Abstract
This study examines the early affective consequences of two close forms of suppression. Participants (N=37) were shown negative, positive, and neutral pictures and cued either to attend to the pictures, or to perform expressive or physiological suppression (i.e. reduce body reactions). Continuous measures of experience, expressivity, and autonomic responses showed that both suppression strategies produced rapid response modulation. Common effects of the two strategies included a transient increase in negative feeling, a durable decrease in positive feeling, and a decrease in expressivity, cardiovascular activity, and oxygenation. The two strategies were significantly different only in response to positive stimuli, with physiological suppression showing a larger decrease in experience intensity and blood pressure. These results suggest a strong overlap between the two suppression strategies in terms of their early impact on emotional responses.
Descriptors: Emotion, Emotion regulation, Suppression, Temporal dynamics
Emotions involve loosely coupled changes in experiential, behavioral (expressive), and autonomic response systems (Buck, 1994; Frijda, 2007; Gross, 2007; Lang, 1995; Levenson, 1994). These changes often emerge very quickly. For example, Dimberg showed that EMG reactions are noticeable within one second of the onset of relevant emotional pictures, and heart rate and skin conductance responses are evident within five seconds from picture onset (Dimberg, Hansson, & Thunberg, 1998). Other studies have shown that emotional experience reliably emerges within four seconds of emotional stimulation (Dan Glauser & Scherer, 2008).
Despite the speed with which emotions unfold, people often try to influence these emotional responses (Tomkins, 1984), and it is becoming clear that these attempts to alter the unfolding emotion response trajectory can powerfully shape the resultant response. Given the crucial role that emotions play in healthy adaptation (Gross & Muñoz, 1995), it is important to know what early effects different forms of emotion regulation have on the response trajectory of emotions.
Emotion Regulation
Emotion regulation refers to “the processes by which individuals influence which emotions they have, when they have them, and how they experience and express these emotions” (Gross, 1998b, p. 275). One framework for analyzing emotion regulation draws attention to the fact that emotions unfold over time (Gross, 2001). According to this framework, each step in the emotion-generative process may be targeted for regulation. For example, the situation that gives rise to emotions may be targeted, either via the process of selecting which situation we expose ourselves to (or not), or via the process of modifying a situation that has already arisen. We can also influence the way situations are attended to, or alter the construal processes that play such a crucial role in emotion regulation. Even after emotional responses have been generated, we can target one or more of these experiential, behavioral, and autonomic responses for regulation, by using the so-called “response-focused strategies” (Gross, 1998b).
In the past decade or so, researchers have begun to empirically contrast different forms of emotion regulation in order to understand whether they have different affective consequences. Typically, quite different forms of emotion regulation have been contrasted, such as reappraisal and suppression (see for example Goldin, McRae, Ramel, & Gross, 2008; Gross, 1998a; Gross & John, 2003; Hermann, Pejic, Vaitl, & Stark, 2009; Hofmann, Heering, Sawyer, & Asnaani, 2009), reappraisal and distraction (see for example McRae, et al., 2010; Sheppes & Meiran, 2008), or reappraisal and rumination (Ray, Wilhelm, & Gross, 2008).
Recently, however, researchers have begun to examine the affective consequences of emotion regulation strategies that are more closely related to one another. For example, Demaree and collaborators (2006) contrasted suppression and exaggeration, which are regulation strategies that have opposing behavioral consequences, but which occur at the same point in the emotion generative process. More recently, Shiota and Levenson (2009) contrasted the affective consequences of detached and positive reappraisals. Comparisons of closely related emotion-regulatory processes (that differ only on limited and well circumvented aspects of their construct) are valuable because they provide insights regarding the exact elements that may be crucial in influencing emotion responses.
One promising – but largely unexplored – target for such investigation concerns the emotion regulation family of response-focused strategies (response modulation). Response modulation is a common form of emotion regulation (Koole, 2009) that intervenes rather late in the emotion process (Gross, 2001), and can be in the service of either up-regulation (exaggeration, see for example Robinson & Demaree, 2009) or down-regulation (suppression). In the process model of emotion regulation (Gross, 2001; Gross & John, 2003), it is hypothesized that response modulation strategies that target different aspects of the emotion may have different consequences. Although testing this prediction would provide critical information regarding the specificity, strength, and interrelations among response elements, few studies have directly contrasted different forms of response modulation.
Most of the research on response modulation has focused on expressive suppression (simply called emotion suppression; Miles & Gross, 1999; Richards & Gross, 1999), which targets the behavioral component of emotion. Physiological suppression, which targets the peripheral physiological response component of emotion, has received much less attention. We define physiological suppression as the ability to modulate the body reactions to emotional stimulations. This form of suppression includes trying to modulate respiration, which can be easily achieved, but also targets more automatic responses like cardiovascular activity. To date, its specific effects on emotional response trajectories are not well-defined, despite evidence of suitability of such a target for emotion regulation and the high likelihood of this strategy employment in everyday life. In the following section, we consider what is known about these two commonly used forms of response modulation that have so far always been studied separately.
Affective Consequences of Response Modulation
Expressive suppression
Participants instructed to suppress emotional expressions while watching films of various emotional contents show reduced expressivity (Gross, 1998a; Gross & Levenson, 1993, 1997; Jackson, Malmstadt, Larson, & Davidson, 2000; Roberts, Levenson, & Gross, 2008), demonstrating people’s ability to successfully hide expressive signs of emotion. For subjective experience, the effects are mixed. In positive emotion contexts, expressive suppression leads to decreased positive emotion reports in all studies (Gross & Levenson, 1993, 1997; John & Gross, 2004; Strack, Martin, & Stepper, 1988). In negative emotion contexts, however, the effects are less clear, with some reports of no effects on subjective experience (Gross & Levenson, 1993, 1997; Roberts, et al., 2008) and other reports of reduced levels of negative emotion experience (Dunn, Billotti, Murphy, & Dalgleish, 2009; Goldin, et al., 2008). Physiologically, expressive suppression leads to decreased heart rate, increased blood pressure, and increased sympathetic activation (this latter measured by finger temperature, pulse, or skin conductance) in positive and negative stimulations (Gross, 1998a; Gross & Levenson, 1993, 1997; Harris, 2001; Kunzmann, Kupperbusch, & Levenson, 2005; Roberts, et al., 2008). Another study found decreased sympathetic activity (as measured by cardiac pre-ejection period and galvanic skin level) during the suppression of sad emotion-expressive behavior (Robinson & Demaree, 2009). Respiratory activity, whether reported as depth or period, is generally not affected by expressive suppression (Demaree, et al., 2006; Roberts, et al., 2008).
Physiological suppression
Voluntary regulation of one’s own cardiac rhythm can be successfully achieved, even in the absence of direct heart rate feedback (Bell & Schwartz, 1973; Victor, Mainardi, & Shapiro, 1978). In this latter study, attempts to control cardiac activity while performing a cold pressure task did not influence subjective experience of pain. Other studies have considered respiratory control, a parameter we have strong awareness of and that can be regulated without too much effort or training (Ley, 1999). Voluntary decrease in respiratory rate have been found to reduce both arousal (see review in Conde Pastor, Javier Menéndez, Sanz, & Vila Abad, 2008) and anxious experience (McCaul, Solomon, & Holmes, 1979). Other research has also shown that focused breathing triggers the maintenance of a positive attitude toward neutral images, as compared to worry or unfocused attention (Arch & Craske, 2006).
The Present Study
The goal of the present study was to directly contrast two closely related forms of suppression, thus investigating similar processes that differ only in the target of the suppressive activity. More specifically, we wanted to investigate common and differential effects of expressive and physiological suppression. Because emotion responses occur in a matter of seconds, our general hypothesis was that both suppression strategies would have rapid detectable consequences on the emotion-response emergence trajectories, which would quickly deviate from unregulated emotion responses. So far, no research has dynamically assessed such a contrast to highlight commonalities and differences in these forms of suppression.
To address these issues, we examined the first eight seconds of participants’ emotional responses across experiential, expressive, and autonomic response channels while they watched a series of well-validated pictures (see for example Bradley & Lang, 2007; and Lang, Greenwald, Bradley, & Hamm, 1993). As previous research has shown different emotion regulation effects for positive and negative emotions, we examined positive and negative stimuli separately. To be able to contrast the suppression strategies, each participant performed the two kinds of suppression on different material. Unregulated emotional reactions were also collected. Emotion responses were obtained with (a) a continuous assessment procedure to measure emotion experience (see also Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005), (b) continuous EMG, to assess Corrugator supercilii and Zygomaticus Major responses, and (c) continuous measures of cardiovascular and respiratory responses.
Based on prior empirical research, three hypotheses were examined. 1. Physiological and expressive suppression would lead to similar decrements in positive emotion experience, but physiological suppression would lead to a greater decrement in negative emotion experience than expressive suppression. 2. Expressive suppression would lead to a greater decrement in expressive behavior than physiological suppression, as expression is not the main regulation target in the latter condition. 3. Signs of physiological arousal, if present, would be lower in the physiological suppression than in the expressive suppression condition.
Method
Participants
Thirty-seven students participated in our study and were given course credit. Their ages ranged between 18 and 40 years old, with a mean age of 20.2 years (SD=3.5). Participation was restricted to right-handed females to eliminate laterality and gender variability. Participants identified as follows: 13 Asians, 10 Caucasians, two African Americans, and two Hispanics (nine participants indicated “other” or “more than one race” and one declined to answer).
Stimuli
One hundred and seventy-five pictures were selected from the International Affective Picture System (IAPS, Lang, Bradley, & Cuthbert, 1999) based on the affective norms for female participants (ratings from 1 to 9, middle point at 5). Of these pictures, 25 were of neutral valence (mean: 5.00, SD=0.07, range: 4.88–5.15), 75 were of negative valence (mean: 2.33, SD=0.37, range: 1.45–3.16), and 75 were of positive valence (mean: 7.44, SD=0.36, range: 6.91–8.34).1 Since highly negative pictures trigger a ceiling effect in the report of emotional experience (as shown by pilot studies), extremely negative pictures were not used in this study. Erotic pictures were also excluded due to their intensity, as well as the variability of impact in culturally heterogeneous populations. A univariate ANOVA on valence ratings revealed expected differences among stimulus categories, F(2,172)=4269.23, p<.001, η2=.98, all contrasts p<.01. Because we focused on valence, we matched arousal for negative (M: 5.57, SD=0.65, range: 3.85–6.52) and positive stimuli (M: 5.43, SD=0.71, range: 4.54–7.35). A univariate ANOVA on arousal ratings showed no significant differences between these groups, F(1,148)=1.69, p=ns.
Measures
Emotion experience
Participants used a rating dial that provided a continuous record of their degree of negative or positive feelings during the emotional picture presentation (see Mauss, et al., 2005, for a study using the same device to record subjective experience). Past research has shown that measuring emotion in this way does not seem to impact the emotion itself (Hutcherson, et al., 2005) and can be a reliable way to measure experience. Moreover, as already mentioned, emotional experience is expected to emerge during the recorded time frame as it has been shown to be reliably reported within 4 seconds of the stimulation onset (Dan Glauser, 2008; Dan Glauser & Scherer, 2008). The dial was anchored at one side with very negative and on the other side with very positive. The voltage output was linearly transformed to a scale from −100 (very negative) to +100 (very positive).
Emotion-expressive behavior
Expressivity is a fast-emerging emotional response, detectable a few hundred milliseconds after emotional onset (Dimberg, et al., 1998; Tamietto, et al., 2009). Facial behavior was assessed using bipolar surface electromyography (EMG). Electrodes were standard 4 mm Ag-AgCl sensors. Left Corrugator Supercilii and left Zygomaticus Major were the two targeted regions, as they have been associated with negative and positive emotions, respectively (e.g. Cacioppo, Petty, Losch, & Kim, 1986; Vrana, 1993). Electrode placement followed recommendations by Fridlund and Cacioppo (1986). Skin was first cleaned with Kendall Webcol® skin cleansing alcohol pads (Tyco healthcare) and gently rubbed with NuPrep® gel (Weaver and Cie). Excess gel was then removed with alcohol pads. Electrodes were filled with Signagel® (Parker Laboratories, Inc).
Autonomic responses
Selected measures (a) have been shown to be involved in negative and positive emotional responding, and (b) were thought to be sensitive to response modulation. The first four measures reflect cardiovascular activity (1 to 4). Respiratory activity was also recorded (5), as this was one of the main targets of physiological suppression.2
Electrocardiography: Rapidly modulated by orientation to emotional stimulation and differentially affected by valence levels (see for example Bradley, 2000), ECG is a key measure of rapid autonomic arousal. Two standard disposable pre-gelled Ag/AgCl electrodes were placed 5 cm below the lower rib on each side of the abdomen. A third electrode, which functioned as a ground, was placed at the level of the xiphoid process.
Blood pressure: Systolic and diastolic blood pressure were recorded by using a continuous inflatable finger cuff with a FINAPRES 2300 (Finger Arterial Pressure) system (Ohmeda, Madison, WI). Cuff size was determined for each participant, and was fitted to the third finger of the non-dominant hand. Note that, although slower responses than other cardiovascular responses have been obtained with blood pressure recordings, several reports show modulation of blood pressure within 4 to 6 seconds of emotional onset (Globisch, Hamm, Esteves, & Öhman, 1999; Sarlo, Palomba, Buodo, Minghetti, & Stegagno, 2005).
Finger pulse: Variation of amplitude of the blood volume at the finger site was recorded with a photoplethysmography transducer from Mindware Technologies (Gahanna, OH) clipped onto the extremity of the second finger of the non-dominant hand.
-
Skin temperature: Finger temperature was recorded with a temperature probe from Mindware Technologies (Gahanna, OH) taped to the palmar surface of the extremity of the fourth finger of the non-dominant hand.
These latter two measures are thought to have a slower arising reaction to emotional stimulation. However, some reports show modulation of such parameters within a 10 second delay (see for example Levenson, Ekman, Heider, & Friesen, 1992).
Respiration: Changes in respiratory parameters during a 15 seconds picture viewing have been previously reported in the literature (Ritz, Alatupa, Thöns, & Dahme, 2002; Ritz, Thöns, Fahrenkrug, & Dahme, 2005). To our knowledge, however, respiratory activity has rarely, if ever, been studied during shorter recording windows. Thus, little is known about whether modulation of respiration can occur as rapidly as within 8 seconds of an emotional stimulation. Respiration was however included in this study as this measure is crucial to the evaluation of physiological suppression effects, participants being instructed in this condition to focus on controlling their respiration. Thoracic and abdominal respiration were recorded with two respiration belts from Mindware Technologies (Gahanna, OH). The abdominal belt was placed around the waist just above the pants, whereas thoracic belt was placed high on the chest just below the armpits. A calibration procedure was conducted once belts were correctly attached. Participants were instructed to breathe at normal pace into a bag of fixed volume (600 ml) for several cycles. Signal recorded during this procedure was used to later estimate respiration amplitude.
All parameters (including rating and EMG measures) were recorded and amplified with a 32-channel Bionex 8-slot chassis from Mindware Technologies (Grahanna, OH). Data were then converted (16 bit A/D) to a computer for viewing and storage. All acquired channels were sampled at 1000 Hz. Further details regarding the conversion of the data are provided in the Data reduction section (see below).
Procedure
Participants were run in individual sessions. After signing the consent form, they were prepared for the physiological recordings, allowed to observe the signals as they were tested, and to ask any questions they might have about the psychophysiological recordings. Respiration calibration was performed and all the signals were checked for artifacts. Participants were then shown how to use the rating dial and left alone in the room. From this point onward, all instructions were presented on a computer screen; although the participants could use an intercom to communicate with the experimenter should they wish. Participants first viewed a neutral 3-minute film clip as a resting baseline period. They then practiced using the rating dial on several negative and positive pictures (which were not used during the experiment). When they were ready to proceed, participants were acquainted with three types of instruction.
For the unregulated condition (cued by the word WATCH), the instruction was: “Observe the picture and let any emotion you may feel come and go naturally, the same way you did during the training. Continue monitoring yourself and use the dial to report your feelings.” For the expressive suppression trials (cued by the phrase DON’T SHOW), participants were instructed: “Observe the picture but don’t let any emotion you may feel on the inside show in your behavior. In other words, try to behave in such a way that a person watching you would not know that you are feeling an emotion. Remember that you don’t show, but you can feel. Continue monitoring yourself and use the dial to report your feelings.” This instruction was similar to the one used in previous studies on expressive suppression (Gross & Levenson, 1993, 1997). For the physiological suppression trials (cued by the phrase DON’T REACT), participants were instructed: “While watching the picture you may feel that your body is reacting to it. Observe the picture but don’t let any emotion you may feel affect your regular physiological responses. In other words, try to behave in such a way that a person watching your physiological reactions on a computer screen would not know that you are feeling an emotion. Try to calm down, breath normally, relax. Remember that you don’t react, but you can feel. Continue monitoring yourself and use the dial to report your feelings.” Thus, beyond directly instructing to try to decrease physiological arousal (mainly by the mean of relaxation), we also suggested participants to focus on respiration. This was motivated by Philippot and colleagues’ observation that changes in respiration “constitute an easy but potent avenue to manipulate the whole physiological state of the organism” (Philippot, Chapelle, & Blairy, 2002, p. 608).
After receiving these instructions, participants were given a training period, using different pictures than those used in the experiment. Participants then had the opportunity to review the instructions, to do the training again (or both), or to start the session. The session was composed of the 175 stimuli described in a previous section. The order of presentation was randomly chosen for each participant and each time a random assignment was made to one of the three conditions. Thus, a particular image (e.g. IAPS number 5600), assigned to an unregulated trial for one participant, could be assigned to an expressive suppression trial or a physiological suppression trial for another participant. The only controlled variable was the number of trials for each condition. Each participant viewed 25 neutral pictures under the unregulated instruction, 75 negative pictures (25 under each instruction), and 75 positive pictures (25 under each instruction).
On each trial, participants saw a black screen (1 s), a fixation cross (1.5 s), a black screen again (0.5 s), the instruction (WATCH, DON’T SHOW, or DON’T REACT, 1.5 s), the emotional picture (8 s), and then an instruction to reset the dial (1.5 s). Half way through the experiment, participants were given a rest break. After the computer session, sensors were removed and participants were asked to summarize what they did when each of the instructions appeared to control their instruction understanding. Participants were then debriefed and thanked for their participation.
Data Reduction
Dependent variables were rating dial responses, emotion-expressive behavior as recorded by the EMG, and each of the autonomic channels as recorded by the physiological equipment. All of these recordings were exported to be analyzed with ANSLAB (Autonomic Nervous System Laboratory), a biosignal analysis program (shareware version available at the software repository of the Society for Psychophysiological Research; Wilhelm & Peyk, 2005). Channels were manually scanned for artifacts which were corrected, interpolated, or marked as artifact.
To be able to assess the temporal dynamics of emotional responding, continuous recordings were segmented into 16 epochs of 0.5 s each, (see Bradley, 2000, for similar segmentation during the investigation of early emotion responses). This segmentation offers sufficient resolution for all the recorded channels, while permitting the analysis of the entire trial with a reasonable number of epochs. In addition to the picture presentation period (8 s), a baseline of 3.5 s was retained for each trial to calculate specific reactions to the picture onset.
Emotion experience
Rating dial data were exported to obtain mean values for each epoch. Rating data were then transformed for presentation into a scale from −100, extremely negative, to +100, extremely positive, 0 being “absence of emotional feeling”.
Emotion-expressive behavior
EMG signals were rectified and smoothed (moving average = 50 ms) before being averaged for each epoch. EMG was then expressed as the percentage of the baseline level (so that values greater than 100 represent an increase of muscle contraction compared to baseline).
Autonomic responses
1) Cardiovascular activity: Heart rate was calculated from the ECG channel by transforming the inter-beat interval (obtained by the calculation of the duration between successive R waves). Systolic and diastolic blood pressure values were averaged to create a measure of mean blood pressure for each epoch. Pulse transit time (i.e. the time interval between the R wave of the ECG and the upstroke of the peripheral pulse at the finger site) and amplitude were first exported as two separate parameters, they were then z-scored and averaged to form a pulse composite. Decrease in pulse composite indicates lower amplitude and transit time, and thus stronger activation of the sympathetic branch of the cardiovascular system. Temperature channel was exported as mean values for each epoch. 2) Respiratory activity: Respiratory rate and respiratory amplitude were calculated for each epoch. Respiratory rate was obtained by converting the duration of the cycle intervals (in milliseconds) into a number of cycles per minute (c/min). Respiratory amplitude was interpolated by using the difference in volts between the point of maximum inspiration and the point of maximum expiration. Volumes in ml were then obtained using the calibration procedure values. All the autonomic response channels were calculated as change in activity with respect to trial baselines.
Data Analyses
Three factors were used in this within-subject event-related design: (a) Time (16 half-second epochs); (b) Regulation (None, Expressive suppression, Physiological suppression); and (c) Valence (Neutral, Negative, Positive). This last factor was mainly used as a manipulation check. In the analyses of regulation effects, separate ANOVAs were performed for negative and positive trials.
Manipulation checks were performed using a within-factor ANOVA (Valence × Time) with only unregulated trials. To evaluate regulation effects, separate ANOVAs for negative and positive trials were performed for each parameter. For each valence, 75 trials were included in the analyses, comparing activity during trials that were unregulated, regulated with expressive suppression strategy, or regulated with physiological suppression strategy (25 trials each). Temporal dynamics were assessed by including the factor of time. The ANOVAs conducted were thus two within-factor analyses (Regulation × Time) on each of the recorded parameter, separately for negative and positive trials. Regulation during neutral viewing was not assessed.
All ANOVA results were Greenhouse-Geisser corrected. Effect sizes are reported using partial eta square (ηp2). Contrasts for the manipulation check were Bonferroni’s post hoc comparisons. Contrasts of interest for the regulation effects were calculated with two Helmert contrasts. The first compared activity between unregulated and regulated trials (mean of expressive and physiological suppression) in order to assess the general or common effects of the regulation strategies. The second compared activity between expressive and physiological suppression in order to assess the differential effects of each type of regulation.
Results
Manipulation Check
To evaluate the success of our emotion induction, repeated measures ANOVAs (Valence × Time) were conducted for each parameter on unregulated trials. Averages of each parameter over the whole trial duration are reported in Table 1 (unregulated columns). The temporal dynamics during unregulated trials across emotion experience, expressive behavior, and two autonomic channels are presented in Figure 1.
Table 1.
Mean (SE) of Experiential, Expressive, and Autonomic Responses by Picture Type and Condition
| Parameters | Neutral |
Negative |
Positive |
||||
|---|---|---|---|---|---|---|---|
| Unregulated | Unregulated | Expressive suppression | Physiological suppression | Unregulated | Expressive suppression | Physiological suppression | |
| Emotion experience | |||||||
| Rating (/±100) | 4.70 (1.12) | −45.51 (1.75) | −47.14 (1.85) | −47.58 (1.68) | 38.49 (2.24) | 35.65 (2.37) | 33.92 (2.16) |
| Emotion-expressive behavior | |||||||
| Corrugator (%) | 100.21 (1.70) | 114.80 (4.00) | 102.60 (2.24) | 101.70 (2.49) | 94.00 (1.68) | 97.63 (2.40) | 98.42 (1.71) |
| Zygomaticus (%) | 102.21 (2.39) | 100.68 (2.35) | 94.36 (2.18) | 92.11 (2.00) | 130.29 (8.39) | 94.85 (2.35) | 96.34 (1.64) |
| Autonomic responses | |||||||
| Cardiovascular activity | |||||||
| Δ HR (bpm) | −1.5 (0.27) | −2.47 (0.31) | −3.77 (0.43) | −3.77 (0.38) | −1.28 (0.31) | −2.90 (0.33) | −3.27 (0.32) |
| Δ Mean BP (mmHg) | −0.69 (0.24) | −0.49 (0.27) | −0.62 (0.30) | −0.75 (0.24) | −0.38 (0.25) | −0.43 (0.25) | −0.72 (0.26) |
| Δ PC (z-scores) | −0.04 (0.02) | −0.01 (0.01) | −0.04 (0.03) | −0.02 (0.01) | −0.04 (0.01) | −0.02 (0.01) | −0.02 (0.02) |
| Δ FT (°F) | −0.003 (0.001) | −0.003 (0.001) | −0.004 (0.001) | −0.004 (0.001) | −0.002 (0.001) | 0 (0.001) | 0 (0.001) |
| Respiratory activity | |||||||
| Δ RA (ml) | 4.18 (4.87) | −3.72 (5.58) | −28.22 (8.02) | −22.39 (9.94) | 20.65 (6.06) | −16.74 (5.36) | −9.38 (6.37) |
| Δ RR (c/min) | −0.40 (0.16) | −0.46 (0.15) | −1.04 (0.25) | −0.97 (0.25) | −0.44 (0.18) | −1.04 (0.24) | −0.91 (0.22) |
Note: The experience scale goes from −100 (very negative) to +100 (very positive). Expressivity is expressed as percentage of baseline levels. All other parameters are differences with baseline level. HR=Heart Rate, BP=Blood Pressure, PC=Pulse Composite, FT=Finger Temperature, RA=Respiratory Amplitude, RR=Respiratory Rate.
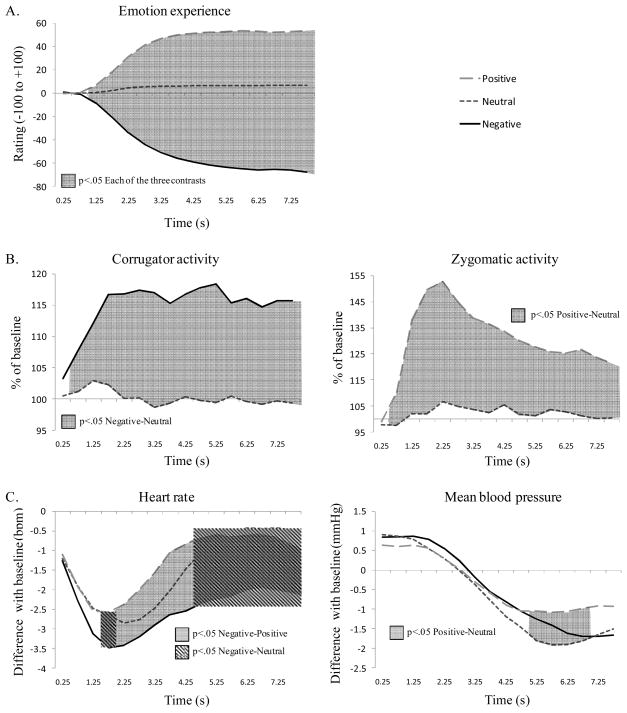
Figure 1.
Manipulation check. Continuous plots of mean emotion experience (Panel A), emotion-expressive behavior (Panel B), and autonomic responses (Panel C) during unregulated trials. Significant contrasts between conditions are represented by shadowed surfaces.
Emotion experience
Analyses of ratings revealed significant effects of valence, F(1,49)=460.92, p<.001, ηp2=.93, and time, F(2,66)=6.22, p<.01, ηp2=.15, as well as a significant Valence × Time interaction, F(2,85)=273.96, p<.001, ηp2=.88. This interaction is shown in Figure 1, Panel A, with significant contrasts for each epoch.
Emotion-expressive behavior
Analyses with the Corrugator Supercilii EMG revealed a significant effect of valence, F(1,44)=27.15, p<.001, ηp2=.43; and a significant Valence × Time interaction, F(5,186)=4.63, p<.001, ηp2=.11. No main effect of time was found (p=.64). A similarly structured ANOVA with the Zygomaticus Major EMG revealed significant effects of valence, F(1,40)=13.93, p<.001, ηp2=.28; and time, F(3,102)=7.69, p<.001, ηp2=.18; as well as a significant Valence × Time interaction, F(4,150)=6.24, p<.001, ηp2=.15. Relevant comparisons are the activity of the corrugator region during negative picture viewing and the activity of the zygomaticus region during positive pictures viewing. Relevant EMG responses for each region are compared to neutral trial activity and are depicted in Figure 1, Panel B, with significant contrasts for each epoch.
Autonomic responses
We first considered cardiovascular responses. For heart rate, we observed significant effects of valence, F(2,67)=9.81, p<.001, ηp2=.21; and time, F(2,68)=17.17, p<.001, ηp2=.32; and a significant interaction of Valence × Time, F(4,145)=4.02, p<.01, ηp2=.1. For mean blood pressure, we found a significant time effect, F(2,62)=37.88, p<.001, ηp2=.51; and a significant Valence × Time interaction, F(4,127)=3.66, p<.05, ηp2=.09. The valence effect was not significant (p=.42). For the pulse composite, a significant time effect was observed, F(3,101)=4.42, p<.01, ηp2=.11. The valence effect and the Valence × Time interaction were not significant (p>.18). For finger temperature, the ANOVA showed a significant time effect, F(1,43)=14.29, p<.001, ηp2=.28. The valence effect and the Valence × Time interaction were not significant (p>.21). Significant interactions (on heart rate and mean blood pressure) are depicted Figure 1, Panel C, with significant contrasts for each epoch. We next considered respiratory activity. No significant effects were found for respiratory rate (p>.28). For respiratory amplitude, we observed significant effects of valence, F(2,69)=5.81, p<.01, ηp2=.14; and time, F(2,74)=3.26, p<.05, ηp2=.08. The interaction was not significant (p=.41).
Regulation Effects on Emotion Experience
To evaluate the effects of suppression on emotional experience, repeated measures ANOVAs (Regulation × Time) were conducted. Separate ANOVAs were performed on the rating values for negative and positive trials. For each condition, the average rating on the whole trial duration is reported in Table 1.
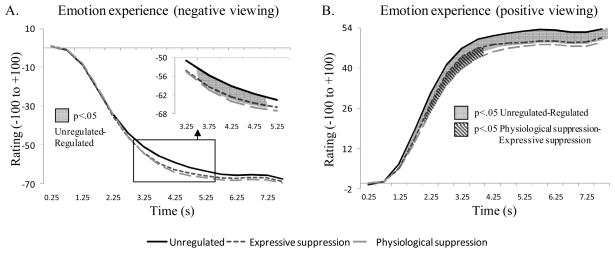
During negative picture viewing, analyses on ratings revealed a significant time effect, F(2,71)=440.92, p<.001, ηp2=.93; and a significant Regulation × Time interaction, F(4,132)=2.98, p<.05, ηp2=.08. Dynamic contrasts are shown Figure 2, Panel A. These findings indicate that both regulation strategies trigger a stronger negative feeling than unregulated viewing for a brief period of time, 3.5 s to 5 s after picture onset. The regulation effect was not significant (p=.14).
Figure 2.
Emotion experience effects for the unregulated and the two regulated conditions during negative picture viewing (Panel A), and positive picture viewing (Panel B). Significant contrasts between conditions are represented by shadowed surfaces.
During positive picture viewing, we observed significant effects of time, F(2,56)=213.02, p<.001, ηp2=.86, regulation, F(2,72)=7.95, p<.01, ηp2=.18, and a significant Regulation × Time interaction, F(4,157)=3.83, p<.01, ηp2=.10. This interaction is shown in Figure 2, Panel B, with significant contrasts for each epoch. Contrasts show that from 1 s after stimulus onset (and until the end of the recorded period) positive feelings are less intense in the regulated conditions. For a brief period (2 to 4 s after picture onset), positive feelings during physiological suppression are less intense than during expressive suppression for the same kind of images.
Regulation Effects on Emotion-expressive Behavior
To evaluate the effects of suppression on expressivity, repeated measures ANOVAs (Regulation × Time) were conducted on EMG values. Separate ANOVAs were performed on negative and positive trials. For each condition and each EMG region, the average activity on the whole trial duration is reported in Table 1.
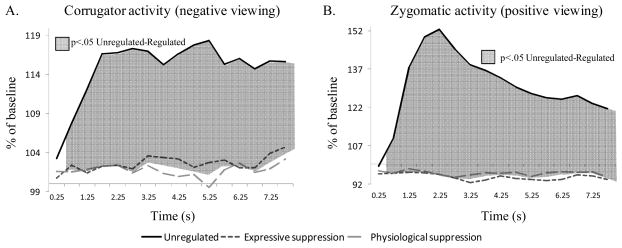
For regulation of negative emotions, expressivity was assessed with the Corrugator Supercilii EMG activity. The ANOVA showed significant effects of regulation, F(1,44)=10.89, p<.01, ηp2=.23; and time, F(3,111)=2.70, p<.05, ηp2=.07; as well as a significant Regulation × Time interaction, F(5,167)=3.20, p<.05, ηp2=.08. Activity of this parameter is presented in Figure 3, Panel A, with significant contrasts for each epoch. These findings indicate that during regulated trials, participants significantly, rapidly, and durably reduced their expressive behavior, with no noticeable difference between the two regulated conditions.
Figure 3.
Emotion-expressive behavior effects for the unregulated and the two regulated conditions during negative picture viewing (Panel A), and positive picture viewing (Panel B). Significant contrasts between conditions are represented by shadowed surfaces.
For regulation of positive emotions, expressivity was assessed with Zygomaticus Major EMG activity. The ANOVA showed significant effects of regulation, F(1,37)=15.67, p<.001, ηp2=.30, time, F(3,120)=7.52, p<.001, ηp2=.17, and a significant Regulation × Time interaction, F(3,120)=7.55, p<.001, ηp2=.17. Activity of this parameter is depicted in Figure 3, Panel B, with significant contrasts for each epoch. Similar to the results on negative trials, regulation during positive trials led to significant, rapid, and durable reduction of expressive behavior, with no noticeable difference between the two regulated conditions.
Regulation Effects on Autonomic Responses
To evaluate the effects of suppression on autonomic responses, repeated measures ANOVAs (Regulation × Time) were conducted for each parameter. Separate ANOVAs were performed on negative and positive trials. For each condition and physiological parameter, the average activity on the whole trial duration is reported in Table 1.
Cardiovascular activity
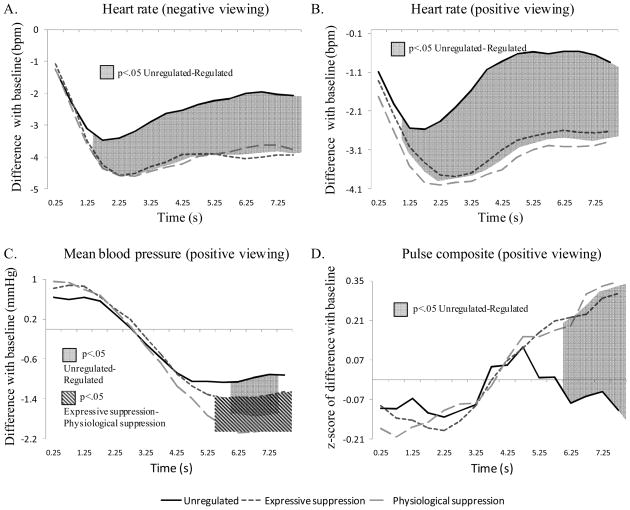
We first considered negative picture viewing. For heart rate, the ANOVA showed significant effects of regulation, F(2,63)=13.36, p<.001, ηp2=.27, time, F(2,69)=15.82, p<.001, ηp2=.31, and a significant Regulation × Time interaction, F(6,200)=7.25, p<.001, ηp2=.17. Dynamic evaluation of this interaction is shown in Figure 4, Panel A. These contrasts indicate that regulated trials show reduced heart rate in comparison to unregulated trials, as early as 1.5 s after picture onset and for the whole subsequent duration of the trial. For mean blood pressure, the ANOVA only showed a significant time effect, F(1,53)=35.88, p<.001, ηp2=.50. The regulation effect and the Regulation × Time interaction were not significant (p>.41). For pulse composite, the ANOVA also only showed a significant time effect, F(2,61)= 5.27, p<.05, ηp2=.13. The regulation effect and the Regulation × Time interaction were not significant (p>.39). Similarly, for finger temperature, the ANOVA performed on negative trials showed only a significant effect of time, F(1,46)=35.48, p<.001, ηp2=.50. The regulation effect and Regulation × Time interaction were not significant (p>.39).
Figure 4.
Cardiovascular effects for the unregulated and the two regulated conditions during negative picture viewing (Panel A), and positive picture viewing (Panel B, C, and D). Significant contrasts between conditions are represented by shadowed surfaces.
We next considered positive picture viewing: For heart rate, the ANOVA showed significant effects of regulation, F(2,61)=22.53, p<.001, ηp2=.39, and time, F(2,67)=11.81, p<.001, ηp2=.25, as well as a significant Regulation × Time interaction, F(5,175)=6.94, p<.001, ηp2=.16. Figure 4, Panel B, shows this interaction with significant contrasts for each epoch. Similar to the results of heart rate for negative picture viewing, regulated positive trials show durable heart rate decrease in comparison to unregulated trials. Differentiation occurs as early as 1 s after stimulus onset. For mean blood pressure, the ANOVA resulted in a significant time effect, F(2,59)=35.25, p<.001, ηp2=.50; and a significant Regulation × Time interaction, F(4,145)=4.64, p<.01, ηp2=.11. Figure 4, Panel C, shows the significant dynamic contrasts of this interaction. These findings indicate that regulated trials trigger à stronger reduction in blood pressure than unregulated trials during a small time window (6 to 7.5 s after picture onset). This result is probably mainly driven by the large decrease in blood pressure that is observed for physiological suppression trials in comparison with the other trials during a 2.5 s-window, starting 5.5 s after picture onset. No main effect of regulation was found (p=.32) for mean blood pressure levels. For pulse composite, the ANOVA resulted in a significant time effect, F(3,105)=10.02, p<.001, ηp2=.22; and a significant Regulation × Time interaction, F(5,166)=4.87, p<.01, ηp2=.12. Figure 4, Panel D, depicts this interaction with highlighted significant contrasts. These contrasts show that from 6 s after picture onset, unregulated trials trigger a significant decrease in pulse composite level, in comparison to the other conditions, highlighting for unregulated trials a stronger activation of the sympathetic branch of the cardiovascular system during this period. No main effect of regulation was found (p=.41). For finger temperature, the ANOVA performed on positive trials showed only a significant effect of time, F(1,45)=6.11, p<.05, ηp2=.15. The regulation effect and the Regulation × Time interaction were not significant (p>.58).
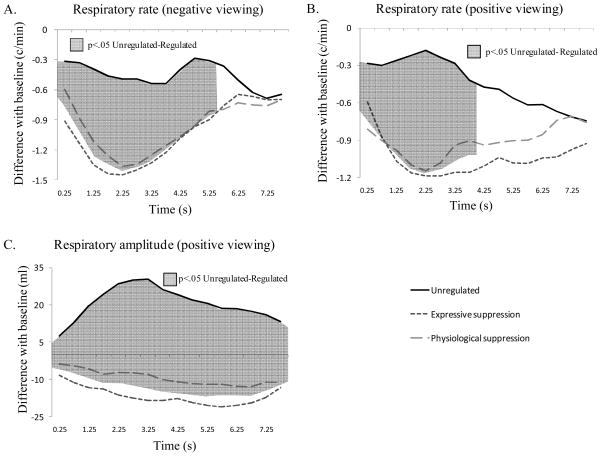
Respiratory Activity
We first considered negative picture viewing. For respiratory rate, the ANOVA resulted in a significant time effect, F(2,63)=3.35, p<.05, ηp2=.09, and a significant Regulation × Time interaction, F(5,191)=3.30, p<.01, ηp2=.08. Figure 5, Panel A, shows this interaction with significant contrasts for each epoch. These findings indicate that as soon as the picture is presented, regulated trials already display differences with unregulated ones (reduced respiratory rate). Differences disappear after 5.5 s of picture viewing. The regulation effect was only marginally significant, F(2,67)=3.17, p<.10, ηp2=.08. For respiratory amplitude, the ANOVA showed significant effects of regulation, F(2,63)=3.56, p<.05, ηp2=.09; and time, F(2,71)=10.39, p<.001, ηp2=.22. The interaction was not significant (p=.36).
Figure 5.
Respiratory effects for the unregulated and the two regulated conditions during negative picture viewing (Panel A), and positive picture viewing (Panel B and C). Significant contrasts between conditions are represented by shadowed surfaces.
We next considered positive picture viewing. For respiratory rate, the ANOVA resulted in a significant Regulation × Time interaction, F(4,153)=2.59, p<.05, ηp2=.07. Figure 5, Panel B, shows this interaction with significant contrasts for each epoch. Similar to what has been shown for negative picture viewing, regulated positive trials differ from unregulated ones by showing reduced respiratory rate as soon as the picture is presented. Differences last for 4 s. The regulation effect on respiratory rate values was only marginally significant, F(2,72)=2.82, p<.10, ηp2=.07, and the time effect was not significant (p=.52). For respiratory amplitude, the ANOVA showed a significant regulation effect, F(2,62)=10.92, p<.001, ηp2=.23; and a significant Regulation × Time interaction, F(5,167)=2.90, p<.05, ηp2=.08. Figure 5, Panel C, shows this interaction with significant dynamic contrasts. These findings indicate that during regulated trials, participants show reduced respiratory amplitude in comparison to what they show during unregulated trials. This effect is already present at time 0 when the picture is presented, and persists for the entire duration of the trial. No effect of time was found (p=.20) on respiratory amplitude values for positive trials.
Discussion
Our goal in this study was to examine the early temporal dynamics and early affective consequences of expressive and physiological suppression. Results demonstrate that both kinds of suppression have early effects on experiential, expressive, and physiological responses (with some effects evident as early as a few hundred milliseconds after emotional onset), and show more common than differential effects on the developing emotion trajectories.
Common Effects of Expressive and Physiological Suppression
Deviations from the unregulated emotion response trajectories were strongly apparent on nearly every recorded parameter during both positive and negative picture viewing. Most deviations were common to both kinds of suppression. Common effects include a transient increase of negative emotion intensity, and a longer decrease of positive emotion experience, only partially confirming our hypothesis. Both forms of suppression also led to decreased expressivity, which was reduced to minimal levels for both suppression types. Cardiovascular activity was reduced, as evidenced by decreased heart rate, blood pressure, and pulse activity, especially during positive picture viewing. Regarding respiratory activity, both suppression types showed anticipatory modulation (i.e. decrease) in respiratory rate and partial reduction of respiratory amplitude (only for positive viewing), globally leading to reduced oxygenation while participants attempted to suppress ongoing emotion responses.3 These results suggest that emotional responses are similarly targeted by suppression efforts, despite differences in the particular target of suppression (behavioral versus physiological responses).
Differential Effects of Expressive and Physiological Suppression
Results being largely dominated by commonalities, relatively few differences were found between these two forms of suppression. Differences were evident only during positive picture viewing and only for two response parameters: experience and blood pressure. Although these differences are limited, they are important, because they suggest that participants indeed executed different strategies, as instructed, and that consequently the two strategies present some slight degree of specificity.
The emergence of positive emotion experience was less rapid during physiological suppression than during expressive suppression. Moreover, emotion experience reports ended at a lower value during physiological response suppression. One could argue this was due to the implicit understanding that decreasing physiological activity means lower experience intensity. If this were true, however, results should show decreased negative experience for physiological suppression trials, which was not the case. Down-regulation of positive emotions thus seems more successful in the physiological suppression condition. During positive trials, mean blood pressure also showed a stronger drop during the second half of the physiological suppression trials, which suggests that efforts to comply with the task had an effect on this parameter, at least during the “easy” task of regulating positive emotion.
Substantial Overlap Between the Suppression Strategies
Our results across all parameters generally show a strong overlap between the two different forms of suppression. By looking at the activity for the two targeted parameters (expression and respiration), it is clearly apparent how overlapping the effects of the two suppression types are.
In fact, and contrary to our hypothesis, expressivity was reduced to a minimum even in the physiological suppression condition. This is a sign that trying to control physiological reactions can be understood as also trying to reduce expressivity, especially that participants were also told to relax as one of the hints for succeeding in physiological suppression. This means that physiological suppression allows control over expressivity as well as other forms of emotional response. Another sign of overlap between the two kinds of suppression is the absence of distinction between the two regulation strategies regarding respiration, respiration being targeted in the expressive suppression condition in a similar manner as in the physiological suppression condition. To summarize, participants suppressed expression while performing physiological suppression and modulate respiration while trying to suppress their emotional expression.
One question that arises is whether those responses can be separately regulated. Physiological theories of neural networks suggest such specificity may be possible since efferent signals seem to be using different pathways. For example different cranial nerves are responsible for facial expressivity (mainly the 7th CN, see e.g. Gillig & Sanders, 2010) and cardiovascular modulation (the 10th CN, or vagus nerve; Holt, 1967; Koizumi, Terui, & Kollai, 1985). Thus, it would be imaginable that separate actions on these different pathways are possible in order to selectively influence emotional responses. From our results, however, it seems that each of these suppression strategies invokes multi-channel regulation. This could in turn be done directly (e.g. individuals try by every means not to show, or not to react, by acting on every possible channel), or indirectly (e.g. voluntary regulation intrinsically addressing different channels). In the latter case, one could imagine that while unconscious, automatic regulation would selectively target emotional responses, increasing voluntary control over emotional responses might spread the influences to several pathways.4 At present, our results lead to the conclusion that in daily life situations, neither expressive suppression nor physiological suppression (e.g. trying to calm down) can be performed solely when they are consciously desired by the individual.
The Use of Brief Static Stimuli Can Highlight Early Processes
Compared to most research on emotion suppression, this study focused on early analyses (just after stimulus appearance) of short lasting (8 s) emotional images (and not films). The use of images gave us a better control over the onset of emotional information, permitting us to lock dynamical assessment of emotional responses. Analyses showed how quickly response modulation strategies can influence body reactions and emotional expression, even for emotional events that are expected to be of short duration.
One important observation is that most prior research on emotion suppression has concentrated on films, whose durations have ranged from one to several minutes, and summary values were often used to report activity over long periods of time. This difference in the induction method might explain some of the discrepancies of the present study results with previous findings. For example, early signs of suppression during picture viewing suggested a decrease in physiological responses rather than the increase in physiological arousal that has been previously suggested. Blood pressure while viewing positive pictures also followed this tendency, especially during the physiological suppression trials. This may mean that participants’ coping ability while facing positive emotional stimuli permitted them to reduce this parameter’s activity about five to six seconds after initiation of the emotional reaction, but that long lasting episodes are less well regulated. Absence of significant effects for negative trials may suggest that coping with negative stimuli may be too difficult to successfully reduce blood pressure activity.
This study nevertheless permitted us to identify recurrent processes that are valid whether for short or long stimulation period, or for static or dynamic stimuli. Thus, facial expression was regulated similarly as previously shown, and the effects of suppression on emotional experience, whether positive or negative, are congruent with part of previous studies on expressive suppression. Heart rate results are also similar to what has been observed in a previous study of expressive suppression during film viewing (Gross & Levenson, 1993, 1997).
The Temporal Dynamics of Emotion Regulation
Reconstructing the temporal unfolding of emotion and emotion regulation is crucial to the understanding of affective processes. When more and more theories highlight synchronization as a key aspect of emotional outbursts (Grandjean, Sander, & Scherer, 2008; Hinrichs & Machleidt, 1992; Scherer, 2000), time becomes one of the most important variables to take into account when portraying emotion trajectories. Modulation of emotion response trajectories is intertwined with the notion of temporal development of emotion, in the same way that the processes related to emotional emergence and those involved in its regulation are inextricably related (Davidson, Jackson, & Kalin, 2000; Hoeksma, Oosterlaan, & Schipper, 2004; Schaefer, et al., 2002).
Recently, psychophysiologists interested in the time course of emotion regulation have captured early processes related to emotion regulation with the help of scalp-recorded event-related potentials (ERPs). Results showed that specific emotion regulation strategies (reappraisal) modulate late positive potentials (LPP) and other components as early as 300 ms after stimulus onset (Hajcak, Moser, & Simons, 2006; Moser, Hajcak, Bukay, & Simons, 2006; Moser, Krompinger, Dietz, & Simons, 2009). The present study shows that suppression effects can also be detected rather early in the emotion process at a peripheral level. The dynamical assessment (0.5 s by 0.5 s) of the emotional emergence in the present study permitted us to (a) precisely delineate the rapidity of emotion regulation intervention, and (b) show that differences between regulated and unregulated emotions are also apparent during emotional emergence and not only on the resulting profile of responses.
Concerning the first point, the present results show for the first time that suppression strategies produce detectable effects as early as a few hundred milliseconds following presentation of an emotional stimulus. Effects on experience are noticeable after 1 to 4 s of stimulation and expressivity can be significantly down-regulated after half a second. Even autonomic activity show rather rapid adaptation (1.5 s for heart rate, 5 to 6 s for blood pressure, and 6 s for pulse activity). Additionally, anticipatory activity was spotted for respiratory activity in early recorded windows (0 to 0.5 s). In the present study, finger temperature is the only parameter not reflecting any changes due to regulation. This may be due to an absence of suppression modulation during the emergence of emotion, or to a longer delay for temperature reactivity. It is possible that regulation effects might be evident with longer recording windows.
As an example for the second point, the three conditions showed no significant difference in negative emotional experience at the end of the observed window. However, differences were observed on how rapidly participants reached this level of experience (see Figure 2, Panel A). Extrapolation of these data beyond the eight second limit can explain why other studies have shown apparently discrepant results concerning emotional experience following suppression. The short time window examined by Goldin et al. (2008) by single value retrospective emotion ratings could have captured this progression in the emerging of emotional experience, whereas in other studies (Gross, 1998a; Gross & Levenson, 1993, 1997), a similar type of retrospective emotional rating taken on larger time windows (about 1 to 3.5 min) may have reflected the common level of experience that can be seen at the end of our recorded period.
Limitations and Future Directions
One limitation of the present study is that we did not focus on particular emotion categories. Instead, we focused on stimuli that are generally negative or positive. This had the advantage of permitting a wider range of stimulation (no repetitive themes), thereby limiting habituation effects. This approach also circumvented the problem of mixed emotions arising from one single stimulation (Ellsworth & Scherer, 2003). However, inasmuch as different emotions have different experiential, behavioral, and physiological response components, one important direction for future research will be to examine reliably induced specific negative and positive emotions at differing intensity levels.
A second limitation is our selection of response measures. Although we were able to continuously assess responses in three domains, and sample several physiological responses, a more complete understanding of the temporal dynamics of emotion regulation will require a broader set of measures of autonomic responses (including for example electrodermal responses and participant’s movements) or the inclusion of neural responses. On this topic, it will be interesting to investigate whether differences in the electro-cortical activation can be shown for suppressive activity, if one can highlight differences between suppression types, and how such activity would relate to the previously found ERP modulations following reappraisal (Hajcak, et al., 2006; Moser, et al., 2006; Moser, et al., 2009).
A third limitation deals with the many different processes that are included in what we called suppression. For example, attentional and other cognitive effects were not separately assessed in this study. This is because we regard such effects as part and parcel of what it means to suppress emotions. However, it bears noting that we required participants to quickly distinguish between two closely related forms of suppression, and it is possible that this required a greater degree of cognitive effort than is usually needed to employ either of these strategies. It has been shown that cognitive loads modulate hormonal and metabolic activities, and increase respiratory and cardiovascular activities (Backs & Seljos, 1994; Carroll, Turner, & Prasad, 1986; Fairclough & Houston, 2004; Fibiger, Evans, & Singer, 1986). Although our results do not seem to reflect such effects, diminishing the load of our task, for example by using a block design (one instruction per block), would be an interesting next step in order to observe the differences in regulatory strategies without the need to rapidly toggle between closely related strategies.
A fourth limitation of the present study has to do with the limited diversity of the participant sample. Focusing on other samples, such as male participants, other age groups, cultures or occupations, as well as special groups like depressed patients, would certainly broaden our understanding of the specific early consequences of response modulation. Also, results could be considered together with data on individual differences, which may have impacted the emotional reactions, especially to negative trials (Taylor, 1991; Watson & Clark, 1984).
Concluding Comment
In this study, we investigated the temporal dynamics of two closely related forms of emotion regulation. We showed that suppression strategies not only affect the overall levels of emotional responses, but also, and most importantly, their dynamics. Snapshots of emotional responses may miss crucial temporal effects, and analyzing emotion emergence and regulation with fine-grained temporal resolution is a key aspect for understanding how those processes work. To our knowledge, this study is the first to document differential and common effects of two kinds of suppression that differ only in the emotional response component that is being targeted. The results showed a strong overlap between the consequences of the two examined strategies. These findings highlight the challenge of disentangling different forms of emotion regulation, particularly in everyday life, where they may be used concurrently.
Acknowledgments
E.D-G. was financed by individual fellowship PBGE11-119321, granted by the Swiss National Science Foundation. Other sources of financing include NIH R01 MH58147 to J.G.
The authors wish to thank Will Dayton for his technical assistance.
Footnotes
Selected IAPS pictures were: a) negative (75 pictures): 2053, 2205, 2710, 2750, 2751, 2800, 2900, 3051, 3061, 3062, 3063, 3064, 3100, 3140, 3160, 3180, 3220, 3230, 3261, 3300, 3350, 3550, 6200, 6212, 6242, 6243, 6312, 6360, 6370, 6530, 6561, 6570, 6571, 6821, 6830, 6831, 7380, 9000, 9006, 9007, 9040, 9050, 9140, 9181, 9220, 9253, 9265, 9280, 9300, 9320, 9331, 9340, 9400, 9405, 9415, 9420, 9421, 9430, 9432, 9433, 9500, 9520, 9560, 9561, 9570, 9571, 9600, 9611, 9620, 9800, 9830, 9910, 9911, 9920, 9921; b) positive (75 pictures): 1440, 1463, 1540, 1590, 1710, 1811, 1999, 2040, 2050, 2057, 2080, 2150, 2160, 2165, 2340, 2352, 2391, 2550, 5260, 5270, 5450, 5460, 5470, 5480, 5600, 5621, 5623, 5629, 5660, 5700, 5820, 5830, 5910, 7200, 7230, 7260, 7270, 7330, 7350, 7400, 7410, 7430, 7470, 7480, 7502, 7570, 7580, 8030, 8034, 8080, 8090, 8120, 8162, 8170, 8180, 8190, 8200, 8210, 8300, 8350, 8370, 8380, 8400, 8420, 8461, 8470, 8490, 8496, 8500, 8501, 8502, 8503, 8510, 8531, 8540; and c) neutral (25 pictures): 2220, 2840, 2890, 5510, 6150, 7000, 7002, 7004, 7006, 7009, 7010, 7020, 7034, 7035, 7050, 7160, 7170, 7185, 7187, 7233, 7235, 7640, 7950, 8160, 9070.
Electrodermal activity would have been a relevant measure to record, but it was not obtained due to the temporary unavailability of this response channel at the time of the study.
Prior studies have not shown such effects of expressive suppression on respiration. However, these studies (Demaree, et al., 2006; Roberts, et al., 2008) have used films to induce emotions and have considered results over one or two minutes of stimulation. Our results show an early dynamic of anticipation (with differences evident during the first 0.5 s of viewing). Differences tended to fade after the eight second period, at least concerning respiratory rate, which helps to reconcile these findings with those of prior researchers.
We thank an anonymous reviewer for suggesting this interpretation.
Contributor Information
Elise S. Dan-Glauser, Department of Psychology, Stanford University;
James J. Gross, Department of Psychology, Stanford University
References
- Arch JJ, Craske MG. Mechanisms of mindfulness: Emotion regulation following a focused breathing induction. Behaviour Research and Therapy. 2006;44:1849–1858. doi: 10.1016/j.brat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Backs RW, Seljos KA. Metabolic and cardiorespiratory measures of mental effort: the effects of level of difficulty in a working memory task. International journal of psychophysiology. 1994;16:57–68. doi: 10.1016/0167-8760(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Bell I, Schwartz G. Cognitive and somatic mechanisms in voluntary control of heart rate. In: Shapiro D, Barber TX, DiCara LV, Kamiya J, Miller NE, Stoyva J, editors. Biofeedback and self-control. Chicago, IL: Aldine; 1973. pp. 503–504. [Google Scholar]
- Bradley MM. Emotion and Motivation. In: Cacioppo JT, Tassinary LG, Bernston GG, editors. Handbook of psychophysiology. New York, NY: Cambridge University Press; 2000. pp. 602–642. [Google Scholar]
- Bradley MM, Lang P. The International Affective Picture System (IAPS) in the study of emotion and attention. In: Coan JA, BAllen JJ, editors. Handbook of emotion elicitation and assessment. New York, NY: Oxford University Press; 2007. pp. 29–46. [Google Scholar]
- Buck R. Social and emotional functions in facial expression and communication: The readout hypothesis. Biological Psychology. 1994;38:95–115. doi: 10.1016/0301-0511(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Petty RE, Losch ME, Kim HS. Electromyographic activity over facial muscle regions can differentiate the valence and intensity of affective reactions. Journal of Personality and Social Psychology. 1986;50:260–268. doi: 10.1037//0022-3514.50.2.260. [DOI] [PubMed] [Google Scholar]
- Carroll D, Turner J, Prasad R. The effects of level of difficulty of mental arithmetic challenge on heart rate and oxygen consumption. International journal of psychophysiology. 1986;4:167–173. doi: 10.1016/0167-8760(86)90012-7. [DOI] [PubMed] [Google Scholar]
- Conde Pastor M, Javier Menéndez F, Sanz M, Vila Abad E. The influence of respiration on biofeedback techniques. Applied Psychophysiology and Biofeedback. 2008;33:49–54. doi: 10.1007/s10484-007-9048-4. [DOI] [PubMed] [Google Scholar]
- Dan Glauser ES. Doctoral Dissertation. University of Geneva; Switzerland: 2008. Vivre son émotion: Mécanismes d’induction et particularités physiologiques d’un processus émotionnel débouchant sur un sentiment subjectif [Living our own emotion: induction mécanisms and physiological particularities of an emotional process leading to a subjective feeling] [Google Scholar]
- Dan Glauser ES, Scherer KR. Neuronal processes involved in subjective feeling emergence: Oscillatory activity during an emotional monitoring task. Brain Topography. 2008;20:224–231. doi: 10.1007/s10548-008-0048-3. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychological Bulletin. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Demaree HA, Schmeichel BJ, Robinson JL, Pu J, Everhart DE, Berntson GG. Up- and down-regulating facial disgust: affective, vagal, sympathetic, and respiratory consequences. Biological Psychology. 2006;71:90–99. doi: 10.1016/j.biopsycho.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Dimberg U, Hansson GÖ, Thunberg M. Fear of snakes and facial reactions: A case of rapid emotional responding. Scandinavian Journal of Psychology. 1998;39:75–80. doi: 10.1111/1467-9450.00054. [DOI] [PubMed] [Google Scholar]
- Dunn B, Billotti D, Murphy V, Dalgleish T. The consequences of effortful emotion regulation when processing distressing material: A comparison of suppression and acceptance. Behaviour Research and Therapy. 2009;47:761–773. doi: 10.1016/j.brat.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth PC, Scherer KR. Appraisal processes in emotion. In: Davidson RJ, Goldsmith HH, Scherer KR, editors. Handbook of Affective Sciences. Oxford, UK: Oxford University Press; 2003. pp. 572–595. [Google Scholar]
- Fairclough SH, Houston K. A metabolic measure of mental effort. Biological psychology. 2004;66:177–190. doi: 10.1016/j.biopsycho.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Fibiger W, Evans O, Singer G. Hormonal responses to a graded mental workload. European journal of applied physiology and occupational physiology. 1986;55:339–343. doi: 10.1007/BF00422730. [DOI] [PubMed] [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Frijda NH. The laws of emotion. New York: Laurence Erlbaum Associates, Inc; 2007. [Google Scholar]
- Gillig PM, Sanders RD. The Trigeminal (V) and Facial (VII) Cranial Nerves: Head and Face Sensation and Movement. Psychiatry (Edgmont) 2010;7:13–16. [PMC free article] [PubMed] [Google Scholar]
- Globisch J, Hamm AO, Esteves F, Öhman A. Fear appears fast: Temporal course of startle reflex potentiation in animal fearful subjects. Psychophysiology. 1999;36:66–75. doi: 10.1017/s0048577299970634. [DOI] [PubMed] [Google Scholar]
- Goldin P, McRae K, Ramel W, Gross J. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean D, Sander D, Scherer K. Conscious emotional experience emerges as a function of multilevel, appraisal-driven response synchronization. Consciousness and Cognition. 2008;17:484–495. doi: 10.1016/j.concog.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Gross J. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998a;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross J. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998b;2:271–299. [Google Scholar]
- Gross J. Emotion regulation in adulthood: Timing is everything. Current Directions in Psychological Science. 2001;10:214. [Google Scholar]
- Gross J. Handbook of emotion regulation. New York: Guilford Press; 2007. [Google Scholar]
- Gross J, John OP. Individual differences in two emotion regulation processes: implication for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross J, Levenson RW. Emotional suppression: Physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64:970–986. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross J, Levenson RW. Hiding Feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106:95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Gross J, Muñoz R. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2:151–164. [Google Scholar]
- Hajcak G, Moser JS, Simons RF. Attending to affect: appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6:517–522. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Harris C. Cardiovascular responses of embarrassment and effects of emotional suppression in a social setting. Journal of Personality and Social Psychology. 2001;81:886–897. doi: 10.1037//0022-3514.81.5.886. [DOI] [PubMed] [Google Scholar]
- Hermann A, Pejic T, Vaitl D, Stark R. Neural correlates of emotion regulation: Differential effects of reappraisal and suppression. NeuroImage. 2009;47:S182. [Google Scholar]
- Hinrichs H, Machleidt W. Basic emotions reflected in EEG-coherences. International Journal of Psychophysiology. 1992;13:225–232. doi: 10.1016/0167-8760(92)90072-j. [DOI] [PubMed] [Google Scholar]
- Hoeksma JB, Oosterlaan J, Schipper EM. Emotion regulation and the dynamics of feelings: a conceptual and methodological framework. Child Development. 2004;75:354–360. doi: 10.1111/j.1467-8624.2004.00677.x. [DOI] [PubMed] [Google Scholar]
- Hofmann S, Heering S, Sawyer A, Asnaani A. How to handle anxiety: The effects of reappraisal, acceptance, and suppression strategies on anxious arousal. Behaviour Research and Therapy. 2009;47:389–394. doi: 10.1016/j.brat.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt GW. Vagus - Nerve Of Heart. American Journal of the Medical Sciences. 1967;253:110–122. [PubMed] [Google Scholar]
- Hutcherson CA, Goldin PR, Ochsner KN, Gabrieli JD, Barrett LF, Gross JJ. Attention and emotion: Does rating emotion alter neural responses to amusing and sad films? NeuroImage. 2005;27:656–668. doi: 10.1016/j.neuroimage.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Jackson D, Malmstadt J, Larson C, Davidson R. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- John OP, Gross J. Healthy and unhealthy emotion regulation: personality processes, individual differences, and life span development. Journal of Personality. 2004;72:1301–1333. doi: 10.1111/j.1467-6494.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- Koizumi K, Terui N, Kollai M. Effect of cardiac vagal and sympathetic nerve activity on heart rate in rhythmic fluctuations. Journal of the Autonomic Nervous System. 1985;12:251–259. doi: 10.1016/0165-1838(85)90065-7. [DOI] [PubMed] [Google Scholar]
- Koole S. The psychology of emotion regulation: An integrative review. Cognition and Emotion. 2009;23:4–41. [Google Scholar]
- Kunzmann U, Kupperbusch CS, Levenson RW. Behavioral inhibition and amplification during emotional arousal: A comparison of two age groups. Psychology and Aging. 2005;20:144–158. doi: 10.1037/0882-7974.20.1.144. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-4. Center for Research in Psychophysiology: University of Florida; 1999. International Affective Picture System (IAPS): Instruction manual and affective ratings. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Levenson RW. Human emotions: A functional view. In: Ekman P, Davidson RJ, editors. The nature of emotion: Fundamental questions. New York, NY: Oxford University Press; 1994. pp. 123–126. [Google Scholar]
- Levenson RW, Ekman P, Heider K, Friesen W. Emotion and autonomic nervous system activity in the Minangkabau of West Sumatra. Journal of personality and social psychology. 1992;62:972–988. doi: 10.1037//0022-3514.62.6.972. [DOI] [PubMed] [Google Scholar]
- Ley R. The modification of breathing behavior: Pavlovian and pperant control in emotion and cognition. Behavior Modification. 1999;23:441–479. doi: 10.1177/0145445599233006. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross J. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5:175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- McCaul K, Solomon S, Holmes D. Effects of paced respiration and expectations on physiological and psychological responses to threat. Journal of Personality and Social Psychology. 1979;37:564–571. doi: 10.1037//0022-3514.37.4.564. [DOI] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli J, Gross J, Ochsner K. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22:248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles H, Gross JJ. Emotion suppression. In: Levinson D, Ponzetti J, Jorgensen P, editors. Encyclopedia of human emotions. New York, NY: Macmillan; 1999. pp. 237–241. [Google Scholar]
- Moser JS, Hajcak G, Bukay E, Simons R. Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology. 2006;43:292–296. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Moser JS, Krompinger JW, Dietz J, Simons RF. Electrophysiological correlates of decreasing and increasing emotional responses to unpleasant pictures. Psychophysiology. 2009;46:17–27. doi: 10.1111/j.1469-8986.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- Philippot P, Chapelle G, Blairy S. Respiratory feedback in the generation of emotion. Cognition and Emotion. 2002;16:605–627. [Google Scholar]
- Ray R, Wilhelm F, Gross J. All in the mind’s eye? Anger rumination and reappraisal. Journal of Personality and Social Psychology. 2008;94:133–145. doi: 10.1037/0022-3514.94.1.133. [DOI] [PubMed] [Google Scholar]
- Richards JM, Gross JJ. Composure at any cost? The cognitive consequences of emotion suppression. Personality and Social Psychology Bulletin. 1999;25:1033–1044. [Google Scholar]
- Ritz T, Alatupa S, Thöns M, Dahme B. Effects of affective picture viewing and imagery on respiratory resistance in nonasthmatic individuals. Psychophysiology. 2002;39:86–94. [PubMed] [Google Scholar]
- Ritz T, Thöns M, Fahrenkrug S, Dahme B. Airways, respiration, and respiratory sinus arrhythmia during picture viewing. Psychophysiology. 2005;42:568–578. doi: 10.1111/j.1469-8986.2005.00312.x. [DOI] [PubMed] [Google Scholar]
- Roberts N, Levenson RW, Gross J. Cardiovascular costs of emotion suppression cross ethnic lines. International Journal of Psychophysiology. 2008;70:82–87. doi: 10.1016/j.ijpsycho.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Demaree HA. Experiencing and regulating sadness: Physiological and cognitive effects. Brain and Cognition. 2009;70:13–20. doi: 10.1016/j.bandc.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Sarlo M, Palomba D, Buodo G, Minghetti R, Stegagno L. Blood pressure changes highlight gender differences in emotional reactivity to arousing pictures. Biological psychology. 2005;70:188–196. doi: 10.1016/j.biopsycho.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Schaefer SM, Jackson DC, Davidson RJ, Aguirre GK, Kimberg DY, Thompson-Schill SL. Modulation of amygdalar activity by the conscious regulation of negative emotion. Journal of Cognitive Neuroscience. 2002;14:913–921. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- Scherer KR. Emotions as episodes of subsystem synchronization driven by nonlinear appraisal processes. In: Lewis MD, Granic I, editors. Emotion, development, and self-organization : Dynamic systems approaches to emotional development. Cambridge, UK: Cambridge University Press; 2000. pp. 70–99. [Google Scholar]
- Sheppes G, Meiran N. Divergent cognitive costs for online forms of reappraisal and distraction. Emotion. 2008;8:870–874. doi: 10.1037/a0013711. [DOI] [PubMed] [Google Scholar]
- Shiota M, Levenson RW. Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychology and Aging. 2009;24:890. doi: 10.1037/a0017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack F, Martin LL, Stepper S. Inhibiting and facilitating conditions of the human smile: A nonobstructive test of the facial feedback hypothesis. Journal of Personality and Social Psychology. 1988;54:768–777. doi: 10.1037//0022-3514.54.5.768. [DOI] [PubMed] [Google Scholar]
- Tamietto M, Castelli L, Vighetti S, Perozzo P, Geminiani G, Weiskrantz L, et al. Unseen facial and bodily expressions trigger fast emotional reactions. Proceedings of the National Academy of Sciences. 2009;106:17661–17666. doi: 10.1073/pnas.0908994106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. Asymmetrical effects of positive and negative events: The mobilization-minimization hypothesis. Psychological Bulletin. 1991;110:67–85. doi: 10.1037/0033-2909.110.1.67. [DOI] [PubMed] [Google Scholar]
- Tomkins SS. Affect theory. In: Ekman P, editor. Emotion in the human face. 2. New York, NY: Cambridge University Press; 1984. pp. 353–395. [Google Scholar]
- Victor R, Mainardi J, Shapiro D. Effects of biofeedback and voluntary control procedures on heart rate and perception of pain during the cold pressor test. Psychosomatic Medicine. 1978;40:216. doi: 10.1097/00006842-197805000-00004. [DOI] [PubMed] [Google Scholar]
- Vrana SR. The psychophysiology of disgust: Differentiating negative emotional contexts with facial EMG. Psychophysiology. 1993;30:279–286. doi: 10.1111/j.1469-8986.1993.tb03354.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. Negative affectivity: The disposition to experience aversive emotional states. Psychological Bulletin. 1984;96:465–490. [PubMed] [Google Scholar]
- Wilhelm FH, Peyk P. ANSLAB: Autonomic Nervous System Laboratory (Version 2.5): Freeware available at the SPR Software Respository. 2005 http://www.sprweb.org.