Abstract
Background
Nearly two-thirds of elderly patients treated for depression fail to achieve symptomatic remission and functional recovery with first-line pharmacotherapy. In this study, we ask whether a mind–body exercise, Tai Chi Chih (TCC), added to escitalopram will augment the treatment of geriatric depression designed to achieve symptomatic remission and improvements in health functioning and cognitive performance.
Methods
One hundred twelve older adults with major depression age 60 years and older were recruited and treated with escitalopram for approximately 4 weeks. Seventy-three partial responders to escitalopram continued to receive escitalopram daily and were randomly assigned to 10 weeks of adjunct use of either 1) TCC for 2 hours per week or 2) health education (HE) for 2 hours per week. All participants underwent evaluations of depression, anxiety, resilience, health-related quality of life, cognition, and inflammation at baseline and during 14-week follow-up.
Results
Subjects in the escitalopram and TCC condition were more likely to show greater reduction of depressive symptoms and to achieve a depression remission as compared with those receiving escitalopram and HE. Subjects in the escitalopram and TCC condition also showed significantly greater improvements in 36-Item Short Form Health Survey physical functioning and cognitive tests and a decline in the inflammatory marker, C-reactive protein, compared with the control group.
Conclusion
Complementary use of a mind–body exercise, such as TCC, may provide additional improvements of clinical outcomes in the pharmacologic treatment of geriatric depression.
Keywords: cognition, escitalopram, geriatric depression, quality of life, resilience, Tai Chi Chih, treatment response
Depression in older adults carries significant risk for decline in health functioning, morbidity, and mortality, including suicide.1–3 Despite the gains in the treatment of major depression, more than 60% of elderly patients treated for depression fail to achieve symptomatic remission and functional recovery with first-line pharmacotherapy.4,5 Older patients with depression are reported to have more physical illness and chronic pain; frailty; psychomotor retardation; or agitation, anxiety, cognitive impairment, anorexia, or weight loss than younger depressed adults.6,7 Late-life depression is also associated with neuroimaging abnormalities, such as cortical atrophy on magnetic resonance imaging and white- and gray-matter hyperintensities,8–11 leading to gait imbalance and psychomotor retardation. In addition to residual depressive symptoms, depressed older adults frequently experience unremitting dysfunction of executive cognitive performance, as well as memory impairments and poor quality of life and health functioning.12–14 Treatments are needed that can complement and add to the benefit of pharmacotherapy, in order for depressed older adults to achieve remission, experience lower rates of residual depressive symptoms, and enjoy the benefits of decreased disability and improved social and health functioning.15
Prior work by our group and others demonstrate that inflammatory mechanisms influence the neural substrates that underlie depression and aging and may prospectively predict depressive symptoms.16,17 Proinflammatory cytokines can also induce the syndrome of sickness behavior18,19 that is typically associated with behavioral changes in humans with symptoms of anhedonia, cognitive dysfunction, anxiety/irritability, psychomotor slowing, anergia/fatigue, anorexia, sleep alterations, and increased sensitivity to pain,20,21 which are all consistent with clinical features of late-life depression. Finally, inflammatory cytokines can alter the metabolism of key monoamines, including serotonin, norepinephrine, and dopamine, that are involved in the pathogenesis of mood disorders,22 which may also be a mechanism that contributes to poor treatment response with pharmacotherapy. Furthermore, our studies have found that mind–body interventions, including Tai Chi and/or exercise, can alter cellular immunity and reduce markers of inflammation.23–27
Tai Chi and other similar alternative and complementary mind–body intervention trials are directed toward combining aerobic exercise with stress reduction and structured, mindful cognitive approaches that may add to improvement in mood, irritability, and self-esteem.28 In addition, Tai Chi can be readily implemented even among older adults with physical limitations due to chronic medical illnesses or poor balance.29 Finally, Tai Chi Chih (TCC) is a brief standardized version of Tai Chi that involves 20 movements or Chi Gong exercises, thus offering manageable and nonstrenuous training and practice schedules for older adults, with an important advantage over prior relaxation response–based therapies.30,31 Whereas Mather et al.32 had suggested that exercise might be used as an adjunct for depression treatment among older adults with poor response,32 no prior studies have employed a randomized controlled trial (RCT) design to test the efficacy of a mind–body intervention that targets both physical activity and stress–response pathways, which are thought to contribute to a perpetuation of depressive symptoms.
In this study, we present the results of our initial study that examined the use of escitalopram and an adjunct use of TCC versus a Health Education program (HE), as it pertains to several outcomes of interest: 1) depression; 2) cognition; 3) physical functioning; and 4) inflammation.
METHODS
Design Overview
This randomized, controlled clinical trial allocated older adults to receive either TCC or HE (active control intervention) in a 1:1 ratio between 2007 and 2009. HE was selected as the control condition, as we deemed it a higher priority to determine in this initial study whether TCC had an active clinical benefit that was independent of nonspecific treatment factors (e.g., expectation, group support, and attention), rather than comparing this approach with isolated treatment components such as relaxation, exercise, or meditation. Participants were recruited through newspaper advertisements that stated the aim of the study as comparing the effects of TCC versus the effects of HE on “depression in older adults.” No additional information about the study hypothesis or about an evaluation of depression was provided. Hence, participants were blinded to the study objectives and outcomes of depression.
Setting and Participants
All subjects met the following inclusion criteria: 1) current episode of major depressive disorder; 2) a 24-item Hamilton Depression Rating Scale (HDRS) score of 16 or higher at baseline; and 3) Mini-Mental State Exam score of 26 or higher. The subjects were excluded if they had 1) a history of any other psychiatric illness or alcohol or substance abuse/dependence (with the exception of generalized anxiety disorder that is frequently comorbid with geriatric depression); 2) severe or acute medical illness; 3) acute suicidal or violent behavior; 4) any other central nervous system diseases or dementia; or 5) were not able to participate in TCC due to mobility problems. Patients were free of psychotropic medications for at least 2 weeks before starting the trial.
Procedures
Participants who responded to the advertisement (N = 332) underwent two assessment phases before they were included. A 15-minute telephonic interview by a trained project coordinator ensured that participants fulfilled the screening eligibility criteria. The second eligibility-assessment phase included an interview to obtain a medical history and current medication use, followed by administration of the structured clinical interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, to diagnose a current episode of major depression. After describing the details of the study to interested subjects, written informed consent was obtained in accordance with the procedures set by the University of California, Los Angeles, institutional review board.
Over a period of 30 months, we screened a total of 332 older, depressed individuals and recruited 112 participants age 60 and older who met criteria for unipolar major depressive disorder (MDD) and the study admission criteria. All subjects underwent the structured clinical interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, administered by one rater (HL) to establish a diagnosis of MDD. The HDRS was administered at baseline and in all follow-up visits to determine the rate of remission and change in the severity of depressive symptoms after the initial MDD diagnosis. All subjects received an initial assessment, including complete physical and neuropsychiatric examinations, electrocardiography, and laboratory testing at baseline, to rule out new-onset medical illnesses that could account for behavioral symptoms.
All eligible subjects received 10 mg of escitalopram per day for the treatment of depression. We selected to use escitalopram because it is one of the most effective currently available SSRIs.33,34 In addition, escitalopram is very easy to use in a single effective dose of 10 mg daily and generally is well tolerated by older adults.35,36 The convenience of the use of escitalopram in the context of our study includes the lack of the dose titration and the lack of the waiting period after each increase in the dose to observe a maximal effect. In addition, there is an evidence of a faster onset of antidepressant and anxiolytic action of escitalopram compared with citalopram in the adult and geriatric samples.35–38
Randomization and Intervention
Of the 112 subjects who entered the trial and started receiving an initial dose of escitalopram as 10 mg/day, those showing little response had an option of increasing the dosage to 20 mg after 4 weeks of treatment. Thirty-nine subjects dropped out during this initial dose-adjustment phase: 4 dropped out due to side-effects, 1 achieved full remission, 25 withdrew consent, and 7 were lost to follow-up. After about 6 weeks, the remaining 73 subjects, who were able to tolerate the effects of escitalopram and who did not achieve remission within the first few weeks of drug treatment alone, were randomized to the complementary interventions. All randomized subjects maintained drug therapy throughout the duration of the study. Participants were randomized to 10 weeks of complementary intervention of either 1) TCC for 2 hours per week or 2) HE for 2 hours per week. Randomization was performed by using a computer-generated schedule, independent of treatment personnel. Allocation concealment was implemented by using sealed, sequentially numbered boxes that were identical in appearance for the two treatment groups. All assessments were performed by the raters blinded to the treatment group assignment. Subjects were asked not to disclose their group assignment to the raters.
TCC protocol
TCC employs “meditation through movement” as a means of helping older adults cope with fatigue, perceived physical limitations, and depression. The rationale given to patients states that TCC constitutes a health management intervention, which incorporates meditation and physical activity to promote a sense of well-being and to foster control over negative symptoms associated with depression. TCC does not address cognitive activity underlying appraisals of depression, but instead, it emphasizes control over arousal-related responsiveness through the performance of repetitious, nonstrenuous, slow-paced movement. The standard detailed protocol for the TCC uses an adapted protocol from the Stone manual,39 and it has been used in several studies conducted by our research group.30,31 TCC sessions were held for a duration of 2 hours, once a week. Each TCC class was conducted in 120 minutes and also included 10 minutes of warm-up (e.g., stretching and breathing) and 5 minutes of cooldown exercises.
Health education protocol
The HE protocol served as an active control for nonspecific treatment elements such as attention and group support that pose rival explanations for the effectiveness of TCC. Similar to the TCC group, participants assigned to HE were informed that this was an intervention for “depression in older adults,” which served to balance expectations for the TCC and HE interventions. The rationale for this intervention is that education about depression, stress, sleep, and health-related issues will play a central role in helping patients with depression understand and self-manage their mood symptoms and the factors affecting their mood (e.g., stress). The trained study staff implemented HE by using a manual that presented educational information and described learning objectives and patient activities to promote an integration of the material. The HE sessions were held once a week for 120 minutes over the 10-week treatment period and followed a didactic format, with lectures on key topics, followed by focused group discussion and postdiscussion self-help quizzes to assess patient learning. This novel use of a nonexercise control intervention, which matches the exercise intervention in duration, frequency, and social contact, represents an important methodologic advance.40
Treatment credibility
It was essential that subjects perceived the two interventions as equally credible and as promoting a similar degree of expectation for improvement. To address this issue, we evaluated treatment satisfaction and adherence by using a 10-point Likert scale.
Involvement in alternate treatments
All subjects were medically stable, and all current major psychiatric disorders (other than depression) were ruled out before entry into the study protocol. If, for medical or psychiatric reasons, subjects engaged in alternate treatments, they were advised to inform both the TCC instructor and the PI of this action. Changes in medical regimen and use of an alternate treatment were monitored through compliance questionnaires at each visit. If subjects preferred to use other treatments, they were discontinued from the study before randomization. The use of concomitant medications during the treatment trial was restricted to the use of lorazepam up to 1 mg day. At the end of the trial, the decisions were made to continue escitalopram or switch to another antidepressant on the basis of treatment response and tolerability.
Outcomes and follow-up
The primary outcome assessments were administered at all visits, and the secondary outcomes were assessed at baseline and after the completion of the trial. The primary outcome variable was depression remission, defined as HDRS scores of 6 or less. Response was defined as HDRS scores of 10 or less. Nonresponse was defined as less than 30% improvement on HDRS scores from baseline. In addition, we compared improvement in residual depressive symptoms in the two treatment groups over time by using the HDRS scores. The assessments of depressive symptoms were obtained during the lead-in phase between baseline assessment and randomization and then repeated every 2 weeks throughout the study. In addition, we assessed the severity of anxiety, apathy, psychomotor retardation, resilience, health-related quality of life, and cognition at baseline and at follow-up. A single rater (HL), who was blind to the treatment group assignment, carried out weekly behavioral assessments.
Assessment instruments
The 24-item HDRS41 was used to quantify mood symptoms. Cognitive performance was measured by the Mini-Mental State Exam and a brief cognitive battery. Medical comorbidity was measured by the Cerebrovascular Risk Factor Prediction Chart42 of the American Heart Association for rating cerebrovascular risk factors, including age, systolic blood pressure, antihypertensive medication use, history of diabetes, smoking, previous strokes, atrial fibrillation, and left-ventricular hypertrophy. The Cumulative Illness Rating Scale–Geriatric43 was used for rating global chronic medical illness burden. Other secondary outcomes measures included the Clinical Global Impression Severity and Improvement scale,44 a measure of the overall severity and clinical improvement over time. Measures of comorbid symptoms were administered at baseline and week 16 and included the Hamilton Anxiety Scale,45 a widely used measure of anxiety symptoms on a 0–5 scale; the Apathy Evaluation Scale,46 a measure of the severity of apathy; the Unified Parkinson’s Disease Rating Scale,47 used for the assessment of psychomotor slowing and extrapyramidal symptoms; the Medical Outcomes Study 36-Item Short Form Health Survey,48 an instrument that measures health-related quality of life and mental, physical, and social functioning; and the Connor-Davidson Resilience scale, as a measure of stress coping ability.49
Vital signs and weight were measured during each visit. Side-effects were assessed by the Udvalg for Kliniske Undersogelser Side-Effect Rating Scale.50 We collected blood samples to measure plasma levels of C-reactive protein (CRP) by using the Dade Behring N High Sensitivity CRP assay (Dade Behring Diagnostics, Marburg, Germany) at three time points (baseline, randomization, and after completion of the study).
A brief neuropsychological assessment battery was developed and administered by Dr. Ercoli to assess the domains likely to show impairment in geriatric depression that may improve with TCC: 1) executive functioning; 2) episodic memory; and 3) information processing speed and attention. The California Verbal Learning Test II51 was used to test verbal memory. Depressed patients tend to show a poor learning curve and deficient recall, yet their recognition rates are similar to controls.52 The California Verbal Learning Test II provides numerous scores for examination, including ratios comparing verbal abilities of the left frontal, ventral, and dorsolateral regions.51 Trail-Making Test A and B53 was used to assess basic and divided attention, motor speed, and executive function. The Stroop Test54 is a group of three tests of 100 items in which patients have to name 1) the printed word, 2) the color of the block, and 3) the color of the ink in which the word is printed. The interference effect is estimated by subtracting Part A from Part C, the interference trial. Scores are calculated to include the time on each part, number of errors on each part, total number of errors, and total near misses (errors that are self-corrected).
Statistical Analysis
Patients in the two treatment groups were compared on all demographic and clinical measures at baseline to assess the success of the randomization procedures by using χ2 tests for the categoric measures and bidirectional t-tests for the continuous measures. Safety analyses were performed by using descriptive statistics and frequency distribution of dropouts. All outcome results used intent-to-treat analyses with mixed linear models analyses; no data were imputed. The proportion of subjects who achieved remission was analyzed by using χ2 test. The primary outcome measure, namely the continuous HDRS score, was analyzed by using a mixed models using SAS version 9.1 (SAS Institute Inc., Cary, NC). Treatment group, time, and the interaction term between time and treatment group were included in the model, with group as a between-subject effect and time as a continuous within-subject variable. For the secondary measures such as anxiety, resilience, distress, and burden, change scores were analyzed by using t-tests. We examined the relationship between the variables by using Pearson correlation coefficients. The level of significance was set at the α level of p ≤0.05, two tailed.
RESULTS
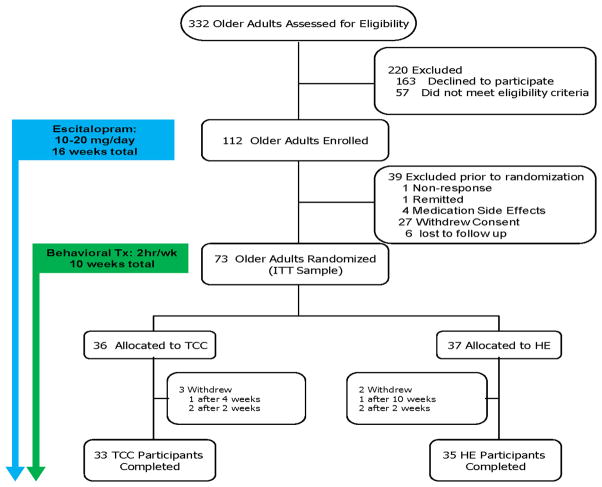
Figure 1 presents the CONSORT flow. Table 1 presents the baseline demographic and clinical characteristics of the intent-to-treat sample of the two subject groups. The baseline characteristics did not differ between the two treatment groups. Although medication was adjusted upward for about 25% of the sample (to 20 mg/day), the two groups did not differ in mean dosage of escitalopram (TCC mean: 12.5 mg/day, SD: 4.4; HE mean: 12.7, SD: 4.5; t[71]= 0.20, p>0.20). Both groups did not differ by adherence to the protocol (t[67]= 0.89, p>0.20) or satisfaction or expectation for benefit with the intervention (t[67]= 0.68, p>0.20).
FIGURE 1.
Participant Flow and Distribution of Subjects in the Study
Table 1.
Comparison of the baseline characteristics in the two comparison groups receiving escitalopram and Tai Chi and escitalorpam and Health Education
| esCIT+ TC (N=36) Baseline | esCIT+ HE (N=37) Baseline | t†(P) | |
|---|---|---|---|
| Demographics | |||
| Women, Number (%) | 23 (64%) | 22 (60%) | 0.15 (0.7) |
| Age in years, mean (sd) | 69.1 (7.0) | 72.0 (7.4) | 1.72 (0.1) |
| Age at depression onset in years, mean (sd) | 41.4 (24.1) | 46.8 (24.1) | 0.95 (0.35) |
| Education in years, mean (sd) | 16.1 (2.5) | 15.1 (2.5) | 1.81 (0.08) |
| Clinical variables, Depression | |||
| Duration of depression in months, mean (sd) | 35.8 (31.3) | 34.9 (36.1) | 0.11 (0.92) |
| Number of episodes, mean (sd) | 4.1 (4.4) | 3.5 (3.8) | 0.65 (0.52) |
| Hamilton Depression Rating Score (Week 6 randomization point), mean (sd) | 8.2 (5.6) | 9.8 (5.8) | −.8 (0.4) |
Chi-squareanalysis is reported for the gender ratio (df=1) and t-testwas performed for the remaining variables (df=71at baseline and df=67 at follow up)
We did not observe any serious adverse events. Common mild-to-moderate side-effects attributable to the medication included nausea, diarrhea, excessive sedation, daytime sleepiness, and rash. Together, the benefits of the complementary treatment coupled with minimal side-effects led to a low dropout rate in this study, about 30%, which primarily, and almost solely, occurred during the lead-in phase before complementary addition of TCC. As shown in Figure 1, only five subjects (7%) dropped out after randomization and during TCC/HE intervention. Two subjects dropped out from the HE group due to the lack of efficacy of the treatment, and three subjects dropped out in the TCC group due to other health reasons, preventing them from performing TCC exercises (N = 3).
Among the TCC participants, depression response rates were high, with 94% of the subjects achieving HAMD scores of 10 or less and 65% achieving remission, as defined by HAMD, with a score of 6 or less, in contrast among the HE participants, with only 77% achieving HAMD scores of 10 or less and only 51% achieving remission (χ2[1] = 3.68; p< 0.06). Figure 2 reports group differences on the mean HAMD scores over time. Both intervention groups demonstrated improvement in the severity of depression, with greater reductions in depressive symptom severity among those taking escitalopram and participating in the TCC compared with those taking escitalopram combined with HE (group × time interaction: F[5, 285] = 2.26; p<0.05).
FIGURE 2.
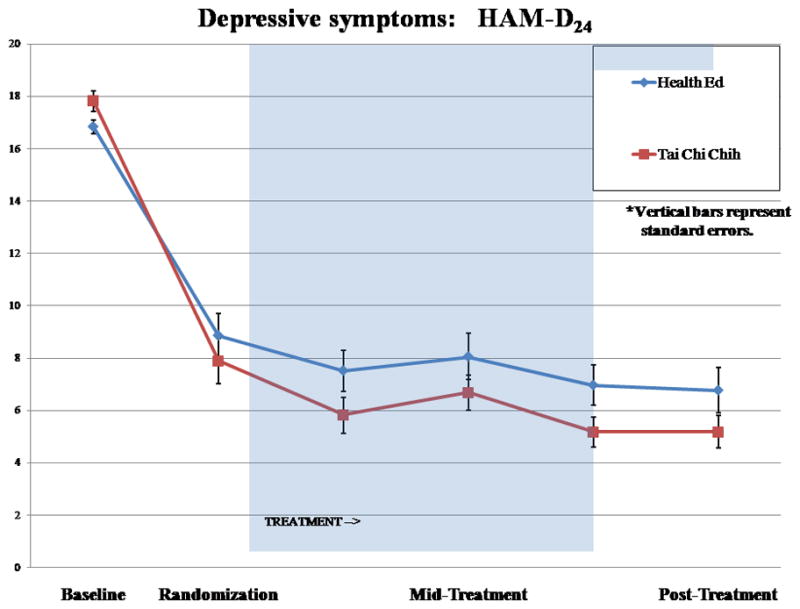
Hamilton Depression Rating Scale Mean Score Over Time in the Two Treatment Group With Randomization Point at Week 6
On the basis of our prior studies, we also hypothesized a beneficial effect of escitalopram and TCC on the secondary outcomes such as health functioning, cognition, and inflammation. Table 2 summarizes primary and secondary outcomes that differentially changed in the two treatment groups. Secondary outcomes reported in Figures 3–5 are particularly promising in terms of the overall benefit for depressed elderly. As compared with escitalopram and HE, escitalopram and TCC yielded greater improvements in 36-Item Short Form Health Survey physical functioning (group × time interaction: F[1, 66] = 5.73; p = 0.02) and cognition (i.e., memory; group × time interaction: F[1, 65] = 5.29; p<0.05) as well as declines in the inflammatory marker, CRP (time effect: F[2, 78] = 3.14, p<0.05 and group × time trend in posttreatment period: F[1, 39] = 2.91; p = 0.10).
Table 2.
Comparison of the outcomes before and after treatment in the two comparison groups receiving escitalopram and Tai Chi and escitalorpam and Health Education
| esCIT+ TC (N=36) Baseline | esCIT+ HE (N=37) Baseline | t(P) | esCIT+ TC (N=33) Mean (SD) End of Treatment | esCIT+ HE (N=35) Mean (SD) End of Treatment | Time (Change Pre-Post Tx) (sig.) | Group × time (sig.) | |
|---|---|---|---|---|---|---|---|
| Clinical variables, Depression | |||||||
| Hamilton Depression Rating Score, mean (sd) | 17.8 (2.0) | 17.0 (1.6) | 1.90 (0.07) | 5.1 (3.5) | 6.7 (4.4) | .001 | .01 |
| Comorbid psychiatric symptoms | |||||||
| Hamilton Anxiety Rating Scale, mean (sd) | 9.1 (2.7) | 9.3 (3.4) | 0.30 (0.77) | 3.5 (2.7) | 4.2 (3.0) | .001 | .27 |
| Apathy Evaluation Scale, mean (sd) | 34.9 (8.2) | 33.1 (8.2) | 0.94 (0.35) | 44.3 (5.8) | 37.9 (8.9) | .007 | .02 |
| Comorbid medical/neurological symptoms | |||||||
| CIRS-G,* mean (sd) | 5.3 (3.0) | 6.1 (3.5) | 1.04 (0.30) | 5.2 (3.1) | 5.6 (3.9) | .73 | .57 |
| CVRF,* mean (sd) | 10.1 (5.1) | 11.1 (4.7) | 0.89 (0.38) | 9.7 (4.9) | 10.6 (3.9) | .53 | .68 |
| UPDRS,* mean (sd) | 2.5 (4.1) | 4.0 (3.6) | 1.61 (0.12) | 1.5 (2.7) | 3.6 (4.4) | .93 | .24 |
| Coping | |||||||
| Resilience, mean (sd) | 60.5 (14.0) | 56.9 (13.4) | 1.13 (0.27) | 71.5 (9.5) | 65.5 (14.3) | .04 | .34 |
| Sleep | |||||||
| Pittsburgh Sleep Quality Index, mean (sd) | 14.4 (6.1) | 13.8 (6.1) | 0.38 (0.71) | 9.0 (6.4) | 10.5 (5.7) | .003 | .08 |
| Health Functioning | |||||||
| SF-36 physical functioning | 93.6 (9.6) | 91.4 (12.0) | 0.89 (0.38) | 97.3 (4.2) | 91.1 (13.1) | .97 | .02 |
| SF-36 role emotional | 48.1 (37.8) | 57.6 (32.1) | 1.16 (0.25) | 83.9 (25.2) | 71.2 (28.3) | .42 | .003 |
| Cognition | |||||||
| MMSE* | 29.2 (1.0) | 29.0 (1.3) | 0.72 (0.48) | 29.2 (1.1) | 29.3 (1.1) | .62 | .24 |
| CVLT long delayed recall, cued* | 11.8 (3.4) | 11.3 (3.6) | 0.28 (0.78) | 12.36 (2.8) | 10.5 (3.4) | .018 | .05 |
| Trails A errors | 0.44 (0.72) | 0.43 (.95) | 0.09 (0.93) | 0.19 (0.47) | 0.54 (1.09) | .37 | .07 |
| Inflamamtory Marker | |||||||
| CRP | 2.9 (2.5) | 2.6 (2.3) | 0.40 (0.69) | 1.8 (1.6) | 2.2 (2.1) | .05 | .10 |
CIRS- medical burden; CVRF- vascular burden; UPDRS- Unified Parkinson’s Disease Rating scale; MMSE- Mini-Mental State examination score; CVLT- California Verbal learning score for verbal memory; Trails A errors- test of executive function.
FIGURE 3.

The Mean SF-36 Physical Functioning Scores Over Time in the Two Treatment Groups
FIGURE 5.
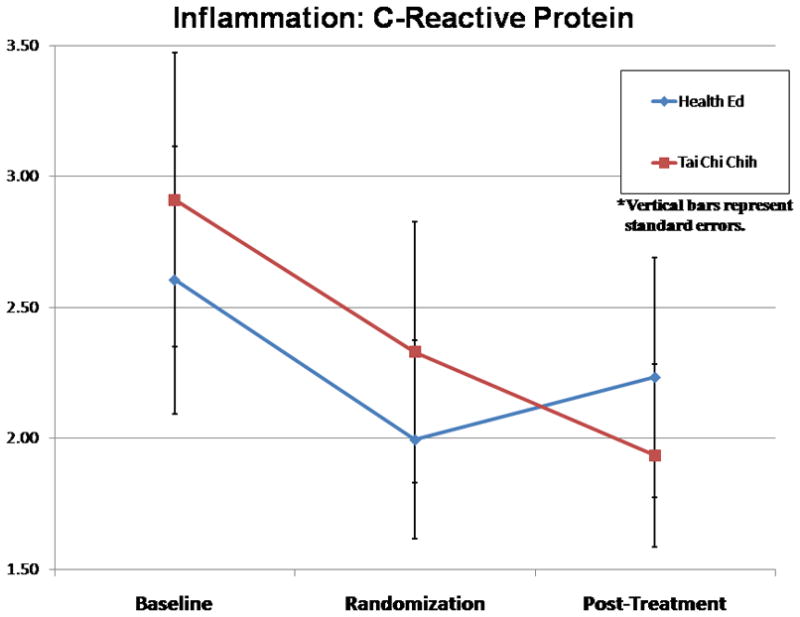
Mean C-Reactive Protein Levels Over Time in the Two Treatment Groups
DISCUSSION
Our study is the first RCT to test the efficacy of the complementary use of TCC versus HE as an adjunct to standard antidepressant medication treatment of geriatric depression. We report greater improvements in depression, health-related quality of life, and memory, as well as decreases in the inflammatory marker, CRP, in older depressed participants receiving escitalopram with TCC compared with those receiving escitalopram and HE. Furthermore, by using a TCC intervention that is easily translatable to the community, the number of benefits from a simple adjunct intervention goes beyond the known benefits of standard antidepressant treatment or adjunctive pharmacologic treatment. However, this study needs to be replicated in a larger, comprehensive study, with consideration of other nonpharmacologic mind–body approaches (i.e., yoga and meditation) in this difficult-to-manage population.
Although there are no comparable studies using Tai Chi as complementary intervention added to the standard antidepressant treatment, a recent review of 36 clinical trials of Tai Chi in older adults with 3,799 participants55 reported significant improvement in physical function, balance, and depression and anxiety. Another review compared 12 RCTs of mindful exercises versus nonmindful exercises,56 indicating that both the mindful and nonmindful physical exercises were effective in their short-term effect in reducing depression levels or depressive symptoms. However, most of the studies had methodologic problems that included small sample size. Whereas Cho13 reported improvement in depression in a small sample of community-dwelling older patients who were randomly assigned to a 3-month Tai Chi intervention with 36 sessions and compared to the wait-list control after adjusting for age, gender, and education,13 social support might be partly responsible for the effect of Tai Chi on depressive symptoms. There is additional literature that Tai Chi may benefit various comorbid medical conditions of aging such as rheumatoid arthritis57 and heart failure58 and improve physical functioning and quality of life, as well as mood and anxiety and sleep quality, in healthy older adults.28,59–63
Several pathways may mediate the effects of TCC on immunity and health functioning. Research interest has focused on two components, relaxation and exercise, without necessarily considering their effects in concert, as they occur during the practice of TCC. In a meta-analysis on the effects of relaxation training, Hyman et al.64 found that various relaxation response-based interventions led to a reduction of clinical somatic symptoms, with additional effects on symptoms of anxiety and depression,65 blood pressure,66 and recovery from immune-mediated diseases.67 Prior research by our group and others have found that the administration of TCC decreases sympathetic output, improves viral-specific immunity and vaccine response, improves sleep quality, and augments overall health functioning in healthy older adults.60,68 In the current study, we found significant differences in the change in the levels of CRP over time, favoring the TCC group over HE group that supports our prior findings of beneficial influence of TCC on inflammatory markers.
The limitations of our study include a relatively small sample size, brief follow-up, and the pilot exploratory evaluation of cognitive measures. In addition, the control group of antidepressant + HE turned into a very powerful intervention that helped to relieve social isolation in older depressed participants and resulted in the significant reduction in depression severity. In the future studies, Usual Care control group can prove to be a less-active intervention. Furthermore, our participants had moderate to major depression, which raises the possibility that these findings might not generalize to a more severely depressed and/or disabled population of older adults. Finally, the cognitive test battery was relatively brief, and we did not have a nondepressed control group to determine whether improvements in cognitive function occurred as a result of TCC or improvements in depression severity. Nevertheless, the results of the study are intriguing and indicate similar benefits of Tai Chi described in other studies of older adults. This relatively simple mind–body exercise can provide substantial additional benefits not only for depression but also for physical functioning, resilience, quality of life, and cognition. Very few interventions in late-life depression manage to improve cognitive functioning in older depressed individuals.12,69 These findings should inform clinical practices and encourage clinicians to consider recommending a mind–body exercise, such as TCC, as an adjunct to the standard antidepressant treatment to improve clinical outcomes of geriatric depression. Future studies should include longer follow-up and control groups that would utilize different components (e.g., exercise and meditation) to understand the behavioral mechanisms that contribute to the benefit of TCC on depression, health functioning, cognitive performance, and inflammation in depressed older adults.
FIGURE 4.

The Mean CVLT Long Delayed Recall (Cued) Scores Over Time in the Two Treatment Groups
Acknowledgments
This work was supported by the grants MH077650, MH86481, and AT003480 to Dr. Lavretsky and NIH grants T32-MH19925, HL079955, AG026364, CA10014152, CA116778, RR00827, and P30-AG028748; General Clinical Research Centers Program, the UCLA Cousins Center at the Semel Institute for Neurosciences; and the UCLA Older Americans Independence Center Inflammatory Biology Core to Dr. Irwin.
References
- 1.Surgeon General Report on Mental Health in Older Adults. 2004. [Google Scholar]
- 2.Report of the 2005 White House Conference on Aging; 2005. http://www.whcoa.gov/ [Google Scholar]
- 3.Retooling for an Aging America. Building the Health Care Workforce; 2008. [PubMed] [Google Scholar]
- 4.Charney DS, Nemeroff CB, Lewis L, et al. National Depressive and Manic-Depressive Association consensus statement on the use of placebo in clinical trials of mood disorders. Arch Gen Psychiatry. 2002;59:262–270. doi: 10.1001/archpsyc.59.3.262. [DOI] [PubMed] [Google Scholar]
- 5.Thase ME. Achieving remission and managing relapse in depression. J Clin Psychiatry. 2003;64(suppl 18):3–7. [PubMed] [Google Scholar]
- 6.Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. Am J Geriatr Psychiatry. 2002;10:98–106. [PubMed] [Google Scholar]
- 7.Caine ED, Lyness JM, King DA, et al. Clinical and etiological heterogeneity of mood disorders in elderly patients, in diagnosis of depression in late life. In: Schneider LS, Reynolds CF, Lebowitz BD, editors. Results of the NIH Consensus Development Conference. 1994. pp. 21–54. [Google Scholar]
- 8.Kumar A, Bilker W, Zhisong J, et al. Atrophy and high intensity lesions: complimentary mechanisms in late-life depression. 1998. [DOI] [PubMed] [Google Scholar]
- 9.Lesser IM, Boone KB, Mehringer CM, et al. Cognition and white matter hyperintensities in older depressed patients. Am J Psychiatry. 1996;153:1280–1287. doi: 10.1176/ajp.153.10.1280. [DOI] [PubMed] [Google Scholar]
- 10.Lavretsky H, Lesser IM, Wohl M, et al. Relationship of age, age at onset, and sex to depression in older adults. Am J Geriatr Psychiatry. 1998;6:248–256. [PubMed] [Google Scholar]
- 11.Lavretsky H, Kurbanyan K, Ballmaier M, et al. Sex differences in brain structure in geriatric depression. Am J Geriatr Psychiatry. 2004;12:653–657. doi: 10.1176/appi.ajgp.12.6.653. [DOI] [PubMed] [Google Scholar]
- 12.Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 13.Cho KL. Effect of Tai Chi on depressive symptoms amongst Chinese older patients with major depression: the role of social support. Med Sport Sci. 2008;52:146–154. doi: 10.1159/000134295. [DOI] [PubMed] [Google Scholar]
- 14.Lavretsky H, Irwin MR. Resilience and aging. Aging Health Perspect. 2007;3:309–323. [Google Scholar]
- 15.Zajecka JM. Treating depression to remission. J Clin Psychiatry. 2003;64(suppl 15):7–12. [PubMed] [Google Scholar]
- 16.Eisenberger NI, Inagaki TK, Mashal NM, et al. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24(4):558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenberger NI, Inagaki TK, Rameson LT, et al. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 19.Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 20.Kent S, Bluthe RM, Kelley KW, et al. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- 21.Kent S, Bret-Dibat JL, Kelley KW, et al. Mechanisms of sickness-induced decreases in food-motivated behavior. Neurosci Biobehav Rev. 1996;20:171–175. doi: 10.1016/0149-7634(95)00037-f. [DOI] [PubMed] [Google Scholar]
- 22.Dantzer R, Wollman EE, Vitkovic L, et al. Cytokines, stress, and depression. Conclusions and perspectives. Adv Exp Med Biol. 1999;461:317–329. doi: 10.1007/978-0-585-37970-8_17. [DOI] [PubMed] [Google Scholar]
- 23.Black S, Humphrey JH, Niven JS. Inhibition of Mantoux reaction by direct suggestion under hypnosis. Br Med J. 1963;1:1649–1652. doi: 10.1136/bmj.1.5346.1642-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fawzy FI, Kemeny ME, Fawzy NW, et al. A structured psychiatric intervention for cancer patients. II. Changes over time in immunological measures. Arch Gen Psychiatry. 1990;47:729–735. doi: 10.1001/archpsyc.1990.01810200037005. [DOI] [PubMed] [Google Scholar]
- 25.Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutgendorf SK, Antoni MH, Kumar M, et al. Changes in cognitive coping strategies predict EBV-antibody titre change following a stressor disclosure induction. J Psychosom Res. 1994;38:63–78. doi: 10.1016/0022-3999(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 27.Segerstrom SC, Taylor SE, Kemeny ME, et al. Optimism is associated with mood, coping, and immune change in response to stress. J Pers Soc Psychol. 1998;74:1646–1655. doi: 10.1037//0022-3514.74.6.1646. [DOI] [PubMed] [Google Scholar]
- 28.Brown DR, Wang Y, Ward A, et al. Chronic psychological effects of exercise and exercise plus cognitive strategies. Med Sci Sports Exerc. 1995;27:765–775. [PubMed] [Google Scholar]
- 29.Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 30.Irwin M, Pike J, Oxman M. Shingles immunity and health functioning in the elderly: Tai Chi Chih as a behavioral treatment. Evid Based Complement Alternat Med. 2004;1:223–232. doi: 10.1093/ecam/neh048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irwin MR, Pike JL, Cole JC, et al. Effects of a behavioral intervention, Tai Chi Chih, on varicella-zoster virus specific immunity and health functioning in older adults. Psychosom Med. 2003;65:824–830. doi: 10.1097/01.psy.0000088591.86103.8f. [DOI] [PubMed] [Google Scholar]
- 32.Mather AS, Rodriguez C, Guthrie MF, et al. Effects of exercise on depressive symptoms in older adults with poorly responsive depressive disorder: randomised controlled trial. Br J Psychiatry. 2002;180:411–415. doi: 10.1192/bjp.180.5.411. [DOI] [PubMed] [Google Scholar]
- 33.Cipriani A, La Ferla T, Furukawa TA, et al. Sertraline versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2009;(2):CD006117. doi: 10.1002/14651858.CD006117.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Cipriani A, Santilli C, Furukawa TA, et al. Escitalopram versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2009:CD006532. doi: 10.1002/14651858.CD006532.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore N, Verdoux H, Fantino B. Prospective, multicentre, randomized, double-blind study of the efficacy of escitalopram versus citalopram in outpatient treatment of major depressive disorder. Int Clin Psychopharmacol. 2005;20:131–137. doi: 10.1097/00004850-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Rampello L, Alvano A, Raffaele R, et al. New possibilities of treatment for panic attacks in elderly patients: escitalopram versus citalopram. J Clin Psychopharmacol. 2006;26:67–70. doi: 10.1097/01.jcp.0000195383.96383.25. [DOI] [PubMed] [Google Scholar]
- 37.Lader M, Andersen HF, Baekdal T. The effect of escitalopram on sleep problems in depressed patients. Hum Psychopharmacol. 2005;20:349–354. doi: 10.1002/hup.694. [DOI] [PubMed] [Google Scholar]
- 38.Stein DJ, Andersen HF, Goodman WK. Escitalopram for the treatment of GAD: efficacy across different subgroups and outcomes. Ann Clin Psychiatry. 2005;17:71–75. doi: 10.1080/10401230590932335. [DOI] [PubMed] [Google Scholar]
- 39.Stone JF. Tai-Chi-Chih! Joy Through Movement. Boston: Good Karma Publishing; 1996. [Google Scholar]
- 40.Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322:763–767. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Heart Association. Stroke Risk Factor Prediction Chart. Dallas: 1990. [Google Scholar]
- 43.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 44.Guy W. Services UDoHaH. ECDEU Assessment Manual for Psychopharmacology. Rockville: Alcohol Drug Abuse and Mental Health Administration, NIMPH Psychopharmacology Research Branch; 1976. Clinical Global Impressions (CGI) pp. 218–222. [Google Scholar]
- 45.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 46.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 47.Montgomery GK, Reynolds NC, Jr, Warren RM. Qualitative assessment of Parkinson’s disease: study of reliability and data reduction with an abbreviated Columbia Scale. Clin Neuropharmacol. 1985;8:83–92. [PubMed] [Google Scholar]
- 48.Ware JEJ, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scores: A User’s Manual. Boston: The Health Institute, New England Medical Center; 1994. [Google Scholar]
- 49.Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC) Depress Anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- 50.Lingjaerde O, Ahlfors UG, Bech P, et al. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 51.Delis DC, Kramer JH, Kaplan E, et al. CVLT-II: California Verbal Learning Test, Adult Version, Manual. San Antonio: the Psychological Corporation; 2000. [Google Scholar]
- 52.Elderkin-Thompson V, Mintz J, Haroon E, et al. Executive dysfunction and memory in older patients with major and minor depression. Arch Clin Neuropsychol. 2006;21:669–676. doi: 10.1016/j.acn.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Reitan RM. Validity of the Trail Making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 54.Golden CJ. A group version of the Stroop Color and Word Test. J Pers Assess. 1975;39:386–388. doi: 10.1207/s15327752jpa3904_10. [DOI] [PubMed] [Google Scholar]
- 55.Rogers CE, Larkey LK, Keller C. A review of clinical trials of tai chi and qigong in older adults. West J Nurs Res. 2009;31:245–279. doi: 10.1177/0193945908327529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsang HW, Chan EP, Cheung WM. Effects of mindful and non-mindful exercises on people with depression: a systematic review. Br J Clin Psychol. 2008;47:303–322. doi: 10.1348/014466508X279260. [DOI] [PubMed] [Google Scholar]
- 57.Wang C. Tai Chi improves pain and functional status in adults with rheumatoid arthritis: results of a pilot single-blinded randomized controlled trial. Med Sport Sci. 2008;52:218–229. doi: 10.1159/000134302. [DOI] [PubMed] [Google Scholar]
- 58.Barrow DE, Bedford A, Ives G, et al. An evaluation of the effects of Tai Chi Chuan and Chi Kung training in patients with symptomatic heart failure: a randomised controlled pilot study. Postgrad Med J. 2007;83:717–721. doi: 10.1136/pgmj.2007.061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chou KL, Lee PW, Yu EC, et al. Effect of Tai Chi on depressive symptoms amongst Chinese older patients with depressive disorders: a randomized clinical trial. Int J Geriatr Psychiatry. 2004;19:1105–1107. doi: 10.1002/gps.1178. [DOI] [PubMed] [Google Scholar]
- 60.Irwin MR, Olmstead R, Motivala SJ. Improving sleep quality in older adults with moderate sleep complaints: a randomized controlled trial of Tai Chi Chih. Sleep. 2008;31:1001–1008. [PMC free article] [PubMed] [Google Scholar]
- 61.Jin P. Changes in heart rate, noradrenaline, cortisol and mood during Tai Chi. J Psychosom Res. 1989;33:197–206. doi: 10.1016/0022-3999(89)90047-0. [DOI] [PubMed] [Google Scholar]
- 62.Li F, Harmer P, McAuley E, et al. Tai Chi, self-efficacy, and physical function in the elderly. Prev Sci. 2001;2:229–239. doi: 10.1023/a:1013614200329. [DOI] [PubMed] [Google Scholar]
- 63.Mustata S, Cooper L, Langrick N, et al. The effect of a Tai Chi exercise program on quality of life in patients on peritoneal dialysis: a pilot study. Perit Dial Int. 2005;25:291–294. [PubMed] [Google Scholar]
- 64.Hyman RB, Feldman HR, Harris RB, et al. The effects of relaxation training on clinical symptoms: a meta-analysis. Nurs Res. 1989;38:216–220. [PubMed] [Google Scholar]
- 65.Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149:936–943. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- 66.Alexander CN, Schneider RH, Staggers F, et al. Trial of stress reduction for hypertension in older African Americans. II. Sex and risk subgroup analysis. Hypertension. 1996;28:228–237. doi: 10.1161/01.hyp.28.2.228. [DOI] [PubMed] [Google Scholar]
- 67.Kabat-Zinn J, Wheeler E, Light T, et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA) Psychosom Med. 1998;60:625–632. doi: 10.1097/00006842-199809000-00020. [DOI] [PubMed] [Google Scholar]
- 68.Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to Varicella zoster virus in older adults: a randomized, controlled trial of Tai Chi. J Am Geriatr Soc. 2007;55:511–517. doi: 10.1111/j.1532-5415.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 69.Butters MA, Bhalla RK, Mulsant BH, et al. Executive functioning, illness course, and relapse/recurrence in continuation and maintenance treatment of late-life depression: is there a relationship? Am J Geriatr Psychiatry. 2004;12:387–394. doi: 10.1176/appi.ajgp.12.4.387. [DOI] [PubMed] [Google Scholar]