Abstract
Baccharis dracunculifolia DC (Asteraceae) is a Brazilian medicinal plant popularly used for its antiulcer and anti-inflammatory properties. This plant is the main botanical source of Brazilian green propolis, a natural product incorporated into food and beverages to improve health. The present study aimed to investigate the chemical profile and intestinal anti-inflammatory activity of B. dracunculifolia extract on experimental ulcerative colitis induced by trinitrobenzenosulfonic acid (TNBS). Colonic damage was evaluated macroscopically and biochemically through its evaluation of glutathione content and its myeloperoxidase (MPO) and alkaline phosphatase activities. Additional in vitro experiments were performed in order to test the antioxidant activity by inhibition of induced lipid peroxidation in the rat brain membrane. Phytochemical analysis was performed by HPLC using authentic standards. The administration of plant extract (5 and 50 mg kg−1) significantly attenuated the colonic damage induced by TNBS as evidenced both macroscopically and biochemically. This beneficial effect can be associated with an improvement in the colonic oxidative status, since plant extract prevented glutathione depletion, inhibited lipid peroxidation and reduced MPO activity. Caffeic acid, p-coumaric acid, aromadendrin-4-O-methyl ether, 3-prenyl-p-coumaric acid, 3,5-diprenyl-p-coumaric acid and baccharin were detected in the plant extract.
1. Introduction
The inflammatory bowel diseases (IBD) refer essentially to two different but closely related conditions, Crohn's disease and ulcerative colitis. Although the etiology of IBD remains unclear, there is evidence that it involves immune, genetic and environmental factors, which are, in turn, related to the initiation and progression of colitis [1, 2]. IBD is related to an abnormally exacerbated immune response to otherwise innocuous stimuli which is not properly abrogated by the feedback system that normally downregulates the mucosal response to luminal factors [3]. As a consequence, increased numbers of inflammatory cells are found in areas of intestine with chronic inflammation, resulting in overproduction of variety of proinflammatory mediators including eicosanoids, platelet-activating factor, cytokines and reactive oxygen and nitrogen metabolites [1–4]. The oxidative stress through an excessive release of reactive oxygen species has been hypothesized to play a key role in IBD pathogenesis [5, 6]. In fact, antioxidant activity may be responsible for the beneficial effects shown by 5-aminosalicylates in human IBD [7] and for benefits derived from different natural compounds in experimental models, mainly phenolic compounds as flavonoids [8], tempol [9], isocoumarin [10] and coumarin derivatives [11].
Baccharis dracunculifolia DC (Asteraceae) is a Brazilian medicinal shrub popularly known as “Alecrim do Campo” that is used against ulcer, inflammation and hepatic disorders [12, 13]. This medicinal plant was found to be the main botanical source of resin and chemical constituents of Brazilian propolis, denominated green propolis, which was recently incorporated into food and beverages to improve health and to prevent several diseases [14–16].
In recent years, there has been growing interest in studying the chemical profile and biological activity of Brazilian green propolis and its main botanical source, B. dracunculifolia. Several studies have identified B. dracunculifolia as an important source of active compounds with trypanocidal [17], antiulcer [18], antimicrobial [19], antimutagenic [20], immunomodulatory [21], anti-inflammatory [22] and radical scavenging [23] activities. Although green propolis composition is more complex and unpredictable than previously assumed [24], it is clear that pharmacological potentialities of green propolis are related to substances previously known as plant constituents, mainly from B. dracunculifolia [14, 16, 24–26].
Therefore, the present work aimed to evaluate the intestinal anti-inflammatory activity of ethyl acetate extract from aerial parts of B. dracunculifolia in the trinitrobenzenesulfonic (TNBS) acid model of rat colitis. For this purpose, we assayed the effects of B. dracunculifolia extract in preventing the inflammatory response induced by TNBS in two different experimental settings, that is, when the colonic mucosa is intact and when the mucosa is healing after an initial insult. Thus, the present work also sought to determine the chemical constituents of the B. dracunculifolia ethyl acetate extract through HPLC analysis.
2. Methods
2.1. Preparation of Plant Extract
The aerial parts of B. dracunculifolia DC were collected in Cajuru city, State of São Paulo, Brazil, in December 2005. The plant material was authenticated by Jimi N. Nakagima, and a voucher specimen (SPFR 06143) was deposited in the Herbarium of the Biology Department of the University of São Paulo (FFCLRP) at Ribeirão Preto, State of São Paulo, Brazil. Fresh plant material was air-dried at 40°C for 48 h. The dried leaves were powdered in a blender and submitted to maceration for 24 h in ethyl acetate at room temperature. This solvent was used due to its polyphenolic content. After solvent evaporation using vacuum <40°C, an ethyl acetate extract of B. dracunculifolia was obtained.
2.2. Experimental Design
Male Wistar rats (200 ± 20 g) obtained from the Laboratory Animal Service of the São Paulo State University (UNESP) were randomly distributed in experimental groups. The study was carried out in accordance with “Guide for the Care and Use of Laboratory Animals” as promulgated by the Animal Experimental Committee of São Paulo State University (protocol number 042/04-CEEA-IB). Animals were housed in makrolon cages and maintained in air-conditioned with a 12 h light-dark cycle and air-filtration, and they were provided with free access to tap water and food. Colitis was induced by the method originally described [27]. Animals were fasted overnight and anaesthetized with halothane. Under anaesthesia, they were given 10 mg of TNBS dissolved in 0.25 ml 50% ethanol (v/v) by means of a Teflon cannula inserted 8 cm through the anus. Rats from the non-colitic group received 0.25 ml of saline.
2.2.1. Acute Colitis
Rats were given 5, 10, 25, 50, 100 and 200 mg kg−1 per day of B. dracunculifolia extract for 72, 48, 24 and 2 h before colitis induction as well as 24 h thereafter. The plant extract was suspended in 1% (v/v) Tween 80 and administered by means of an oesophageal catheter. Rats from the non-colitic group were orally administered with the saline and rats non-treated colitic (TNBS-control group) with the vehicle (1% Tween 80). Animals from all groups (n = 6) were killed 48 h after colitis induction.
2.2.2. Chronic Colitis
In this protocol, colitis was induced with 10 mg of TNBS in 50% ethanol, as previously described. The animals were divided in three groups; two groups were daily orally treated with 5 and 50 mg kg−1 of plant extract, one group received instead 25 mg kg−1 of sulfasalazine suspended in the same vehicle. Treatments started 2 h after the first administration of TNBS and continued until the day before the animals were killed. Two additional groups were included for reference: a non-colitic group and a colitic group, receiving the latter only the first dose of TNBS (TNBS control group). The animals of these groups were given the vehicle (5 ml kg−1 1% Tween 80) orally. Animals from each group were killed after 1 or 2 weeks of the colitis induction.
2.2.3. Assessment of Colonic Damage
Animal body weights, occurrence of diarrhoea and adherence of adjacent organs were recorded. The animals were euthanized by an overdose of halothane, and colonic segments were obtained after laparotomy. Colon were placed on an ice-cold plate, cleaned of fat and mesentery, and blotted on filter paper, weighed and its length measured under a constant load (2 g). The colon was longitudinally opened and scored for macroscopically visible damage on a 0–10 scale by two observers unaware of the treatment, according to the criteria described [28] in Table 1. The colon was subsequently divided longitudinally into different pieces to be used for the biochemical determinations: total glutathione (GSH) content [29], myeloperoxidase (MPO) activity [30] and alkaline phosphatase (AP) activity [31].
Table 1.
Criteria for assessment of macroscopic colonic damage
| Score | Criteria |
|---|---|
| 0 | No damage |
| 1 | Hyperemia, no ulcers |
| 2 | Linear ulcer with no significant inflammation |
| 3 | Linear ulcer with inflammation at one site |
| 4 | Two or more sites of ulceration/inflammation |
| 5 | Two or more major sites of ulceration and inflammation or one site of ulceration/inflammation extending >1 cm along the length of the colon |
| 6–10 | If damage covers >2 cm along the length of the colon, the score is increased by 1 for each additional centimeter of involvement |
Additional in vitro experiments were performed in order to test the antioxidant activity of different concentrations of the ethyl acetate of B. dracunculifolia (12.5–1600 μg ml−1). These were evaluated by the assay of lipid peroxidation in the rat brain membrane modified from the original protocol [32]. Briefly, rat brain samples were obtained from 4-month-old male Wistar rats. All samples were diluted upto 1 : 10 (w/v) in a buffer containing (mmol l−1) Tris 50.0, NaCl 100, KCl 0.5, CaCl2 0.5, MgSO4 1.0, KH2PO4 0.55 and sucrose (pH 7.4) for preparation of a membrane-enriched fraction. This membrane concentration was diluted upto 1 : 4 v/v in the above-mentioned buffer solution but with 20 mol l−1 Tris. Then, buffer (in the assays without inhibitors) or different concentrations of plant extract were added. Lipid peroxidation was induced via nonenzymatic way with 100 μm ol l−1 of both ferrous sulfate and ascorbic acid. After incubation at 37°C for 45 min, the reaction was stopped and malondialdehyde (MDA) was analysed using 0.5% thiobarbituric acid in 20% trichloroacetic acid. The amount of MDA produced after agitation, incubation at 100°C for 15 min and centrifugation at 1000 g for 15 min at 4°C, was determined by measuring the spectrophotometric absorbance of the supernatant at 532 nm. The flavonoid quercetin was used as reference and tested in the same assay system.
2.3. Statistical Analysis
All results are expressed as the mean ± SEM. Differences between means were tested for statistical significance using one-way analysis of variance (ANOVA) and post hoc least significance tests. Nonparametric data (score) are expressed as median (range) and were analysed with the Kruskal–Wallis test. Statistical significance was set at P < .05.
2.4. Phytochemical Analysis of Plant Extract by HPLC
The ethyl acetate extract of the aerial parts from B. dracunculifolia was submitted to HPLC analysis using the following equipment and conditions: a Shimadzu high performance liquid-chromatograph (SCL-10Avp system controller, three LC-10AD pumps, SPD-M10Avp photodiode array detector and Shimadzu Class-VP 5.02 software). A CLC-ODS (M) column (4.6 mm i.d. × 250 mm, 5 μm particle diameter) and a CLC G-ODS guard column were used as the stationary phase. The mobile phase had the following composition: A–B: 25–100% (B) in 60 min, A: 93.9% water, 0.8% acetic acid, 0.3% ammonium acetate, 5.0% methanol; B: acetonitrile; detection: 280 nm, flow rate of 1 ml min−1. The compounds detected were identified by comparison with authentic standards available, comparing UV spectra and considering both the maximum lambda and the relative area obtained with the use of two wavelengths (A280/320). The ethyl acetate extract was dissolved in methanol (HPLC grade) to obtain a concentration of 1 mg ml−1. Before analysis, all samples were centrifuged at 1300 rpm and filtered through a 45 μm filter.
3. Results
Administration of TNBS/ethanol resulted in colonic inflammation that presented several mucosal necrosis extending along the colon accompanied by bowel wall thickening, hyperemia and focal adhesions to adjacent organs (Table 2). This inflammatory process was associated with an increase of the colonic weight/length ratio and with signs of diarrhea in 80% of the colitic animals (Table 2). In addition, the colonic MPO and AP activities increased by 8-fold and 2-fold, respectively, whereas glutathione levels were reduced by 56% in comparison with non-colitic rats (Table 3).
Table 2.
Effects of B. dracunculifolia extract (5–200 mg kg−1) and sulfasalazine (25 mg kg−1) on damage score, changes in colonic weight/length, diarrhea and adherence incidence in TNBS acute colitis
| Group | Damage scorea (0–10) | Colonic weight/lengthb (mg cm−1) | Diarrhoea (%) | Adherence (%) |
|---|---|---|---|---|
| Non-colitic | 0*** | 82.30 ± 3.86** | 0 | 0 |
| TNBS-control | 8.5 (7–9) | 146.99 ± 8.78 | 80.0 | 50.0 |
| B. dracunculifolia | ||||
| 5 mg kg−1 | 6.0 (5–8)* | 127.44 ± 8.06 | 50.0 | 50.0 |
| 10 mg kg−1 | 7.5 (6–8) | 127.82 ± 3.94 | 50.0 | 66.7 |
| 25 mg kg−1 | 7.5 (5–10) | 135.28 ± 6.33 | 66.7 | 66.7 |
| 50 mg kg−1 | 6.5 (5–8)* | 139.10 ± 9.05 | 33.3* | 0* |
| 100 mg kg−1 | 6.5 (4–8) | 131.53 ± 7.34 | 83.3 | 33.3 |
| 200 mg kg−1 | 7.0 (4–8) | 134.62 ± 9.11 | 50.0 | 33.3 |
| Sulfasalazine | 6.0 (2–8)** | 133.58 ± 12.38 | 16.7 | 50.0 |
aScore data are expressed as median (range).
bColonic weight data are expressed as mean ± SEM.
*P < .05; **P < .01; ***P < .001 versus TNBS control group.
Table 3.
Effects of B. dracunculifolia extract (5–200 mg kg−1) and sulfasalazine (25 mg kg−1) on GSH content, MPO activity and AP activity in TNBS acute colitis
| Group | GSH content (nmol g−1 tissue) | MPO activity (U g−1 tissue) | AP activity (mU mg−1 protein) |
|---|---|---|---|
| Non-colitic | 1357.5 ± 86.5** | 84.2 ± 8.7** | 6.24 ± 0.36* |
| TNBS control | 759.6 ± 31.9 | 684.2 ± 62.8 | 12.62 ± 1.72 |
| B. dracunculifolia | |||
| 5 mg kg−1 | 967.3 ± 55.0* | 324.9 ± 89.9** | 10.53 ± 1.21 |
| 10 mg kg−1 | 850.3 ± 27.8 | 532.8 ± 96.3 | 14.58 ± 1.36 |
| 25 mg kg−1 | 878.5 ± 82.7 | 576.4 ± 79.8 | 14.27 ± 2.02 |
| 50 mg kg−1 | 809.4 ± 55.0 | 216.9 ± 31.6** | 6.16 ± 0.63* |
| 100 mg/kg−1 | 873.8 ± 25.8 | 245.8 ± 39.9** | 7.45 ± 0.93 |
| 200 mg kg−1 | 930.9 ± 113.8 | 380.1 ± 84.4* | 9.54 ± 1.76 |
| Sulfasalazine | 1006.2 ± 87.0* | 419.8 ± 80.1* | 9.02 ± 1.56 |
Data are expressed as mean ± SEM. *P < .05, **P < .01 versus TNBS control group.
Oral administration of the plant extract at the doses of 5 and 50 mg kg−1 significantly attenuated the macroscopic mucosal damage in the TNBS/ethanol model of colitis in rats (Table 2). Administration of 5 mg kg−1 of the B. dracunculifolia extract to colitic animals was able to counteract the colonic glutathione depletion that took place as a result of the colonic oxidative damage induced by TNBS (Table 3). Colonic tissue's MPO activity differed significantly between controls and treated colitic rats at the doses 5, 50, 100 and 200 mg kg−1 (Table 3). AP activity was also reduced in rats treated with 50 mg kg−1 of plant extract. The effects of plant extract were similar to those produced by sulfasalazine (Tables 2 and 3). These results prompt us to select both doses (5 and 50 mg kg−1) for testing with the chronic protocol.
In the chronic colitis, the inflammatory process induced by intracolonic instillation of 10 mg of TNBS in 50% ethanol (v/v) progressed over time with characteristics similar to those previously reported [10, 11]. Indeed, an alteration of the colonic absorptive function was noted since 67% of rats evidenced signs of diarrhea (Table 4).
Table 4.
Effects of B. dracunculifolia extract (5 and 50 mg kg−1) and sulfasalazine (25 mg kg−1) on damage score, changes in colonic weight/length, diarrhea and adherence incidence in TNBS chronic colitis with relapse
| Group | Damage scorea (0–10) | Colonic weight/lenghtb (mg cm−1) | Diarrhoea (%) | Adherence (%) |
|---|---|---|---|---|
| 1 week | ||||
| Non-colitic | 0*** | 100.57 ± 8.03** | 0* | |
| TNBS-control | 8.0 (7–9) | 276.10 ± 31.17 | 83.3 | |
| B. dracunculifolia 5 mg kg−1 | 2.0 (0–4)* | 140.53 ± 8.61** | 0* | |
| B. dracunculifolia 50 mg kg−1 | 1.0 (0–5)* | 171.00 ± 22.91** | 33.3 | |
| Sulfasalazine 25 mg kg−1 | 3.5 (2–7) | 143.25 ± 20.56** | 0 | |
| 2 week | ||||
| Non-colitic | 0*** | 87.16 ± 4.32** | 0 | |
| TNBS-control | 2.5 (2–3) | 185.40 ± 22.21 | 16.7 | |
| B. dracunculifolia 5 mg kg−1 | 3.0 (0–4) | 117.50 ± 6.45** | 0 | |
| B. dracunculifolia 50 mg kg−1 | 4.0 (2–5) | 141.90 ± 12.02 | 33.3 | |
| Sulfasalazine 25 mg kg−1 | 4.0 (1–6) | 137.95 ± 10.73 | 0 |
aScore data are expressed as median (range).
bColonic weight data are expressed as mean ± SEM.
*P < .05, **P < .01, ***P < .001 versus TNBS control group.
Administration of 5 and 50 mg kg−1 of B. dracunculifolia extract to colitic animals reduced damage score, lesion extension, diarrhoea signs and adherence of colon to adjacent organs after the first week (Table 4). Thus, both doses of B. dracunculifolia extract were able to counteract GSH content and to reduce MPO and AP activities by the end of the first week (Table 5). After 2 weeks, both doses of plant extract were actively counteracting the GSH content and reducing AP activity (Table 5).
Table 5.
Effects of B. dracunculifolia extract (5 and 50 mg kg−1) and sulfasalazine (25 mg kg−1) on GSH content, MPO activity and AP activity in TNBS chronic colitis with relapse
| Group | GSH content (nmol g−1 tissue) | MPO activity (U g−1 tissue) | AP activity (mU mg−1 protein) |
|---|---|---|---|
| 1 week | |||
| Non-colitic | 2357.6 ± 311.3* | 127.6 ± 14.5** | 4.20 ± 0.36** |
| TNBS control | 1590.1 ± 53.6 | 1045.2 ± 231.2 | 19.15 ± 4.38 |
| B. dracunculifolia 5 mg kg−1 | 2386.1 ± 177.1* | 221.1 ± 59.2* | 7.93 ± 1.13** |
| B. dracunculifolia 50 mg kg−1 | 2218.6 ± 97.4* | 167.6 ± 12.7* | 8.31 ± 1.51** |
| Sulfasalazine 25 mg kg−1 | 1992.2 ± 254.2 | 951.0 ± 328.0 | 7.45 ± 0.92** |
| 2 week | |||
| Non-colitic | 2233.9 ± 192.5 | 115.9 ± 12.9 | 5.79 ± 0.70** |
| TNBS control | 1881.3 ± 146.8 | 220.6 ± 42.9 | 13.31 ± 1.65 |
| B. dracunculifolia 5 mg kg−1 | 2228.3 ± 132.7* | 167.5 ± 31.7 | 8.39 ± 1.07* |
| B. dracunculifolia 50 mg kg−1 | 2538.2 ± 132.8** | 178.5 ± 34.6 | 8.78 ± 1.13* |
| Sulfasalazine 25 mg kg−1 | 1776.6 ± 63.5 | 262.4 ± 59.4 | 11.20 ± 1.29 |
Data are expressed as mean ± SEM.
*P < .05, **P < .01 versus TNBS control group.
The in vitro experiments performed showed that the plant extract exerts an inhibitory effect on the lipid peroxidation induced in rat brain membranes, with an IC50 value of 26.33 μg ml−1. The corresponding IC50 value of quercetin was 1.51 μm corresponding to 0.46 μg ml−1.
HPLC analysis of the ethyl acetate extract of B. dracunculifolia permitted the identification of only the following compounds: caffeic acid, p-coumaric acid, aromadendrin-4-O-methyl ether, 3-prenyl-p-coumaric acid, 3,5-diprenyl-p-coumaric acid and baccharin (Figures 1 and 2).
Figure 1.
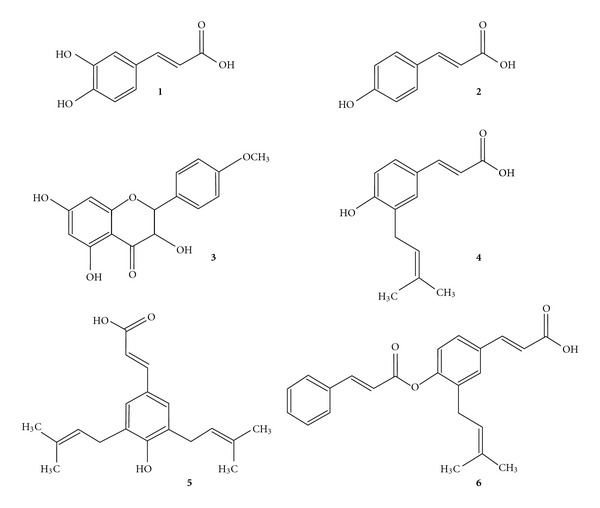
Structures of chemical constituents in the ethyl acetate extract of B. dracunculifolia. (1) caffeic acid; (2) p-coumaric acid; (3) aromadendrin-4-O-methyl ether; (4) 3-prenyl-p-coumaric acid (drupanin); (5) 3,5-diprenyl p-coumaric acid (artepillin C); (6) baccharin.
Figure 2.
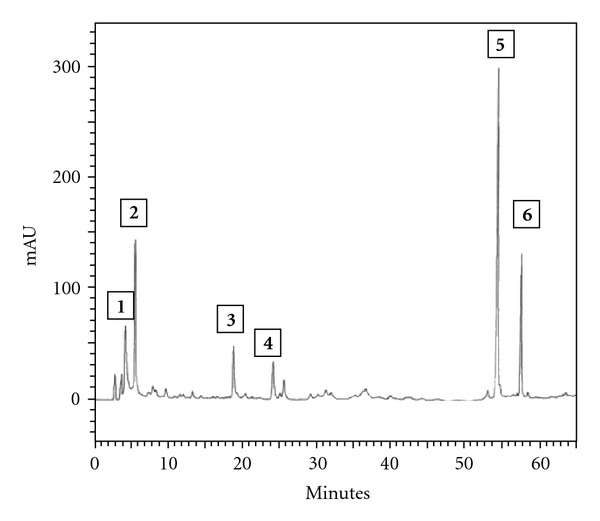
HPLC profile of B. dracunculifolia: (1) caffeic acid; (2) p-coumaric acid; (3) aromadendrin-4-O-methyl ether; (4) 3-prenyl-p-coumaric acid (drupanin); (5) 3,5-diprenyl p-coumaric acid (artepillin C); (6) baccharin.
4. Discussion
The role of reactive metabolites of oxygen and nitrogen in the pathophysiology of IBD has been well reported in humans as experimental models of colitis [6, 10, 33, 34]. The inflammatory process in the intestine is probably derived from the chronic presence of numerous, activated, MPO-containing phagocytes in the inflamed intestine. These are responsible for the overproduction of reactive oxygen species that overwhelm the antioxidant defences, such as glutathione and its related enzymes that normally participle in protecting colonic tissue from oxidative damage. In fact, it has been reported that the production of reactive oxygen species is considerably increased in colonic biopsy specimens from ulcerative colitis and Crohn's disease patients in comparison with normal control mucosa, in a manner positively correlated with inflammatory bowel disease activity. This phenomenon appears to be neutrophil derived [35]. Thus, antioxidant therapy can constitute an interesting approach in the downregulation of this inflammatory condition. In the context of this effect, the beneficial protection exerted by the 5-aminosalicylic derivatives has been attributed to their antioxidant and free radical scavenger properties [7]. Thus, plant extracts containing antioxidant compounds such as flavonoids and other phenolic compounds may be of prime interest [36].
The present study shows the preventive effect exerted by an ethyl acetate extract of B. dracunculifolia in ameliorating the colonic insult induced after intracolonic administration of TNBS to rats. The preventive intestinal anti-inflammatory activity of this plant extract (5 and 50 mg kg−1) was evidenced macroscopically by reducing the damage score of the lesions in the acute phase of the TNBS-induced inflammatory process. Similar effects were produced by oral administration of 25 mg kg−1 of sulfasalazine, a drug of choice for treating IBD. Both B. dracunculifolia extract and sulfasalazine failed to decrease the colonic weight/length ratio. The lack of effect on this ratio can be explained by the severe and extensive colonic damage induced by TNBS/ethanol, which is difficult to overcome by pharmacological treatment as has been previously suggested [37]. In the acute phase of the colonic inflammatory process, the protective effects of B. dracunculifolia extract were not dose dependent, since higher doses of plant extract resulted in the loss of activity. A dual effect has already been described for flavonoids, such as diosmin and hesperidin [38], quercetin [33] and silymarin [39], isocoumarin paepalantine [10] as well as coumarin and 4-hydroxycoumarin [11], and may be related to the known capacity of these compounds to behave as antioxidants at lower doses and pro-oxidants at high doses in rat colon. On the other hand, inflammation is a multicomponent system that involves a network of cellular crosstalk and control. However, the anti-inflammatory effect produced by B. dracunculifolia may be related to distinct mechanisms of these compounds acting on distinct inflammatory mediators.
MPO activity has been widely used to detect and monitor intestinal inflammation, so that a reduction in the activity of this enzyme can be interpreted as a manifestation of the anti-inflammatory property of a given compound [36, 40]. Similarly, AP activity can also be considered as a sensitive marker of inflammation in the intestine, since this enzyme's activity is invariably augmented in experimental conditions [32, 41]. One of these mechanisms could be the antioxidant properties of B. dracunculifolia extract. This property may play a crucial role in the intestinal anti-inflammatory effect of the plant extract, given that intense oxidative insult is a common feature in human IBD [42, 43] and in the different experimental models of rat colitis including TNBS [32, 44, 45]. In this aspect, in the present study, B. dracunculifolia extract treatment of TNBS colitic rats counteracted the depletion of colonic glutathione levels that took place in control colitic animals in both experimental treatment protocols. The effect exerted by B. dracunculifolia extract in preserving the colonic mucosa from oxidative insult may collaborate to decrease the neutrophil infiltration that occurs in response to TNBS. Free radical generation has been proposed as playing an important early role in the pathogenesis of IBD [6], and could contribute to the initial neutrophil infiltration in the inflamed colonic mucosa. The recruitment and activation of these cells result in an increase in free radical production that overwhelms the tissue's antioxidant protective mechanisms, resulting in a situation of oxidative stress, which definitively perpetuates colonic inflammation [42]. As a consequence, a rapid inhibition of free radical generation could contribute to a lower level of leukocyte infiltration into the inflamed tissue, thus preventing colonic tissue becoming inflamed. The antioxidant properties ascribed to this B. dracunculifolia extract could also contribute to its intestinal anti-inflammatory effect, similarly to other reputed drugs used in the treatment of IBD, such as 5-aminosalicylic derivates [46], thus supporting additional studies aiming to evaluate the application of this plant extract in the treatment of human IBD.
In the second set of experiments, the effects of B. dracunculifolia were evaluated after induction of the inflammatory process. The results demonstrated that oral administration of B. dracunculifolia extract (5 and 50 mg kg−1) after colitis induction significantly reduced the macroscopic colonic damage score and lesion extension after the first week when compared to the corresponding TNBS control group. In addition, these compounds were able to alter the extent of neutrophil infiltration into the colon 1 week after TNBS administration, as determined by tissue MPO activity reduction. The reduction of neutrophil infiltration seemed to be a consequence of the accelerated healing of colonic ulcers, thus facilitating the elimination of neutrophil accumulation from the inflamed colon. Similarly to the acute experimental setting, the anti-inflammatory effect exerted by B. dracunculifolia extract at doses of 5 and 50 mg kg−1 in chronic colitis was related to the protective effect against GSH depletion and inhibition of AP activities in the first and second week.
HPLC analysis of the ethyl acetate extract of B. dracunculifolia permitted the identification of such cinnamic acid derivatives such as caffeic, p-coumaric, 3-prenyl-p-coumaric (drupanin), 3,5-diprenyl-p-coumaric (artepillin C) and 3-prenyl-4-dihydrocinnamoiloxy–cinnamic (baccharin) acids, as well as the flavonoid aromadendrin-4-O-methyl ether (Figure 2). Artepillin C was detected as a major component of B. dracunculifolia. This compound is also the main constituent in Brazilian green propolis and an efficient radical scavenger [21]. Artepillin C is a potent anti-inflammatory compound that decreases the number of neutrophils during peritonitis and reduces the prostaglandin E2 level, nitric oxide production and NF-κB activity [22]. In addition, artepillin C also suppresses the formation of aberrant crypt foci induced by azoxymethane in mouse colon after oral administration [47]. Phenolic compounds including cinnamic acid derivatives detected in B. dracunculifolia extract have been identified as responsible for antiulcerogenic [18], immunomodulatory [21], antitumoural [48, 49], neuroprotective and antioxidant [23, 50] activities. Polyphenolic compounds of higher plants are known to be excellent in vitro antioxidants, while numerous studies suggest that dietary intake of plant phenolics may produce positive effects on oxidative stress-related diseases [51]. The compounds of B. dracunculifolia presented free radical scavenging activity similar to that of green propolis, and phenolic compounds are found abundantly in both [52].
In conclusion, the present study showed that B. dracunculifolia prevents colonic damage induced by TNBS in rats with either acute or chronic colitis. This anti-inflammatory effect may be associated with improved intestinal oxidative stress (Figure 3), mainly due to reduced MPO activity, augmented endogenous antioxidant defences in the inflamed colon, such as glutathione content and inhibited lipid peroxidation. This intestinal anti-inflammatory activity is related to the phenolic compounds present in the plant extract, mainly artepillin C. In this manner, the use of B. dracunculifolia, the main source of active compounds in Brazilian green propolis, as a dietary product, can be an important supplement in the treatment and prevention of human IBD.
Figure 3.
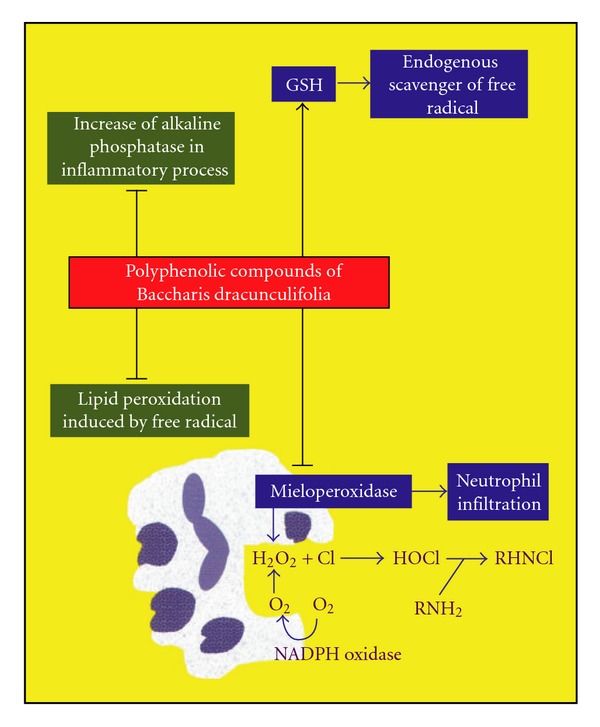
Main effects of the B. dracunculifolia on the inflammatory intestinal process induced by TNBS in rats. Black lines indicate inhibition and black arrows indicate counteraction of depletion induced by the inflammatory process.
Funding
CNPq (Science and Technology Ministry, Brazil); CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazilian Ministry of Education); and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo).
References
- 1.Podolsky DK. Pride and prejudice: inflammatory bowel disease models and drug development. Current Opinion in Gastroenterology. 2000;16(4):295–296. doi: 10.1097/00001574-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109(4):1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 3.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115(1):182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 4.Katz JA, Itoh J, Fiocchi C. Pathogenesis of inflammatory bowel disease. Current Opinion in Gastroenterology. 1999;15(4):291–297. doi: 10.1097/00001574-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Buffinton GD, Doe WF. Depleted mucosal antioxidant defences in inflammatory bowel disease. Free Radical Biology and Medicine. 1995;19(6):911–918. doi: 10.1016/0891-5849(95)94362-h. [DOI] [PubMed] [Google Scholar]
- 6.Pavlick KP, Laroux FS, Fuseler J, et al. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radical Biology and Medicine. 2002;33(3):311–322. doi: 10.1016/s0891-5849(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 7.Grisham MB, Specian RD, Zimmerman TE. Effects of nitric oxide synthase inhibition on the pathophysiology observed in a model of chronic granulomatous colitis. Journal of Pharmacology and Experimental Therapeutics. 1994;271(2):1114–1121. [PubMed] [Google Scholar]
- 8.Gálvez J, Sánchez de Medina F, Jimenez J, Zarzuelo A. Effects of flavonoids on gastrointestinal disorders. In: Atta-ur-Rahman X, editor. Studies in Natural Products Chemistry. Amsterdam, The Netherlands: Elsevier Science; 2001. pp. 607–49. [Google Scholar]
- 9.Cuzzocrea S, McDonald MC, Mazzon E, et al. Tempol, a membrane-permeable radical scavenger, reduces dinitrobenzene sulfonic acid-induced colitis. European Journal of Pharmacology. 2000;406(1):127–137. doi: 10.1016/s0014-2999(00)00623-3. [DOI] [PubMed] [Google Scholar]
- 10.Di Stasi LC, Camuesco D, Nieto A, Vilegas W, Zarzuelo A, Galvez J. Intestinal anti-inflammatory activity of paepalantine, an isocoumarin isolated from the capitula of Paepalanthus bromelioides, in the trinitrobenzenesulphonic acid model of rat colitis. Planta Medica. 2004;70(4):315–320. doi: 10.1055/s-2004-818942. [DOI] [PubMed] [Google Scholar]
- 11.Luchini AC, Rodrigues-Orsi P, Cestari SH, et al. Intestinal anti-inflammatory activity of coumarin and 4-hydroxycoumarin in the trinitrobenzenesulphonic acid model of rat colitis. Biological and Pharmaceutical Bulletin. 2008;31(7):1343–1350. doi: 10.1248/bpb.31.1343. [DOI] [PubMed] [Google Scholar]
- 12.Correa MP. Dicionário das plantas úteis do Brasil e das exóticas cultivadas. Brasília, Brazil: IBDF; 1984. [Google Scholar]
- 13.Rodrigues VEG, Carvalho DA. Plantas medicinais no domínio dos Cerrados. Lavras, Brazil: Editora UFLA; 2001. [Google Scholar]
- 14.Bankova V, Boudourova-Krasteva G, Sforcin JM, et al. Phytochemical evidence for the plant origin of Brazilian propolis from Sao Paulo state. Zeitschrift fur Naturforschung. Section C. 1999;54(5-6):401–405. doi: 10.1515/znc-1999-5-616. [DOI] [PubMed] [Google Scholar]
- 15.Kumazawa S, Yoneda M, Shibata I, Kanaeda J, Hamasaka T, Nakayama T. Direct evidence for the plant origin of Brazilian propolis by the observation of honeybee behavior and phytochemical analysis. Chemical and Pharmaceutical Bulletin. 2003;51(6):740–742. doi: 10.1248/cpb.51.740. [DOI] [PubMed] [Google Scholar]
- 16.Park YK, Paredes-Guzman JF, Aguiar CL, Alencar SM, Fujiwara FY. Chemical constituents in Baccharis dracunculifolia as the main botanical origin of Southeastern Brazilian propolis. Journal of Agricultural and Food Chemistry. 2004;52(5):1100–1103. doi: 10.1021/jf021060m. [DOI] [PubMed] [Google Scholar]
- 17.da Silva Filho AA, Pires Bueno PC, Gregório LE, Andrade Silva ML, Albuquerque S, Bastos JK. In vitro trypanocidal activity evaluation of crude extract and isolated compounds from Baccharis dracunculifolia DC (Asteraceae) Journal of Pharmacy and Pharmacology. 2004;56(9):1195–1199. doi: 10.1211/0022357044067. [DOI] [PubMed] [Google Scholar]
- 18.Lemos M, De Barros MP, Sousa JPB, Da Silva Filho AA, Bastos JK, De Andrade SF. Baccharis dracunculifolia, the main botanical source of Brazilian green propolis, displays antiulcer activity. Journal of Pharmacy and Pharmacology. 2007;59(4):603–608. doi: 10.1211/jpp.59.4.0017. [DOI] [PubMed] [Google Scholar]
- 19.Da Silva Filho AA, De Sousa JPB, Soares S, et al. Antimicrobial activity of the extract and isolated compounds from Baccharis dracunculifolia D. C. (Asteraceae) Zeitschrift fur Naturforschung. Section C. 2008;63(1-2):40–46. doi: 10.1515/znc-2008-1-208. [DOI] [PubMed] [Google Scholar]
- 20.Munari CC, Resende FA, Alves JM, De Sousa JPB, Bastos JK, Tavares DC. Mutagenicity and antimutagenicity of Baccharis dracunculifolia extract in chromosomal aberration assays in Chinese hamster ovary cells. Planta Medica. 2008;74(11):1363–1367. doi: 10.1055/s-2008-1081306. [DOI] [PubMed] [Google Scholar]
- 21.Missima F, Da Silva Filho AA, Nunes GA, et al. Effect of Baccharis dracunculifolia D.C. (Asteraceae) extracts and its isolated compounds on macrophage activation. Journal of Pharmacy and Pharmacology. 2007;59(3):463–468. doi: 10.1211/jpp.59.3.0017. [DOI] [PubMed] [Google Scholar]
- 22.Paulino N, Abreu SRL, Uto Y, et al. Anti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolis. European Journal of Pharmacology. 2008;587(1–3):296–301. doi: 10.1016/j.ejphar.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 23.Nakanishi I, Uto Y, Ohkubo K, et al. Efficient radical scavenging ability of artepillin C, a major component of Brazilian propolis, and the mechanism. Organic and Biomolecular Chemistry. 2003;1(9):1452–1454. doi: 10.1039/b302098c. [DOI] [PubMed] [Google Scholar]
- 24.Teixeira ÉW, Negri G, Meira RMSA, Message D, Salatino A. Plant origin of green propolis: bee behavior, plant anatomy and chemistry. Evidence-Based Complementary and Alternative Medicine. 2005;2(1):85–92. doi: 10.1093/ecam/neh055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Funari CS, de Oliveira Ferro V, Mathor MB. Analysis of propolis from Baccharis dracunculifolia DC. (Compositae) and its effects on mouse fibroblasts. Journal of Ethnopharmacology. 2007;111(2):206–212. doi: 10.1016/j.jep.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Salatino A, Teixeira ÉW, Negri G, Message D. Origin and chemical variation of Brazilian propolis. Evidence-Based Complementary and Alternative Medicine. 2005;2(1):33–38. doi: 10.1093/ecam/neh060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96(3):795–803. [PubMed] [Google Scholar]
- 28.Bell CJ, Gall DG, Wallace JL. Disruption of colonic electrolyte transport in experimental colitis. American Journal of Physiology. 1995;268(4):G622–G630. doi: 10.1152/ajpgi.1995.268.4.G622. [DOI] [PubMed] [Google Scholar]
- 29.Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods in Enzymology. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 30.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87(6):1344–1350. [PubMed] [Google Scholar]
- 31.Bessey OA, Lowry OH, Brook MJ. Rapid colorimetric method for the determination of alkaline phosphatase in five cubic milliliters of serum. The Journal of Biological Chemistry. 1946;164:321–329. [PubMed] [Google Scholar]
- 32.Galvez J, de la Cruz JP, Zarzuelo A, de la Cuesta FS. Flavonoid inhibition of enzymic and nonenzymic lipid peroxidation in rat liver differs from its influence on the Glutathione-Related enzymes. Pharmacology. 1995;51(2):127–133. doi: 10.1159/000139325. [DOI] [PubMed] [Google Scholar]
- 33.Fermin Sanchez De Medina L-H, Gálvez J, Romero JA, Zarzuelo A. Effect of Quercitrin on acute and chronic experimental colitis in the rat. Journal of Pharmacology and Experimental Therapeutics. 1996;278(2):771–779. [PubMed] [Google Scholar]
- 34.Cruz T, Gálvez J, Crespo E, Ocete MA, Zarzuelo A. Effects of silymarin on the acute stage of the trinitrobenzenesulphonic acid model of rat colitis. Planta Medica. 2001;67(1):94–96. doi: 10.1055/s-2001-10620. [DOI] [PubMed] [Google Scholar]
- 35.Lih-Brody L, Powell SR, Collier KP, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Digestive Diseases and Sciences. 1996;41(10):2078–2086. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 36.Halliwell B. Antioxidants in human health and disease. Annual Review of Nutrition. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 37.Veljaca M, Lesch CA, Pllana R, Sanchez B, Chan K, Guglietta A. BPC-15 reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. Journal of Pharmacology and Experimental Therapeutics. 1995;272(1):417–422. [PubMed] [Google Scholar]
- 38.Crespo ME, Gálvez J, Cruz T, Ocete MA, Zarzuelo A. Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Medica. 1999;65(7):651–653. doi: 10.1055/s-2006-960838. [DOI] [PubMed] [Google Scholar]
- 39.Cruz T, Gálvez J, Crespo E, Ocete MA, Zarzuelo A. Effects of Silymarin on the acute stage of the trinitrobenzenesulphonic acid model of rat colitis. Planta Medica. 2001;67(1):94–96. doi: 10.1055/s-2001-10620. [DOI] [PubMed] [Google Scholar]
- 40.Yamada T, Marshall S, Specian RD, Grisham MB. A comparative study of two models of experimental colitis in rats. Gastroenterology. 1992;102:1524–1534. doi: 10.1016/0016-5085(92)91710-l. [DOI] [PubMed] [Google Scholar]
- 41.Cooper HS, Steplewski Z. Immunohistologic study of ulcerative colitis with monoclonal antibodies against tumor-associated and/or differentiation antigens. Gastroenterology. 1988;95(3):686–693. doi: 10.1016/s0016-5085(88)80015-5. [DOI] [PubMed] [Google Scholar]
- 42.Grisham MB. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344(8926):859–861. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- 43.McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. Journal of Clinical Investigation. 1996;98(1):136–141. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lauterburg BH, Bilzer M, Rowedder E, Inauen W. Decresead glutathione in inflamed colonic mucosa in man. A possible role of hypochlorous acid and prevention by 5-aminosalicylic acid. In: MacDermott E, editor. Inflammatory Bowel Disease: Current Status and Future Approach. Amsterdam, The Netherlands: Elsevier Science; 1988. pp. 273–277. [Google Scholar]
- 45.Gálvez J, Coelho G, Crespo ME, et al. Intestinal anti-inflammatory activity of morin on chronic experimental colitis in the rat. Alimentary Pharmacology and Therapeutics. 2001;15(12):2027–2039. doi: 10.1046/j.1365-2036.2001.01133.x. [DOI] [PubMed] [Google Scholar]
- 46.Travis SPL, Jewell DP. Salicylates for ulcerative colitis—their mode of action. Pharmacology and Therapeutics. 1994;63(2):135–161. doi: 10.1016/0163-7258(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu K, Das SK, Babab M, Matsuura Y, Kanazawa K. Dietary artepillin C suppresses the formation of aberrant crypt foci induced by azoxymethane in mouse colon. Cancer Letters. 2005;240:135–142. doi: 10.1016/j.canlet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Akao Y, Maruyama H, Matsumoto K, Ohguchi K, Nishizawa K, Sakamoto T, et al. Cell growth inhibitory effect of cinnamic acid derivatives from Propolis on human tumor cell lines. Biological and Pharmaceutical Bulletin. 2003;26:1057–1059. doi: 10.1248/bpb.26.1057. [DOI] [PubMed] [Google Scholar]
- 49.Mishima S, Ono Y, Araki Y, Akao Y, Nozawa Y. Two related cinnamic acid derivatives from Brazilian honey bee propolis, baccharin and drupanin, induce growth inhibition in allografted sarcoma S-180 in mice. Biological and Pharmaceutical Bulletin. 2005;28:1025–1030. doi: 10.1248/bpb.28.1025. [DOI] [PubMed] [Google Scholar]
- 50.Nakajima Y, Shimazawa M, Mishima S, Hara H. Water extract of propolis and its main constituents, caffeoylquinic acid derivatives, exert neuroprotective effects via antioxidant actions. Life Sciences. 2007;80(4):370–377. doi: 10.1016/j.lfs.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 51.Urquiaga I, Leighton F. Plant polyphenol antioxidants and oxidative stress. Biological Research. 2000;33(2):55–64. doi: 10.4067/s0716-97602000000200004. [DOI] [PubMed] [Google Scholar]
- 52.Park YK, Paredes-Guzman JF, Aguiar CL, Alencar SM, Fujiwara FY. Chemical constituents in Baccharis dracunculifolia as the main botanical origin of southeastern Brazilian propolis. Journal of Agricultural and Food Chemistry. 2004;52(5):1100–1103. doi: 10.1021/jf021060m. [DOI] [PubMed] [Google Scholar]


