Abstract
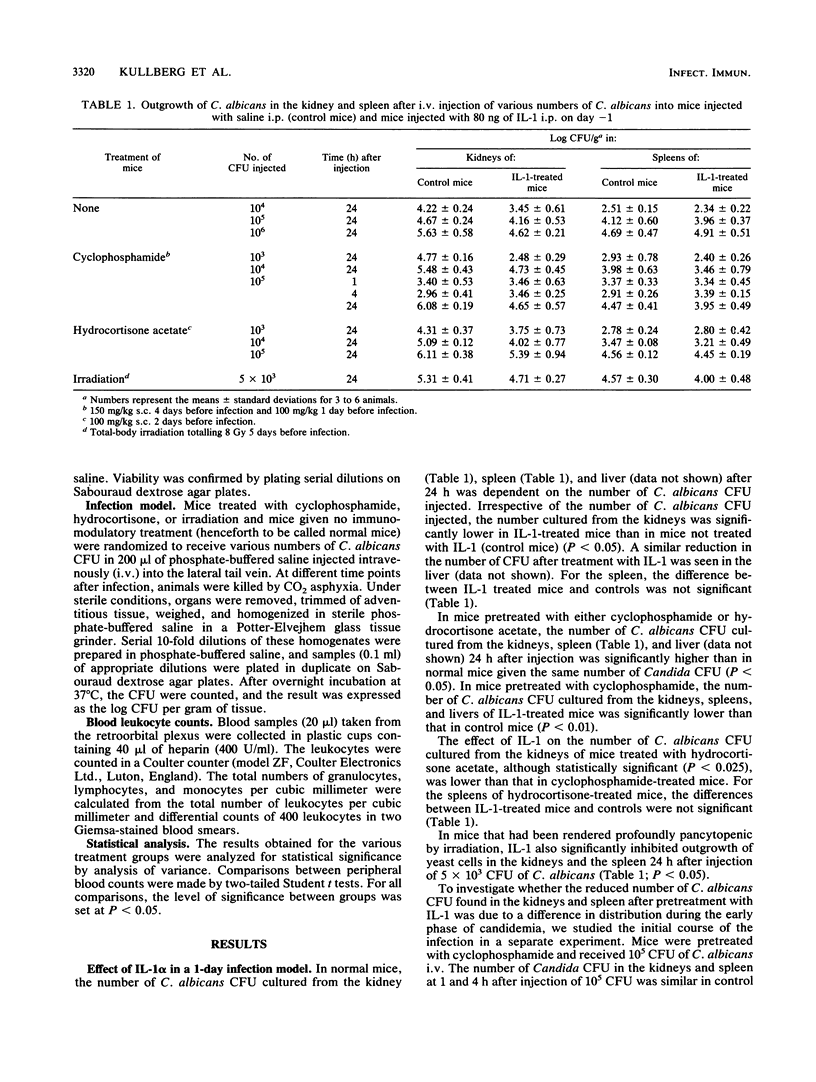
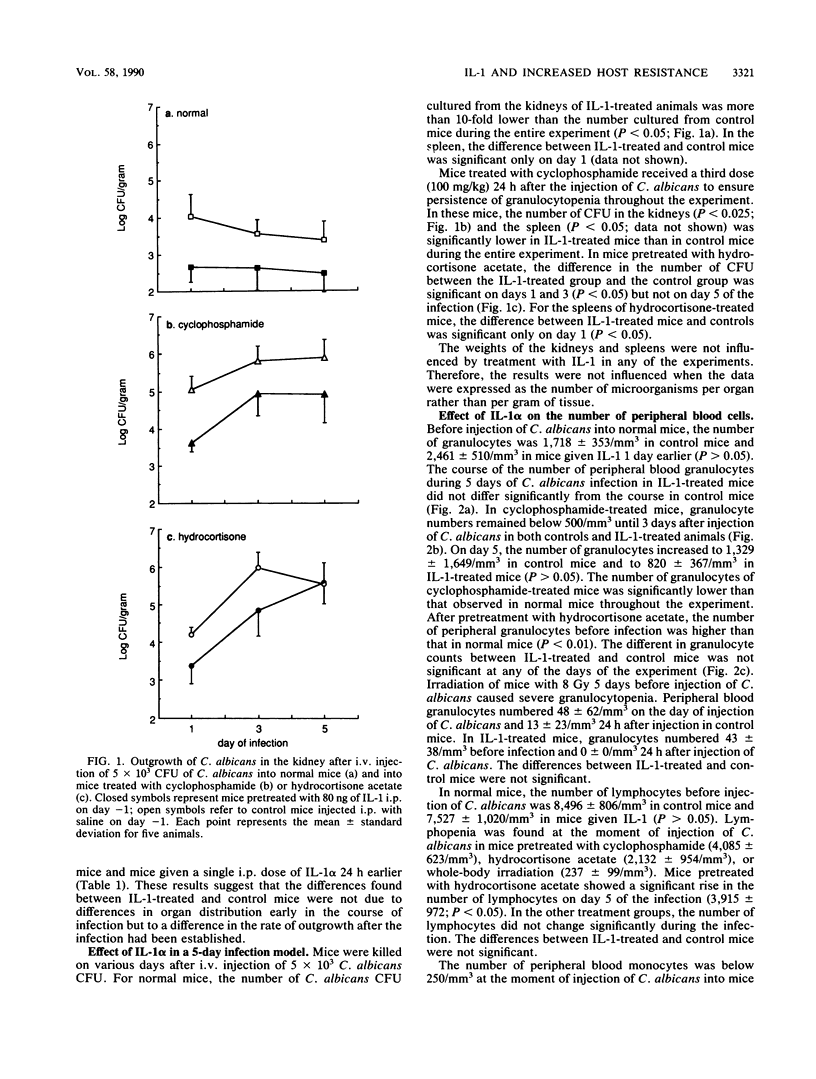
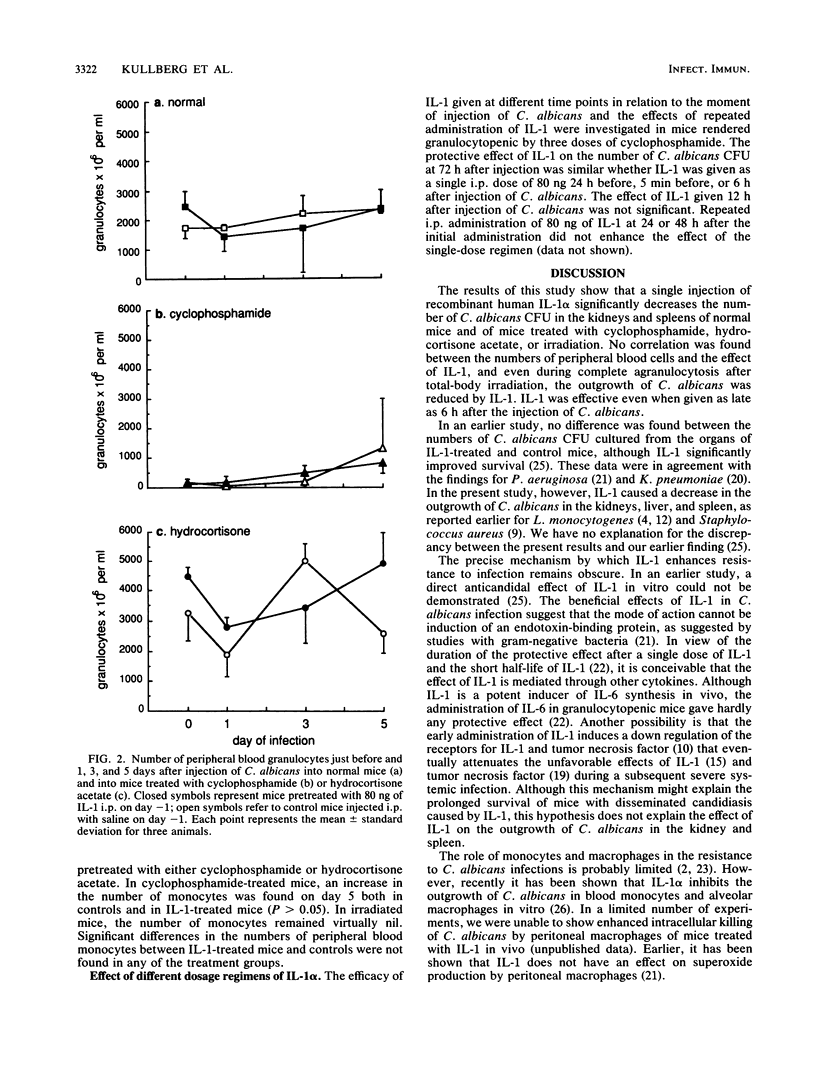
The effect of human recombinant interleukin-1 alpha (IL-1 alpha) on a systemic candidal infection in mice under various conditions of immunosuppression was investigated. In normal mice and in mice pretreated with cyclophosphamide, hydrocortisone acetate, or sublethal total body irradiation, the outgrowth of Candida albicans in the kidney was significantly reduced by the administration of a single intraperitoneal dose of 80 ng of IL-1 (P less than 0.05). In mice treated with either cyclophosphamide or irradiation, IL-1 also significantly reduced the outgrowth of C. albicans in the spleen. The protective effect of IL-1 was present when given 24 h before injection of C. albicans but also when IL-1 was given simultaneously with or 6 h after injection of C. albicans in cyclophosphamide-treated mice. The effect of IL-1 was independent of the presence or recruitment of granulocytes, since IL-1 inhibited the outgrowth of C. albicans in the kidneys and spleens of mice that were rendered severely granulocytopenic (less than 50 granulocytes per mm3) throughout the duration of the infection by either repeated injections of cyclophosphamide or sublethal total body irradiation. These results indicate that the enhancement of host resistance by IL-1 is not due solely to increased granulocytopoiesis or chemotaxis of granulocytes but strongly suggest that other mechanisms play a role in the protective effect of IL-1 against systemic infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodey G. P. Candidiasis in cancer patients. Am J Med. 1984 Oct 30;77(4D):13–19. [PubMed] [Google Scholar]
- Brummer E., McEwen J. G., Stevens D. A. Fungicidal activity of murine inflammatory polymorphonuclear neutrophils: comparison with murine peripheral blood PMN. Clin Exp Immunol. 1986 Dec;66(3):681–690. [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F. Purified human and recombinant murine interleukin-1 alpha induced accumulation of inflammatory peritoneal neutrophils and mononuclear phagocytes: possible contributions to antibacterial resistance. Microb Pathog. 1987 Nov;3(5):377–386. doi: 10.1016/0882-4010(87)90007-6. [DOI] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F. Recombinant murine interleukin-1 alpha enhancement of nonspecific antibacterial resistance. Infect Immun. 1987 Sep;55(9):2061–2065. doi: 10.1128/iai.55.9.2061-2065.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F., Young K. M., Cooley A. J., Kurtz R. S. Effects of murine recombinant interleukin 1 alpha on the host response to bacterial infection. J Immunol. 1988 Feb 1;140(3):962–968. [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Mier J. W., Bernheim H. A., LoPreste G., Lynn D. L., Love R. N., Webb A. C., Auron P. E., Reuben R. C. Multiple biological activities of human recombinant interleukin 1. J Clin Invest. 1986 Jun;77(6):1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Fibbe W. E., van Damme J., Billiau A., Voogt P. J., Duinkerken N., Kluck P. M., Falkenburg J. H. Interleukin-1 (22-K factor) induces release of granulocyte-macrophage colony-stimulating activity from human mononuclear phagocytes. Blood. 1986 Dec;68(6):1316–1321. [PubMed] [Google Scholar]
- Gladue R., Girard A., Newborg M. Enhanced antibacterial resistance in neutropenic mice treated with human recombinant interleukin-1 beta. Agents Actions. 1988 Jun;24(1-2):130–136. doi: 10.1007/BF01968091. [DOI] [PubMed] [Google Scholar]
- Holtmann H., Wallach D. Down regulation of the receptors for tumor necrosis factor by interleukin 1 and 4 beta-phorbol-12-myristate-13-acetate. J Immunol. 1987 Aug 15;139(4):1161–1167. [PubMed] [Google Scholar]
- Horn R., Wong B., Kiehn T. E., Armstrong D. Fungemia in a cancer hospital: changing frequency, earlier onset, and results of therapy. Rev Infect Dis. 1985 Sep-Oct;7(5):646–655. doi: 10.1093/clinids/7.5.646. [DOI] [PubMed] [Google Scholar]
- Kurtz R. S., Young K. M., Czuprynski C. J. Separate and combined effects of recombinant interleukin-1 alpha and gamma interferon on antibacterial resistance. Infect Immun. 1989 Feb;57(2):553–558. doi: 10.1128/iai.57.2.553-558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre K. W., Unowsky J., DeLorenzo W., Benjamin W. Enhancement of antibacterial resistance of neutropenic, bone marrow-suppressed mice by interleukin-1 alpha. Infect Immun. 1989 Jan;57(1):48–54. doi: 10.1128/iai.57.1.48-54.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Togawa A., Chedid L., Mizel S. Components of mycobacteria and muramyl dipeptide with adjuvant activity induce lymphocyte activating factor. Cell Immunol. 1980 Mar 1;50(1):71–81. doi: 10.1016/0008-8749(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Ozaki Y., Ohashi T., Minami A., Nakamura S. Enhanced resistance of mice to bacterial infection induced by recombinant human interleukin-1a. Infect Immun. 1987 Jun;55(6):1436–1440. doi: 10.1128/iai.55.6.1436-1440.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecyk R. A., Fraser-Smith E. B., Matthews T. R. Efficacy of interleukin-1 beta against systemic Candida albicans infections in normal and immunosuppressed mice. Infect Immun. 1989 Oct;57(10):3257–3258. doi: 10.1128/iai.57.10.3257-3258.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Van der Meer J. W., Helle M., Aarden L. Comparison of the effects of recombinant interleukin 6 and recombinant interleukin 1 on nonspecific resistance to infection. Eur J Immunol. 1989 Feb;19(2):413–416. doi: 10.1002/eji.1830190229. [DOI] [PubMed] [Google Scholar]
- Van't Wout J. W., Van der Meer J. W., Barza M., Dinarello C. A. Protection of neutropenic mice from lethal Candida albicans infection by recombinant interleukin 1. Eur J Immunol. 1988 Jul;18(7):1143–1146. doi: 10.1002/eji.1830180728. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Todisco T., Puliti M., Dottorini M., Bistoni F. Modulation of anti-Candida activity of human alveolar macrophages by interferon-gamma or interleukin-1-alpha. Am J Respir Cell Mol Biol. 1989 Jul;1(1):49–55. doi: 10.1165/ajrcmb/1.1.49. [DOI] [PubMed] [Google Scholar]
- van 't Wout J. W., Linde I., Leijh P. C., van Furth R. Contribution of granulocytes and monocytes to resistance against experimental disseminated Candida albicans infection. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):736–741. doi: 10.1007/BF01975039. [DOI] [PubMed] [Google Scholar]
- van der Meer J. W., Barza M., Wolff S. M., Dinarello C. A. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1620–1623. doi: 10.1073/pnas.85.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. W. The effects of recombinant interleukin-1 and recombinant tumor necrosis factor on non-specific resistance to infection. Biotherapy. 1988;1(1):19–25. doi: 10.1007/BF02170132. [DOI] [PubMed] [Google Scholar]
- van't Wout J. W., Linde I., Leijh P. C., van Furth R. Effect of irradiation, cyclophosphamide, and etoposide (VP-16) on number of peripheral blood and peritoneal leukocytes in mice under normal conditions and during acute inflammatory reaction. Inflammation. 1989 Feb;13(1):1–14. doi: 10.1007/BF00918959. [DOI] [PubMed] [Google Scholar]



