Abstract
Background
Colorectal cancer (CRC) screening reduces CRC incidence and mortality but is underutilized. Effective interventions to increase screening that can be implemented broadly are needed.
Methods
We conducted a controlled trial to evaluate patient and practice-level intervention to increase use of recommended CRC screening tests among health plan members. The patient-level intervention was a patient decision aid and stage-targeted brochures, mailed to health plan members. Intervention practices received academic detailing to prepare practices to facilitate CRC testing once patients were activated by the decision aid. We used patient surveys and claims data to assess CRC screening test completion.
Results
Among 443 active participants, 75.8% were 52–59 years of age, 80.9% were White, 62.1% were women and 46.4% had college degrees or greater education. Among 380 active participants with known screening status at 12 months based on survey results, 39.0% in the intervention group reported receiving CRC screening compared with 32.2% in the usual care group (unadjusted odds ratio (OR) 1.34, 95% CI 0.88, 2.05, p=0.17). After adjusting for baseline differences and accounting for clustering, the effect was somewhat larger (OR 1.64, 95% CI 0.98, 2.73, p=0.06). Claims analysis produced similar effects for active participants. Intervention was more effective in those with income over $50,000 (OR 2.16, 95% CI 1.07, 4.35) than those with lower income (OR 1.25, 95% CI 0.53, 2.94, p = 0.03 for interaction).
Conclusions
An intervention combining a patient-directed decision aid and practice-directed academic detailing had a modest, though non-statistically significant, effect on colon cancer screening rates among active participants.
Keywords: primary prevention, colorectal cancer, cancer screening, colonic neoplasms
INTRODUCTION
CRC screening is effective, cost-effective, and a high priority among preventive services.1–3 Although use of CRC screening has increased over the past ten years, only 50–60% of age-eligible U.S. adults were up-to-date with screening in 2006.4, 5 Effective and efficient methods are needed to increase CRC screening utilization.
Recent systematic reviews have identified several effective techniques for increasing CRC screening, including reminder systems, audit and feedback, and small media.6 Multi-component interventions, which target physicians' practices and patients and thus can address multiple barriers, may be more effective than interventions that focus only on patients or physicians.7 Our team previously had found that a videotape decision aid, delivered during routine primary care visits, increased CRC screening test ordering and completion.8 Other research has shown that practice-directed interventions, including academic detailing and organizational change interventions, could improve quality of care, including some studies that demonstrated increases in cancer screening rates.9–11
To bring the value of this research to larger populations, it is important to test whether interventions that are efficacious in controlled trials performed in selected environments can be implemented effectively in broader, less-controlled settings, such as health plans and community practices. We sought to test whether an intervention that combined two effective techniques (patient decision aids and academic detailing) could improve CRC screening among health plan members in primary care practices.
METHODS
CHOICE (Communicating Health Options through Information and Cancer Education) was a practice-level controlled trial to evaluate the effect of a patient-level intervention, provision of a mailed patient decision aid on CRC screening, combined with a practice-level intervention, academic detailing. The study was conducted among members of a large health plan (Aetna's HMO product) from selected metropolitan areas in Georgia and Florida. Details of the methods and baseline findings have been previously reported.12
Practice Recruitment
Potential practices for participation were identified from a list of primary care physicians in the Atlanta, Tampa and Orlando areas who participated in the Aetna HMO product. Medical practices recruited to the study each had a minimum of 50 Aetna members between 52 and 75 years of age.
Enrolled practices were grouped into three waves of 10 practices each in order to facilitate timely entry into the trial. The first wave, which were all Georgia practices, was block-randomized into intervention and usual care groups, based on two variables: size of the study-eligible member population and rural vs. urban location. The second wave was randomly allocated in pairs based on practice size and state (Georgia or Florida). As the third wave was recruited, we noted that the intervention and control groups from Waves 1 and 2 were unbalanced in practice size. We therefore used purposive assignment in Wave 3 to balance intervention and control groups with respect to practice size. Two additional practices that were originally intended to be pilot sites (one intervention, one control) also were included without randomization. Detailed information about practice recruitment and characteristics of enrolled practices has been reported previously.12
Study Population and Recruitment
Potentially eligible patient participants in participating practices were Aetna members between ages 52 and 80 who were not current with CRC screening based on claims data. We excluded individuals at increased CRC risk (personal history of CRC or polyps, known history of CRC or polyps in a first-degree relative, or personal history of inflammatory bowel disease); those with medical conditions that might limit study participation or that might cause them not to be considered appropriate candidates for screening (e.g. dementia, heart failure); those unable to effectively communicate in English; and those who were no longer insured by Aetna or who were no longer receiving care in one of the participating practices. Members were considered up-to-date with screening, and therefore ineligible, if claims data indicated that they had received fecal occult blood testing (FOBT) within the last year or a sigmoidoscopy, colonoscopy, or barium enema during the period covered by claims data.
Potentially eligible members were sent a brief eligibility survey to obtain information about receipt of CRC screening not found in claims data and to identify people at increased-risk for CRC for whom the decision aid would not be appropriate (e.g. persons with a first degree relative with a history of CRC). Results of the eligibility survey and agreement with prior claims data have been reported previously.13 Patients deemed eligible after review of claims and completion of the eligibility survey were mailed the baseline survey. Those who agreed to participate and completed the baseline survey were enrolled and considered active participants in the trial.
This study received approval from Emory University and the University of North Carolina Committees on the Protection of Human Subjects. A partial Health Insurance Portability and Accountability Act (HIPAA) waiver was granted to allow access to information in Aetna's claims data repository to identify eligible health plan members. In addition, a full HIPAA Waiver was obtained to permit access to claims data for survey non-responders in both study arms (n=638) to allow us to test, in a separate sub-study, the effect of sending the decision aid mailing to the non-responders in intervention practices.
Practice-Level Intervention: Academic Detailing
The academic detailing intervention was modeled after previously successful interventions to improve use of appropriate clinical services.9 The goal of detailing was to prepare practices to facilitate CRC screening once patients were activated by the decision aid. Because practices also saw patients covered by multiple other health plans (and thus not eligible for the CHOICE trial), we did not focus on changing the overall approach to cancer screening in the practice, as has been reported in previous, more intensive interventions.11
The detailing sessions have been described elsewhere.12 Briefly, two physician detailers (drawn from a group of 4 trained physician detailers) conducted two sessions for each practice which included: information about colon cancer and screening tests; practice-specific screening rates, clips of the decision aid, development of practice specific plans to address requests for screening.
Member-Level Intervention: Decision Aid
The decision aid used in the CHOICE trial is a modified version of a previously tested decision aid, which was effective at increasing CRC screening rates in previous practice-based trials.8, 14 The updated version's total duration is 22 minutes. It contains 1) general information on colorectal cancer and the benefits of screening; 2) information about specific screening tests (FOBT, sigmoidoscopy, colonoscopy, combination of FOBT and sigmoidoscopy, and double contrast barium enema); 3) comparative information on screening tests (efficacy, frequency of testing, preparation, discomfort, likelihood of major complications, and costs); 4) color-coded stage-targeted brochures.
Decision Aid Mailing
The intervention mailing contained a personalized letter; decision aid in DVD and VHS formats with instructions for viewing; stage-targeted brochures; Aetna-specific co-payment and referral information; CRC screening options chart; and the decision aid survey. The survey assessed use of and reactions to the materials, and evaluated changes in knowledge as a result of viewing the decision aid.
Usual Care Condition
Usual care practices received no academic detailing; participants in these practices did not receive the decision aid. All Aetna members (including those in our study's intervention and usual care groups) annually received brief mailed reminders from Aetna encouraging them to obtain CRC screening.
Sub-trial for initial non-responders
We performed a separate sub-trial for members that did not respond to eligibility or baseline surveys and hence were not considered active participants for the main analysis. Those from intervention practices were mailed the decision aid materials. The effect of the decision aid mailing on screening among these non-respondents was assessed with claims data (see below).
Outcomes
Our primary outcome was self-reported completion of any CRC screening test at 12 months. Screening status at 12 months for active participants was determined by response to a mailed survey at 12 months after the baseline survey or by screening reported on the baseline survey.
For secondary analyses of active participants, and for analysis of the effect of the decision aid in the sub-trial of survey non-respondents, we used claims data to assess CRC screening test completion. Receipt of CRC screening was defined as having a claim with a procedure code or diagnosis code for fecal occult blood testing, sigmoidoscopy, double contrast barium enema, or colonoscopy.
Analysis
Screening test completion – active participants with survey data
Our main analysis included participants who had a known screening status based on their response to the 12 month survey or a report of screening test completion on the baseline survey. In our main analysis, we included those reporting screening on the baseline survey as screened at year 1, since screening at baseline may have been the result of earlier study contact. We also performed a sensitivity analysis excluding those who reported screening on the baseline survey.
We compared screening outcomes in intervention and usual care groups using mixed effects logistic regression models. Because assignment of practices did not completely balance patient characteristics at the individual level,12 the analysis controlled for characteristics that differed between intervention and usual care groups at baseline, including state, race, and general beliefs about colorectal cancer screening. The analysis also controlled for gender, age category (<60 years vs. ≥60 years), and education. We accounted for clustering of patients within practices through inclusion of a random intercept for practice in the models. The intracluster correlation coefficient (ICC) for screening outcomes at 12 months within practices was estimated to be 0.03, using a version of the analysis of variance ICC estimator adapted for use with binary variables.15
To assess whether the intervention had different effectiveness among different demographic groups, we considered models that included terms for the interaction between the intervention and common demographic variables, including age, gender, education, income, and race. Interaction with each of these factors was assessed individually, in separate models. For the analysis of interaction with income, we conducted statistical analysis with and without imputation of missing values for income only. Imputation was done 35 times using the logistic regression method, with the imputation model including all variables in the analytical model. SAS PROC MI was used to impute income values, PROC NLMIXED was used to conduct analysis of the resulting data sets with income imputation, and PROC MIANALYZE was used to combine the results of the analyses of the imputations (SAS Institute, Cary, NC). Interaction analyses controlled for baseline differences and other demographic characteristics, and included a random intercept for practice. Statistical significance of the set of interaction terms was assessed using the likelihood ratio test.
Claims analysis for screening test completion
We used mixed effects logistic regression models for all claims analyses. We conservatively assumed that health plan members who had incomplete follow-up claims data due to leaving Aetna coverage did not receive any additional screening after the end of their claims data availability. All claims analyses accounted for clustering of patients within practices through inclusion of a random intercept for practice in the models.
Because the nature of our study design made it difficult to set a “time zero” for assessing the effect of the intervention among active participants, we conducted claims analyses with two different time frames. First, for active participants only, we identified receipt of colorectal cancer screening through examination of any claims with dates of service 6 months before to 12 months after receipt of baseline surveys. This time frame was chosen to be as comparable as possible to results from the 12-month surveys, which asked about screening within the previous 18 months. The random effects logistic regression models for this analysis controlled for the same variables used in the survey analysis (see above).
For our second claims analysis, receipt of colorectal cancer screening was identified through examination of any claims for dates of service within 30 months (912 days) after the mailing date for the eligibility survey. Patients who were determined to have been screened based on claims data before the date when their eligibility surveys were sent were not included in these analyses. Random effects logistic regression models for this analysis controlled for age category (< 60 years vs. ≥60 years), gender, state, and response to the baseline survey (as a marker of participation in the survey portion of the study).
Agreement between survey and claims data was good for those in whom both sources of data were available (n=374): simple agreement was 82.6%, and kappa 0.58.
For the separate sub-trial of survey non-respondents, our claims analysis used the second method of identifying any claims within 30 months of the mailing of the eligibility survey.
All analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC). Random effects models used SAS PROC NLMIXED, with the degrees of freedom based on the number of practices, and using the assumption of normally distributed random effects.
RESULTS
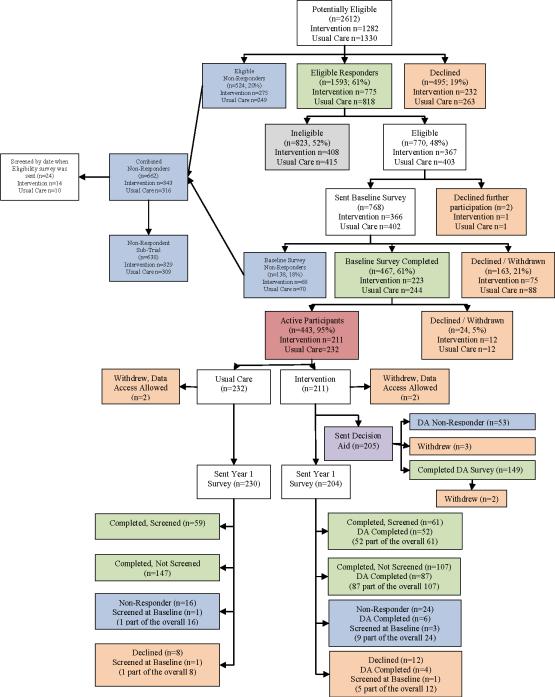
The study flow is shown in Figure 1. Characteristics of the 443 active study participants (211 intervention group, 232 usual care group) have been described in detail previously.12 Most (75.8%) were 52–59 years of age, 62.1% were female, 80.9% were White, 15.2% African-American, 37.1% reported income under $50,000 per year, and 46.4% had graduated from college or had an advanced degree. There were few demographic differences between intervention and usual care groups except for race, with a smaller proportion of white/Non-Hispanics in the intervention group.
Figure 1.
Study flow of the active patients
Of 443 active participants, 374 (84.4%) completed 12-month surveys, including 168 in the intervention group and 206 in the usual care group. In addition, the 12-month analysis also included 6 participants (4 in the intervention group and 2 in the usual care group) who did not respond to the year 1 survey but reported having been screened on the baseline survey, for a total of 380 active participants in the analysis. Those without a known screening status at 12 months (n=63) were statistically more likely to be older, non-white, and not to have graduated from college than those who completed 12-month surveys. Intervention group members, those with no doctor's visits in the past year, those with low perceived risk for colorectal cancer at baseline, and those expressing agreement with statements about colon cancer and CRC screening that would denote less belief in the benefits of CRC screening were also more likely to have an unknown screening status at 12 months (data not shown).
Main outcome: screening test completion at 12 months
Among 380 active participants (172 intervention group, 208 usual care group) for whom 12 month outcome data were available, screening rates were 39.0% for intervention group members compared with 32.2% of participants in the usual care group, resulting in an unadjusted difference in the percentage screened between the groups of 6.7 percentage points (unadjusted 95% CI −3.5%, +16.9%; unadjusted odds ratio (OR) 1.34, 95% CI.0.88, 2.05, p=0.17). After adjusting for baseline differences and accounting for clustering of patients by practice, the overall effect was somewhat larger (OR 1.64, 95% CI 0.98, 2.73, p=0.06).
Excluding those with screening on the baseline survey (whether or not they completed 12 month surveys), screening rates among the 336 remaining participants (150 intervention group, 186 usual care group) were 30.0% for intervention group members compared with 24.2% of participants in the usual care group (adjusted OR 1.82, 95% CI 1.02–3.25, p=0.04).
Colonoscopy was the most frequently reported screening test, followed by FOBT. Type of screening test completed did not differ appreciably between intervention and usual care groups (data not shown).
Differences in effect by demographic characteristics
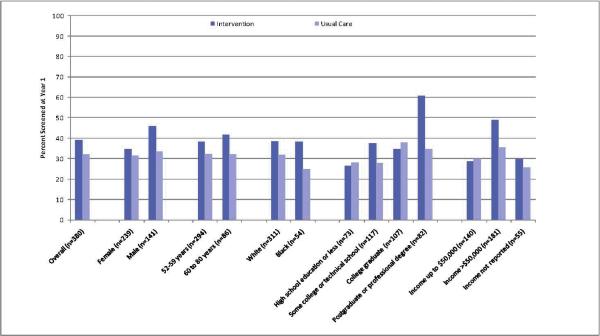
The unadjusted percentages screened, stratified by demographic group and intervention status, are shown in Figure 2, and the adjusted odds ratios are shown in Table 1. Those with income over $50,000 per year had a larger effect from the intervention (adjusted OR 2.16, 95% CI 1.07, 4.35) than those with lower income (adjusted odds ratio 1.25, 95% CI 0.53, 2.94). Interactions with gender, age, race, and education were not statistically significant, although the effect of the intervention in the highest education group (those with a postgraduate or professional degree or higher) was substantially larger than the effect in other educational groups.
Figure 2.
Analysis of Interaction between Intervention and Demographic Variables for Screening at 12 Months
Table 1.
Analysis of Interaction between Intervention and Demographic Variables (n=380)
| Overall n | Screened at year 1 or at baseline | OR for screening at year 1 survey Intervention vs. Usual care* | LR test p-value | ||||
|---|---|---|---|---|---|---|---|
| # | % | OR | 95% CI | p-value | |||
| Gender (missing= 0) | 0.44 | ||||||
| Female | |||||||
| Intervention | 109 | 38 | 34.9 | 1.43 | 0.75–2.71 | 0.26 | |
| Usual Care | 130 | 41 | 31.5 | ||||
| Male | |||||||
| Intervention | 63 | 29 | 46.0 | 2.04 | 0.91–4.55 | 0.08 | |
| Usual Care | 78 | 26 | 33.3 | ||||
| Age (missing= 0) | 0.53 | ||||||
| 52 to 59 | |||||||
| Intervention | 136 | 52 | 38.2 | 1.75 | 0.99–3.10 | 0.05 | |
| Usual Care | 158 | 51 | 32.3 | ||||
| 60 to 80 | |||||||
| Intervention | 36 | 15 | 41.7 | 1.28 | 0.45–3.65 | 0.64 | |
| Usual Care | 50 | 16 | 32.0 | ||||
| Race (missing= 3) | 0.45 | ||||||
| White Non-Hispanic | |||||||
| Intervention | 135 | 52 | 38.5 | 1.45 | 0.84–2.52 | 0.17 | |
| Usual Care | 176 | 56 | 31.8 | ||||
| Black/African-American | |||||||
| Intervention | 34 | 13 | 38.2 | 3.26 | 0.80–13.24 | 0.10 | |
| Usual Care | 20 | 5 | 25.0 | ||||
| Other | |||||||
| Intervention | 3 | 2 | 66.7 | 3.44 | 0.16–74.02 | 0.42 | |
| Usual Care | 9 | 4 | 44.4 | ||||
| Education (missing= 1) | 0.32 | ||||||
| High school, GED, or less | |||||||
| Intervention | 34 | 9 | 26.5 | 0.98 | 0.31–3.13 | 0.97 | |
| Usual Care | 39 | 11 | 28.2 | ||||
| Some college or technical school | |||||||
| Intervention | 56 | 21 | 37.5 | 1.81 | 0.73–4.52 | 0.19 | |
| Usual Care | 61 | 17 | 27.9 | ||||
| College graduate | |||||||
| Intervention | 49 | 17 | 34.7 | 1.16 | 0.47–2.88 | 0.74 | |
| Usual Care | 58 | 22 | 37.9 | ||||
| Postgraduate or professional degree | |||||||
| Intervention | 33 | 20 | 60.6 | 3.26 | 1.16–9.17 | 0.03 | |
| Usual Care | 49 | 17 | 34.7 | ||||
| Income (missing= 4) | 0.03 | ||||||
| Up to $50,000 | |||||||
| Intervention | 63 | 18 | 28.6 | 1.25 | 0.53–2.94 | 0.60 | |
| Usual Care | 77 | 23 | 29.9 | ||||
| Over $50,000 | |||||||
| Intervention | 88 | 43 | 48.9 | 2.16 | 1.07–4.35 | 0.03 | |
| Usual Care | 93 | 33 | 35.5 | ||||
| Prefer not to answer | |||||||
| Intervention | 20 | 6 | 30.0 | 1.34 | 0.33–5.44 | 0.67 | |
| Usual Care | 35 | 9 | 25.7 | ||||
Adjusted for baseline differences between groups (state, race and general beliefs) and other demographic factors (gender, age category and education); and accounting for clustering by practice.
Decision aid use in intervention group
Among 205 active participants in the intervention group; 149 (72.7%) completed decision aid surveys; 83.2% reported watching at least some or all of the video; 77.9% reported reading any of the readiness stage-targeted brochures, and 69% reported reading the co-payment and referral information sheets. Eleven respondents (7.4%) reported no use of the materials. Knowledge was higher among those who reported watching the decision aid than among those who reported not watching it. (Table 2)
Table 2.
Decision Aid Use and Knowledge about CRC Screening among Decision Aid Survey Respondents in the Intervention Group
| Watched video (n=124) | Did not watch video (n=25) | Read Brochures (n=116) | Did not Read Brochures (n=33) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Question | Response | n | Col % | n | Col % | p value* | n | Col % | n | Col % | p-value* |
| Colon cancer is the second leading cause of cancer deaths in the United States. | Correct | 109 | 87.9 | 13 | 52.0 | <0.01 | 100 | 86.2 | 22 | 66.7 | 0.01 |
| Incorrect, Don't know or Missing | 15 | 12.1 | 12 | 48.0 | 16 | 13.8 | 11 | 33.3 | |||
| For most people, colon cancer screening should start at age 60. | Correct | 117 | 94.4 | 18 | 72.0 | <.01 | 107 | 92.2 | 28 | 84.8 | 0.20 |
| Incorrect, Don't know or Missing | 7 | 5.6 | 7 | 28.0 | 9 | 7.8 | 5 | 15.2 | |||
| Colon cancer is most curable when it is found early by a screening test. | Correct | 122 | 98.4 | 25 | 100.0 | 0.52 | 115 | 99.1 | 32 | 97.0 | 0.34 |
| Incorrect, Don't know or Missing | 2 | 1.6 | 0 | 0.0 | 1 | 0.9 | 1 | 3.0 | |||
| In general, once someone starts doing home stool blood tests (FOBT), about how often should they do them? | Correct | 108 | 87.1 | 14 | 56.0 | <.01 | 102 | 87.9 | 20 | 60.6 | <0.01 |
| Incorrect, Don't know or Missing | 16 | 12.9 | 11 | 44.0 | 14 | 12.1 | 13 | 39.4 | |||
| If someone has a colonoscopy, and the results show no signs of colon cancer, when should they have another colon cancer screening test? | Correct | 96 | 77.4 | 8 | 32.0 | <0.01 | 91 | 78.4 | 13 | 39.4 | <0.01 |
| Incorrect, Don't know or Missing | 28 | 22.6 | 17 | 68.0 | 25 | 21.6 | 20 | 60.6 | |||
| You can prevent colon cancer with regular screening tests. | Correct | 84 | 67.7 | 10 | 40.0 | <.01 | 76 | 65.5 | 18 | 54.5 | 0.25 |
| Incorrect, Don't know or Missing | 40 | 32.3 | 15 | 60.0 | 40 | 34.5 | 15 | 45.5 | |||
| You should be tested for colon cancer even if you don't have any symptoms. | Correct | 122 | 98.4 | 24 | 96.0 | 0.44 | 115 | 99.1 | 31 | 93.9 | 0.06 |
| Incorrect, Don't know or Missing | 2 | 1.6 | 1 | 4.0 | 1 | 0.9 | 2 | 6.1 | |||
Among 140 decision aid survey respondents with known screening status at 12 months, screening rates did not differ between those who did or did not report decision aid viewing (39.5% vs. 38.1%, p=0.90) or between those who reported reading brochures and those who did not (38.2% vs. 43.3%, p=0.61). These results were unchanged when the analysis was repeated considering decision aid survey non-respondents with known screening status at 12 months (n=32) to have not watched the decision aid and to have not read the brochures.
Screening test completion based on claims analysis- active participants
Among active study participants (n = 443), we identified claims for CRC screening within 6 months prior to or 12 months after the date when baseline surveys were received for 27.5% in the intervention group and 24.6% in the control group (adjusted OR 1.50, 95% CI 0.91, 2.48, p=0.11)
Among active study participants (n = 433 with 10 excluded because they were screened by claims on or before the date when the eligibility survey was sent), the percentage screened within 30 months of eligibility survey mailing date was 34.3% (71/207) in the intervention group and 31.0% (70/226) in the usual care group (OR adjusting for age category, gender, state, and accounting for clustering by practice=1.60, 95% CI 1.01–2.51, p=0.04).
Among those in the sub-study of eligibility and baseline survey non-respondents (n=638), the percentage screened within 30 months of the eligibility survey mailing date was 28.6% (94/329) in the intervention group and 27.8% (86/309) in the usual care group (OR adjusting for age category, gender, and state, and accounting for clustering by practice=1.08, 95% CI=0.75–1.55, p=0.67).
DISCUSSION
We found that a combined intervention, including a patient-directed decision aid and practice-directed academic detailing, had modest-sized but non-statistically significant overall effects on colorectal cancer screening test completion at 12 months follow-up among active trial participants who completed surveys. The observed effect appeared to be present mainly for those with higher levels of education and income. Claims-based analyses of active participants found similar effect sizes. Screening test completion did not differ based on self-reported use of intervention materials. Among those completing surveys about intervention materials, decision aid use was high, and those who reported viewing the decision aid had higher knowledge than those that did not. Our sub-trial of mailing decision aid materials to survey non-responders, however, had no effect on screening rates.
Our observed effect on CRC screening rates is similar to other recent interventions that have sought to increase screening through patient- and provider-directed interventions.6 In a previous trial of mailing decision aids to patients in one academic internal medicine practice, we found an 11 percentage point increase in screening, with an estimated cost of $94 per additional patient screened.16 Sequist and colleagues found that mailed reminders to patients increased CRC screening by 6 percentage points, with no additional effect from a physician reminder.17 Ganz and colleagues found no difference in CRC screening rates with a multi-modal intervention directed to health plan members.18
More intensive interventions that include telephone-based counseling may have somewhat larger effects. Ling and colleagues reported that enhanced office management (including telephone-based motivational interviewing by a health educator who also facilitated test ordering and implementation of office systems) increased the odds of participants completing colonoscopy or flexible sigmoidoscopy (OR 1.63, 95% confidence interval, 1.11, 2.41; p = 0.01). In the same trial, however, use of tailored reminder letters to patients did not increase screening (OR 1.08, 95% confidence interval, 0.72, 1.62; p = 0.71).19 Lasser and colleagues showed that mailed letter reminders about the need for screening, followed by telephone-based counseling from a patient navigator, increased screening (31% vs. 9%) among community health center patients.20 However, Myers and colleagues found that while a targeted, mailed intervention increased screening by 13% points, addition of tailored messages or a phone reminder did not increase screening more than the mailed intervention.21
Our results should be interpreted with several limitations in mind. Because we used a hybrid allocation procedure performed at the practice-level, we could not completely control confounders at the individual patient level. We accounted for this situation by performing adjustment for key variables that were not equally distributed at baseline (e.g. race), and also accounted for clustering of patients within practices and heterogeneity of screening rates between practices through inclusion of a random intercept for practice. However, it is possible that incomplete adjustment, unmeasured confounding, or misspecification of the distribution of random effects could affect our results.
Our recruitment and data collection procedures required repeated contacts with active participants in both intervention and control groups. Such contact may have acted as an “intervention” to the control group and thus reduced potential differences between groups. In spite of the selected nature of our relatively highly educated and insured survey population, overall screening rates were not particularly high, suggesting that we were not limited by a ceiling effect.
Another important limitation is that our practice-level intervention was not strong enough to produce important changes in practice behavior. We designed our practice intervention with the limited goal of helping to ensure that patients who requested screening could easily have tests scheduled and ordered, but our limited contact (two visits), and the fact that only a small proportion of the practice's patients would be health plan members and thus eligible to receive the intervention may have limited practice engagement and reduced our ability to facilitate screening. Successful cancer screening practice change interventions have employed more frequent and intensive practice contact.6
The “real-world” nature of our research also posed some analytic challenges. We had to determine eligibility and baseline characteristics through separate questionnaires and could not easily define a “time 0”for analyses of test completion. Our main analysis of screening outcome included those who reported screening on the baseline survey (but who were unscreened at eligibility assessment) to recognize that screening at baseline may have been the result of the study contact for either intervention or control group participants. Secondary analyses excluding those screened at baseline, however, produced similar results.
We also did not have complete 12-month survey results for all active participants, and the loss to follow-up differed by intervention status. Our claims analyses, however, did not suffer from this limitation, and produced similar results (OR 1.5–1.6) to the survey-based analyses for active participants. The availability of both claims and survey data is a particular strength of our study.
In conclusion, we found that our combined intervention to increase CRC screening among health plan members likely had a modest, non-statistically significant overall effect on screening test completion, but produced larger effects for those with higher incomes and advanced education. We designed and pre-tested our decision aid so that it would be accessible to a wide range of users; however, our results suggest that in its current form of dissemination, it may be more effective in those with fewer socio-economic barriers. Additional, larger studies are required to determine if these observed differences in effect by income and education are real or due to chance.
Even small improvement in screening rates (net increase of 5 percentage points or more), if applied broadly, could translate into considerable reduction in deaths from colorectal cancer.22 It appears that the increase in screening observed came mainly from the mailed intervention serving as a cue to action among active participants. Based on the experience of our detailing teams, it is unlikely that the practice intervention produced meaningful changes in screening itself. In addition, the practice-level intervention was labor intensive and expensive, and thus would not be a good candidate for broad implementation.
Our results and those of other recent studies suggest that a reasonable next step might be to study the effect of an inexpensive mailed reminder (with access to a decision aid for those who want more information) followed by phone-based telephone counseling and navigation for those who do not respond to the reminder or who need more help in facilitating test scheduling and completion.
Acknowledgements
Drs. Pignone and Glanz had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors wish to thank the following for their assistance with the study: Chris DeLeon, Raquel Vazquez Ludwig, Lauren Taglialatela, Jennifer Griffith, Alison Brenner, Renata Hilson, Murtaza Cassoobhoy, and Lisa Bernstein.
This research was supported by grant number PH000018 from the Centers for Disease Control and Prevention and is registered as NCT00134589 at Clinical Trials.gov.
Dr. Pignone was also supported by a National Cancer Institute Established Investigator Award (K05 CA129166) and the Foundation for Informed Medical Decision Making. Dr. Glanz was also supported by a Georgia Cancer Coalition Distinguished Scholar Award. Dr. Lewis was supported by a K07 Mentored Career Development Award (5K07CA104128) from the National Cancer Institute.
Footnotes
There are no financial disclosures from any authors.
REFERENCES
- 1.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):638–58. doi: 10.7326/0003-4819-149-9-200811040-00245. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18838718. [DOI] [PubMed] [Google Scholar]
- 2.Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(2):96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. Available from http://www.annals.org/cgi/reprint/137/2/96.pdf. [DOI] [PubMed] [Google Scholar]
- 3.Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Colorectal cancer screening: health impact and cost effectiveness. Am J Prev Med. 2006;31(1):80–9. doi: 10.1016/j.amepre.2006.03.009. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16777546. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1623–30. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 5.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15(2):389–94. doi: 10.1158/1055-9965.EPI-05-0678. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16492934. [DOI] [PubMed] [Google Scholar]
- 6.Sabatino SA, Habarta N, Baron RC, Coates RJ, Rimer BK, Kerner J, et al. Interventions to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers systematic reviews of provider assessment and feedback and provider incentives. Am J Prev Med. 2008;35(1 Suppl):S67–74. doi: 10.1016/j.amepre.2008.04.008. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18541190. [DOI] [PubMed] [Google Scholar]
- 7.Baron RC, Rimer BK, Coates RJ, Kerner J, Kalra GP, Melillo S, et al. Client-directed interventions to increase community access to breast, cervical, and colorectal cancer screening a systematic review. Am J Prev Med. 2008;35(1 Suppl):S56–66. doi: 10.1016/j.amepre.2008.04.001. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18541188. [DOI] [PubMed] [Google Scholar]
- 8.Pignone M, Harris R, Kinsinger L. Videotape-based decision aid for colon cancer screening. A randomized, controlled trial. Ann Intern Med. 2000;133(10):761–9. doi: 10.7326/0003-4819-133-10-200011210-00008. Available from http://www.annals.org/cgi/reprint/133/10/761.pdf. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard-Jensen J, Kristoffersen DT, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2007;(4):CD000409. doi: 10.1002/14651858.CD000409.pub2. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17943742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baron RC, Rimer BK, Breslow RA, Coates RJ, Kerner J, Melillo S, et al. Client-directed interventions to increase community demand for breast, cervical, and colorectal cancer screening a systematic review. Am J Prev Med. 2008;35(1 Suppl):S34–55. doi: 10.1016/j.amepre.2008.04.002. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18541187. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich AJ, Carney PA, Winchell CW, Sox CH, Reed SC. An office systems approach to cancer prevention in primary care. Cancer Pract. 1997;5(6):375–81. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9397706. [PubMed] [Google Scholar]
- 12.Lewis C, Pignone M, Schild LA, Scott T, Winquist A, Rimer BK, et al. Effectiveness of a patient- and practice-level colorectal cancer screening intervention in health plan members: design and baseline findings of the CHOICE trial. Cancer. 2010;116(7):1664–73. doi: 10.1002/cncr.24962. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20143439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pignone M, Scott TL, Schild LA, Lewis C, Vazquez R, Glanz K. Yield of claims data and surveys for determining colon cancer screening among health plan members. Cancer Epidemiol Biomarkers Prev. 2009;18(3):726–31. doi: 10.1158/1055-9965.EPI-08-0751. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19273480. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Whitney A, Hayter S, Lewis C, Campbell M, Sutherland L, et al. Development and initial testing of a computer-based patient decision aid to promote colorectal cancer screening for primary care practice. BMC Med Inform Decis Mak. 2005;5:36. doi: 10.1186/1472-6947-5-36. Available from http://www.biomedcentral.com/1472-6947/5/36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridout MS, Demetrio CG, Firth D. Estimating intraclass correlation for binary data. Biometrics. 1999;55(1):137–48. doi: 10.1111/j.0006-341x.1999.00137.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11318148. [DOI] [PubMed] [Google Scholar]
- 16.Lewis CL, Brenner AT, Griffith JM, Pignone MP. The uptake and effect of a mailed multi-modal colon cancer screening intervention: A pilot controlled trial. Implement Sci. 2008;3:32. doi: 10.1186/1748-5908-3-32. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18518990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sequist TD, Zaslavsky AM, Marshall R, Fletcher RH, Ayanian JZ. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2009;169(4):364–71. doi: 10.1001/archinternmed.2008.564. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19237720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganz PA, Farmer MM, Belman MJ, Garcia CA, Streja L, Dietrich AJ, et al. Results of a randomized controlled trial to increase colorectal cancer screening in a managed care health plan. Cancer. 2005;104(10):2072–83. doi: 10.1002/cncr.21434. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16216030. [DOI] [PubMed] [Google Scholar]
- 19.Ling BS, Schoen RE, Trauth JM, Wahed AS, Eury T, Simak DM, et al. Physicians encouraging colorectal screening: a randomized controlled trial of enhanced office and patient management on compliance with colorectal cancer screening. Arch Intern Med. 2009;169(1):47–55. doi: 10.1001/archinternmed.2008.519. [DOI] [PubMed] [Google Scholar]
- 20.Lasser KE, Murillo J, Medlin E, Lisboa S, Valley-Shah L, Fletcher RH, et al. A multilevel intervention to promote colorectal cancer screening among community health center patients: results of a pilot study. BMC Fam Pract. 2009;10:37. doi: 10.1186/1471-2296-10-37. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19480698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers RE, Sifri R, Hyslop T, Rosenthal M, Vernon SW, Cocroft J, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110(9):2083–91. doi: 10.1002/cncr.23022. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17893869. [DOI] [PubMed] [Google Scholar]
- 22.Subramanian S, Bobashev G, Morris RJ. Modeling the cost-effectiveness of colorectal cancer screening: policy guidance based on patient preferences and compliance. Cancer Epidemiol Biomarkers Prev. 2009;18(7):1971–8. doi: 10.1158/1055-9965.EPI-09-0083. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19567507. [DOI] [PubMed] [Google Scholar]