeficiency syndrome. J Am Acad Dermatol. 1984 Feb;10(2 Pt 2):387–390. doi: 10.1016/s0190-9622(84)80013-4. [DOI] [PubMed] [Google Scholar]
- Cantorna M. T., Balish E. Mucosal and systemic candidiasis in congenitally immunodeficient mice. Infect Immun. 1990 Apr;58(4):1093–1100. doi: 10.1128/iai.58.4.1093-1100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley L. K., Murphy J. W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979 Mar;23(3):644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler F. W. Pathology of the mycoses in patients with the acquired immunodeficiency syndrome (AIDS). Curr Top Med Mycol. 1985;1:1–23. doi: 10.1007/978-1-4613-9547-8_1. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Root R. K., Bennett J. E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972 Apr;125(4):367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- Fidel P. L., Jr, Murphy J. W. Characterization of a cell population which amplifies the anticryptococcal delayed-type hypersensitivity response. Infect Immun. 1990 Feb;58(2):393–398. doi: 10.1128/iai.58.2.393-398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Schwamberger G., Kaufmann S. H. Fungicidal activity of IFN-gamma-activated macrophages. Extracellular killing of Cryptococcus neoformans. J Immunol. 1989 May 1;142(9):3219–3224. [PubMed] [Google Scholar]
- Fodstad O., Hansen C. T., Cannon G. B., Boyd M. R. Immune characteristics of the beige-nude mouse. A model for studying immune surveillance. Scand J Immunol. 1984 Sep;20(3):267–272. doi: 10.1111/j.1365-3083.1984.tb01002.x. [DOI] [PubMed] [Google Scholar]
- Fodstad O., Hansen C. T., Cannon G. B., Statham C. N., Lichtenstein G. R., Boyd M. R. Lack of correlation between natural killer activity and tumor growth control in nude mice with different immune defects. Cancer Res. 1984 Oct;44(10):4403–4408. [PubMed] [Google Scholar]
- Fung P. Y., Murphy J. W. In vitro interactions of immune lymphocytes and Cryptococcus neoformans. Infect Immun. 1982 Jun;36(3):1128–1138. doi: 10.1128/iai.36.3.1128-1138.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Macrophage-mediated fungistasis in vitro: requirements for intracellular and extracellular cytotoxicity. J Immunol. 1986 Jan;136(2):672–680. [PubMed] [Google Scholar]
- Graybill J. R., Mitchell L., Drutz D. J. Host defense in cryptococcosis. III. Protection of nude mice by thymus transplantation. J Infect Dis. 1979 Oct;140(4):546–552. doi: 10.1093/infdis/140.4.546. [DOI] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Natural cellular resistance of beige mice against Cryptococcus neoformans. J Immunol. 1986 Dec 1;137(11):3624–3631. [PubMed] [Google Scholar]
- Iacobellis F. W., Jacobs M. I., Cohen R. P. Primary cutaneous cryptococcosis. Arch Dermatol. 1979 Aug;115(8):984–985. [PubMed] [Google Scholar]
- Kärre K., Klein G. O., Kiessling R., Klein G., Roder J. C. In vitro NK-activity and in vivo resistance to leukemia: studies of beige, beige//nude and wild-type hosts on C57BL background. Int J Cancer. 1980 Dec 15;26(6):789–797. doi: 10.1002/ijc.2910260613. [DOI] [PubMed] [Google Scholar]
- Levitz S. M., DiBenedetto D. J. Differential stimulation of murine resident peritoneal cells by selectively opsonized encapsulated and acapsular Cryptococcus neoformans. Infect Immun. 1988 Oct;56(10):2544–2551. doi: 10.1128/iai.56.10.2544-2551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W., Cauley L. K. Host-etiological agent interactions in intranasally and intraperitoneally induced Cryptococcosis in mice. Infect Immun. 1980 Aug;29(2):633–641. doi: 10.1128/iai.29.2.633-641.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect Immun. 1980 Oct;30(1):5–11. doi: 10.1128/iai.30.1.5-11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis G., Montplaisir S., Pelletier M., Mousseau S., Auger P. Genetic resistance to murine cryptococcosis: increased susceptibility in the CBA/N XID mutant strain of mice. Infect Immun. 1985 Jan;47(1):282–287. doi: 10.1128/iai.47.1.282-287.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis G., Montplaisir S., Pelletier M., Mousseau S., Auger P. Genetic resistance to murine cryptococcosis: the beige mutation (Chédiak-Higashi syndrome) in mice. Infect Immun. 1985 Jan;47(1):288–293. doi: 10.1128/iai.47.1.288-293.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. F., Mitchell T. G., Storkus W. J., Dawson J. R. Human natural killer cells do not inhibit growth of Cryptococcus neoformans in the absence of antibody. Infect Immun. 1990 Mar;58(3):639–645. doi: 10.1128/iai.58.3.639-645.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaji M., Nishimura K. Studies on organ specificity in experimental murine cryptococcosis. Mycopathologia. 1981 Dec 11;76(3):145–154. doi: 10.1007/BF00437195. [DOI] [PubMed] [Google Scholar]
- Mody C. H., Lipscomb M. F., Street N. E., Toews G. B. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J Immunol. 1990 Feb 15;144(4):1472–1477. [PubMed] [Google Scholar]
- Murphy J. W., Cox R. A. Induction of antigen-specific suppression by circulating Cryptococcus neoformans antigen. Clin Exp Immunol. 1988 Aug;73(2):174–180. [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W. Influence of cryptococcal antigens on cell-mediated immunity. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S432–S435. doi: 10.1093/cid/10.supplement_2.s432. [DOI] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Rook A. H., Frederick W. R., Manischewitz J. F., Epstein J. S., Siegel J., Masur H., Macher A. M., Mitchell C., Armstrong G. Prevalence, clinical manifestations, and immunology of herpesvirus infections in the acquired immunodeficiency syndrome. Ann N Y Acad Sci. 1984;437:200–206. doi: 10.1111/j.1749-6632.1984.tb37138.x. [DOI] [PubMed] [Google Scholar]
- Ray T. L. Fungal infections in the immunocompromised host. Med Clin North Am. 1980 Sep;64(5):955–968. doi: 10.1016/s0025-7125(16)31576-0. [DOI] [PubMed] [Google Scholar]
- Rhodes J. C., Wicker L. S., Urba W. J. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect Immun. 1980 Aug;29(2):494–499. doi: 10.1128/iai.29.2.494-499.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico M. J., Penneys N. S. Cutaneous cryptococcosis resembling molluscum contagiosum in a patient with AIDS. Arch Dermatol. 1985 Jul;121(7):901–902. [PubMed] [Google Scholar]
- Roder J. C., Lohmann-Matthes M. L., Domzig W., Wigzell H. The beige mutation in the mouse. II. Selectivity of the natural killer (NK) cell defect. J Immunol. 1979 Nov;123(5):2174–2181. [PubMed] [Google Scholar]
- Saxena R. K., Saxena Q. B., Adler W. H. Defective T-cell response in beige mutant mice. Nature. 1982 Jan 21;295(5846):240–241. doi: 10.1038/295240a0. [DOI] [PubMed] [Google Scholar]
- Talmadge J. E., Herberman R. B., Chirigos M. A., Maluish A. E., Schneider M. A., Adams J. S., Philips H., Thurman G. B., Varesio L., Long C. Hyporesponsiveness to augmentation of murine natural killer cell activity in different anatomical compartments by multiple injections of various immunomodulators including recombinant interferons and interleukin 2. J Immunol. 1985 Oct;135(4):2483–2491. [PubMed] [Google Scholar]
- Walzer P. D., Kim C. K., Linke M. J., Pogue C. L., Huerkamp M. J., Chrisp C. E., Lerro A. V., Wixson S. K., Hall E., Shultz L. D. Outbreaks of Pneumocystis carinii pneumonia in colonies of immunodeficient mice. Infect Immun. 1989 Jan;57(1):62–70. doi: 10.1128/iai.57.1.62-70.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]




