Abstract
Curcumin has wound healing attributes mediated through a plethora of biological activities that in general are not ascribed to specific receptors. Recently we have demonstrated that i.v. curcumin limits burn injury progression in a rat model. Since decreased microvascular perfusion is a central element of burn injury progression, we hypothesized that curcumin may induce vasodilation in peripheral arterioles, to improve perfusion. Using mucosal microcirculation as an in situ assay, cheek pouch tissue was exteriorized in anesthetized (phentobarbital 70 mg/kg i.p.) male hamsters (N=60) to observe the terminal feed arterioles (~8μm diameter) and the immediately upstream arcade arterioles (~20μm). Curcumin (10−12 – 10−4mol/L) was applied dose-wise (micropipette, 60 seconds). Subnanomolar curcumin dilated whereas micromolar doses constricted the arterioles. For the terminal arteriole: vasodilation logEC50 −10.3±0.2, peak dilation +39±1%; vasconstriction logEC50 −8.0±0.4, peak constriction −14±2%. Simultaneous atropine (muscarinic antagonist) or PD142893 (endothelin antagonist) had no effect. Propranolol (β-Ad antagonist) enhanced constriction by removing the vasodilation response to curcumin. Phentolamine (α-Ad antagonist) enhanced dilation to curcumin by removing the vasoconstriction response. Thus, the curcumin vasomotor activity on microcirculation was α-Ad and β-Ad receptor-dependent and its net vasoactive effect was concentration and time dependent.
Introduction
Curcuma longa (turmeric), an herb belonging to the ginger family, has wound healing properties attributable to a plethora of specific biologic activities that have not for the most part been ascribed to a known receptor (Aggarwal and Sung, 2009). Recently, we have shown that administration of curcumin to rats before (gastric gavage) or 1 hour after (intravenous) hot comb burns will limit injury progression (Singer, McClain, Romanov, et al., 2007; Singer, Taira and Lin, 2010) in a manner unrelated to its anti-oxidant properties (Singer, Taira and Lin, 2010). Alternatively, blood vessel occlusion by red blood cell plugging and thrombosis formation is found in the ischemic tissue surrounding a burn (Moritz, 1947; Regas and Ehrlich, 1992; Vo, Papword, Delaney, 1998) and may be why tissue necrosis extends from the primary burn site. As curcumin is vasoactive in large arteries from some tissues and species (Ahn, Park, Woo et al., 2009; Gilani, Shah, Ghayur et al., 2005; Sasaki, Gogo, Tohda et al., 2003; Xu, Long, Dai and Liu, 2007), we wondered whether some of the beneficial effect of curcumin was related to a vasomotor effect on the microvasculature.
Few studies have shown that curcumin is vasoactive; no previous report has investigated curcumin's action on the microcirculation. Studies in macrovessels typically tested micromolar levels of various curcuminoid preparations, and found a decrease in tension development for artery rings from porcine coronary arteries, rat aorta, and rabbit basilar arteries (Ahn, Park, Woo et al., 2009; Sasaki, Gogo, Tohda et al., 2003; Xu, Long, Dai and Liu, 2007), but not rabbit aorta (Gilani, Shah, Ghayur et al., 2005); decreased tension is interpreted as dilation. From the limited literature, the mechanism by which curcumin decreases tension appears to be at most half NO-mediated (half attributed to β-Ad, Xu, Long, Dai and Liu, 2007), yet the vasoactivity appears to widely differ by tissue and species, or perhaps by curcuminoid preparation or extraction procedure. An important consideration from the cell culture literature is the well-documented cytotoxicity of curcumin, beginning at concentrations of 10−5mol/L (Foresti, Hoque, Monti et al., 2005; Gilani, Shah, Ghayur et al., 2005; Kunwar, Barik, Mishra et al., 2008). Thus, any response, or lack thereof, to curcumin in the micromolar range should be interpreted carefully.
In the present study, we define the vasoactive response to curcumin in peripheral mucosal arterioles using the hamster cheek pouch in situ assay. The buccal mucosa of the hamster has a 2 dimensional microcirculation, which greatly facilitates direct microscopic examination of vasoactive responses. In humans, both the buccal mucosa and cutaneous microcirculation consist of 3 dimensional capillary loops, which arise from deeper feeding arterioles (Braverman, 1997; Scardina, Ruggieri and Messina, 2009). However, the diverse tissues display a similar vasoactive response capability (Gyorfi, Fazekas, Rosivall, 1992; Oaklander and Siegel, 2005) to what is found for the hamster cheek pouch (Frame and Mabanta, 2007; Frame, Rivers, Altland et al., 2007; Georgi, Dewar and Frame, 2010), likely due to a similar vascular receptor distribution that includes adrenergic, muscarinic and endothelin receptors (Hardman and Limbird, 2001). Thus, the hamster cheek pouch microcirculation was used as an in situ assay model system to facilitate direct investigation of possible curcumin vasoactivity on the microcirculation and, if any, of the receptor system by which it acts.
In the present study we hypothesized that ethanol extracted curcumin I (99% purity, Singer, McClain, Romanov, et al., 2007) would induce a robust vasodilation in peripheral arterioles. In the hamster cheek pouch, we found that small arterioles do dilate to subnanomolar concentrations of curcumin. We also report that higher concentrations induce a robust constriction unrelated to vasoactivity of the vehicle ethanol. Both responses are recoverable. Further, the constrictor response is mediated by α-Ad receptors, and the dilatory response is entirely mediated by β-Ad receptors and requires cGMP, yet, not all dilation appears to require NO-formation.
Results
Over the 60 second exposure time, low nanomolar and picomolar concentrations of curcumin induced a sustained dilation. At higher concentrations, initial vasodilation at 20 sec was followed by vasoconstriction at 60 seconds. One experiment is shown in Figure 1 to illustrate the time and concentration dependence of this response. [Illustrative examples of control, dilated and constricted arterioles are shown in Figure 1.] Thus, we examined two separate concentration response relationships: the early response at 20 seconds of exposure and the late response at 60 seconds of exposure (Figure 2A). Corresponding EC50 and maximal values are given in Table 1 (Supplemental Data). Peak dilation was significantly greater for terminal arterioles vs. arcade arterioles.
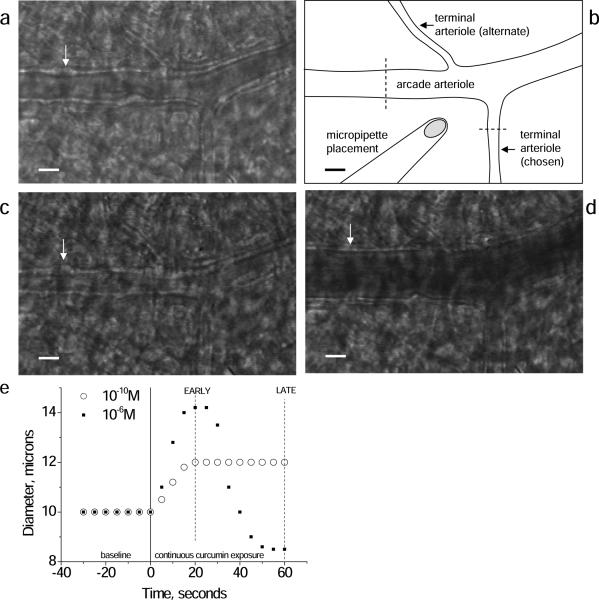
Figure 1.
Representative images of the arcade arteriole-terminal arteriole junction with micropipette placement for curcumin administration (schematic, B) in the basal control state (A), constricted with phenylephrine (C), and dilated with adenosine (D). Dashed lines in (B) indicate typical locations for diameter measurements. White arrow points to a bulge, which is a vascular smooth muscle cell in cross section; especially in (C) the cells are seen as a `string of pearls' along the vascular wall. With dilation (D) the cells flattens and are not apparent. (E) Time course of the diameter change in response to continuous curcumin application (micropipette) in one terminal arteriole. Low dose curcumin induced a sustained vasodilation, peaking by 20 seconds. Higher dose curcumin initiated dilation, peaking by 20 seconds, followed by a secondary constriction, peaking by 60 seconds. Data for concentration response analysis was thus taken at both the EARLY (20 second) and LATE (60 second) time periods. Scale bar in A,B,C,D is 20 microns.
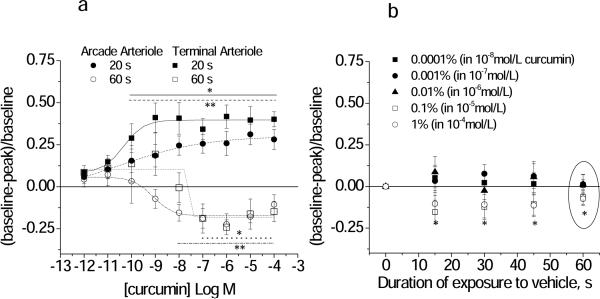
Figure 2.
Concentration response relationship for curcumin+vehicle (A) or vehicle alone (B, ethanol) applied to arcade and terminal feed arterioles (Protocol 1). (A) The Early response was obtained at 20 seconds and the Late response was obtained at 60 seconds of continuous exposure to curcumin. The EC50 and maximal values are given in Table 1. *terminal **arcade: individual responses differ from baseline, p<0.05. (B) Time course of the response to ethanol alone, showing the percent ethanol (in the corresponding curcumin solution). Terminal and arcade arterioles are combined. The response at 60 s (circled) corresponds to the Late time point in (A) for all ethanol concentrations. *differs from baseline for 0.1 and 1% ethanol only, p<0.05.
Two important controls were performed to determine whether the biphasic nature of the vasomotor response to curcumin was attributable to vehicle (ethanol) or to a cytotoxic effect of curcumin over the course of the experiments. Although vehicle (ethanol) alone caused significant constriction at 0.1% and 1% (Figure 2B), only the highest concentration of ethanol (1%) yielded a constriction that could not be distinguished from constriction to curcumin. Furthermore, the initial vasodilation response cannot be explained as a response to vehicle, and therefore must be a response to curcumin itself. To determine whether vasoconstriction responses to curcumin were attributable to a cytotoxic effect, we demonstrated that both constrictor and dilatory (cGMP and cAMP mediated) responses were unchanged before vs. after repeated exposure to curcumin (Table 2, Supplemental Data). Thus, curcumin stimulated a recoverable dose and time dependent dilation/constriction response in hamster cheek pouch arterioles.
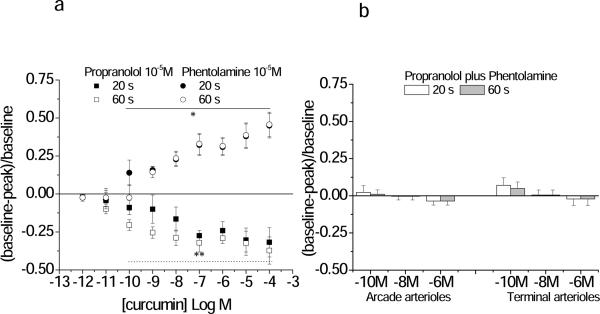
Adrenergic blockade suppressed the response to curcumin. Blockade was confirmed (Table 3, Supplemental Data). Figure 3A shows that the dilation response to curcumin in the terminal arteriole was abolished by propranolol and the constriction response abolished by phentolamine. Propranolol and phentolamine applied together blocked all response to curcumin (Figure 3B). We eliminated the possibility that curcumin was initiating part or all of these vasoactive effects through stimulation of the sympathetic nerves causing norepinephrine release by showing that although 6-OHDA did significantly increase baseline diameters (terminal arteriole, from 9.4±0.8 to 11.2±0.6 μm; arcade arteriole, from 19±3 to 29±4 μm), it did not remove the response to curcumin (Table 1, Supplemental Data). Thus the response to curcumin was prevented with total adrenergic blockade, and not by blocking sympathetic nerves, suggesting a direct action on the vascular wall by curcumin.
Figure 3.
Propanolol, a β-adrenergic antagonist, inhibited curcumin-induced vasodilation, while phentolamine, an α-adrenergic antagoist inhibited curcumin-induced vasoconstriction. Shown are the Early (20 seconds) and Late (60 seconds) diameter changes in response to curcumin in the presence of 10−5M propranolol or 10−5M phentolamine separately (Panel A) or together (Panel B) (Protocol 2). Three concentrations of curcumin were tested in panel B: 10−10M, 10−8M and 10−6M. Responses are shown only for the terminal arteriole in Panel A, and for both arcade and terminal feed arterioles in Panel B. The EC50 and maximal values for both the terminal arteriole and arcading arteriole are given in Table 1. *Phentolamine **Propranolol: individual responses differ from baseline, p<0.05.
Atropine (muscarinic antagonist) or PD142893 (Endothelin antagonist) each diminished the maximal dilation and enhanced the maximal constriction to curcumin (Table 1, Supplemental Data), yet had no effect on baseline diameters. Blockade was confirmed (Table 3, Supplemental Data). While muscarinic or endothelin receptor-mediated-actions may be involved in modulating the response to curcumin, they are not required.
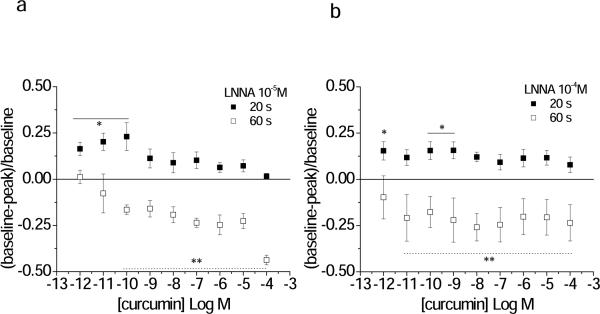
The β-Ad receptors may be present on endothelial cells, where they induce a NO, cGMP mediated dilation. Alternatively, or in addition, β-Ad receptors may be present on vascular smooth muscle cells where they induce a cAMP mediated dilation (Hardman and Limbird, 2001). The nitric oxide dependence of dilation to curcumin was tested by blocking endogenous NO formation with LNNA. With 10−5M LNNA, dilation to curcumin was attenuated to an equivalent extent in both the terminal feed and arcade arterioles; however, a significant dilation remained at the 10−12 to 10−10M curcumin concentrations (Figure 4A). At higher 10−4M LNNA, significant residual dilation remained for the lower concentrations of curcumin (Figure 4B). Blockade was confirmed (Table 3, Supplemental Data). These results suggested that dilation was mediated by NO at least in the nanomolar and micromolar range, but other mechanism(s) contributed to the microcirculation response in the picomolar range, perhaps consistent with low concentrations acting through an alternate G-protein system, or the high concentrations preferably acting directly on the downstream kinase elements (Hardman and Limbird, 2001; Sun, McGarrigle and Huang, 2007).
Figure 4.
LNNA partially inhibits curcumin-induced vasodilation. Shown are the Early (20 s) and Late (60 s) diameter changes in response to curcumin in the presence of LNNA (A, 10−5M; B 10−4M; Protocol 2). Responses are shown for the terminal arteriole only. The EC50 and maximal values for both the terminal arteriole and arcading arteriole are given in Table 1. *Early **Late: individual responses differ from baseline, p<0.05.
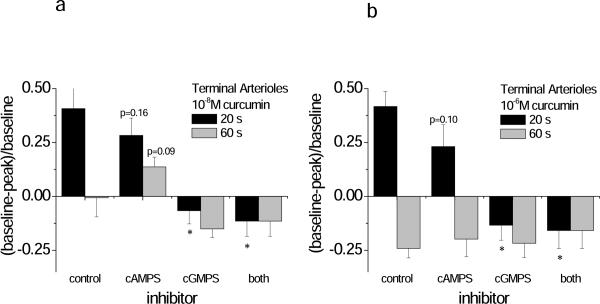
Next, we blocked cGMP and cAMP directly, using the Rp isomers, Rp-8-br-cGMPS and Rp-8-br-cAMPS (Figure 5). Blockade was confirmed (Table 3, Supplemental Data). Blocking cAMP significantly suppressed dilation to curcumin at 10−6mol/L for the arcade, but not terminal arterioles (Figure 5). Further, in arcade arterioles the constrictor response to 10−8mol/L curcumin was attenuated with Rp-8-br-cAMPS. Blocking cGMP prevented all dilation to curcumin in both the terminal and arcade arterioles (Figure 5). Thus, the dilation to curcumin appears to require cGMP for both classes of vessels, yet a significant role for cAMP-mediated dilation is suggested for the larger arcade arterioles.
Figure 5.
Inhibition of cGMP abolished curcumin-induced vasodilation. Shown are the Early (20 s) and Late (60 s) diameter changes in response to curcumin in the presence of 10−5M Rp isomers to block cGMP (Rp-8-br-cGMPS) or cAMP (Rp-8-br-cAMPS) (Protocol 3). Two concentrations of curcumin were tested: (A) 10−8M, and (B) 10−6M. Responses are shown from both the terminal arterioles (panels A and B) and arcade arterioles (panels C and D). *differs from control for the associated early vs. late response.
Discussion
Here we show that picomolar to low nanomolar concentrations of curcumin I induced a sustained vasodilation in hamster cheek pouch peripheral arterioles that was dependent on β-Ad receptor activity (Figure 6). Dilation was consistent with the response to curcumin observed for coronary arteries (Xu, Long, Dai and Liu, 2007). However, curcumin in the micromolar range induced sustained microvasculature constriction that was dependent on α-Ad receptor activity. In contrast, other studies using large arteries demonstrated that micromolar concentrations of curcumin induced decrease tension that was interpreted as vasodilation (Ahn, Park, Woo et al., 2009; Gilani, Shah, Ghayur et al., 2005; Sasaki, Gogo, Tohda et al., 2003; Xu, Long, Dai and Liu, 2007). Persistent vasoconstriction in vascular tissue to alcohol-extracted curcumin I is to our knowledge a previously unreported finding, which cannot be attributed to transient responses to vehicle alone.
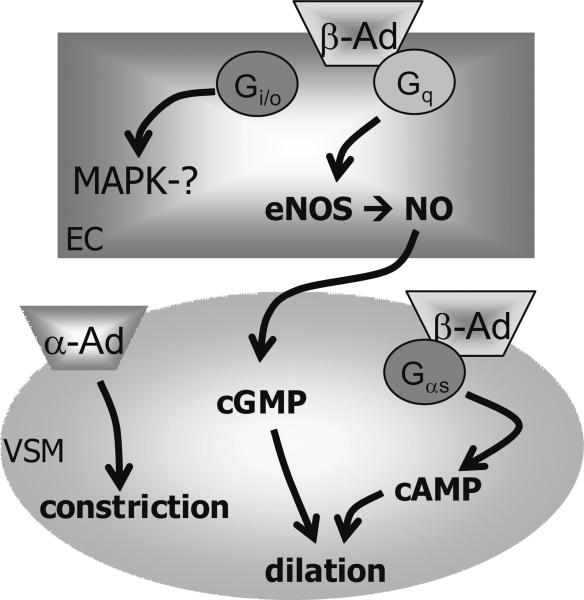
Figure 6.
Schematic of hypothesized mechanism of vasoaction for purified curcumin I in the peripheral microcirculation for terminal (nutritive) arterioles or arcade arterioles that feed them. EC, endothelial cell; VSM, vascular smooth muscle cell; β-AD, beta adrenoceptor (EC or VSM dilation); α-AD, alpha adrenoceptor (VSM constriction). Curcumin induces vasoconstriction that requires α-AD, and vasodilation that requires β-AD. EC dependent dilation occurs through eNOS (endothelial nitric oxide synthase), NO (nitric oxide), and cGMP (cyclic guanosine monophosphate) accounts for most of the dilation; we hypothesize that this is linked to the receptor G-protein subunit, Gq and independent of Gi or MAPK (mitogen activating protein kinase) activity. VSM dependent dilation occurs through cAMP (cyclic adenosine monophosphate) accounts for a smaller portion of the dilation, significant in only the arcade arterioles; based on the data presented here, we hypothesize that this is linked to the G-protein subunit, Gαs.
Propranolol blocked all dilation to curcumin. In this tissue, β2-Ad are present (Koo, 1984), but it was unknown whether they resided on endothelial and/or vascular smooth muscle cell types. While α2-Ad can induce dilation in some tissues and species (e.g., Crassous, Flavahan and Flavahan, 2009), their role was not investigated secondarily due to our conclusive findings with propranolol. We investigated the cell type pharmacologically with LNNA (to block endothelial dependent dilation) and then with Rp-isomers. With LNNA, dilation remained significant for the picomolar concentrations of curcumin, suggesting that the NO-cGMP pathway was required. Supporting this possibility, abrogating cGMP formation directly, prevented significant dilation. We did not investigate whether curcumin was acting on a kinase downstream of the β-Ad receptor (e.g., MAPK, ERK 1/2, and have not ruled out an inverse agonism effect (Hardman and Limbird, 2001; Sun, McGarrigle and Huang, 2007; Woo, Jung, Kim et al., 2005). Our finding is strikingly different from that of Xu, et al., (Xu, Long, Dai and Liu, 2007) who reported only half of the dilation to (synthesized) curcumin I in porcine coronary arteries is blocked with propranolol.
Dilation to curcumin was greater for the terminal arterioles than the arcade arterioles. This is consistent with findings in the microvasculature of the rat cremaster, in which the terminal arterioles exhibited a more robust dilation via cGMP than did the arcade arterioles (Frame, Fox, Kim et al., 2002), and may underlie a fundamental difference in the responses for these two classes of arterioles. The arcade arterioles were sensitive to cAMP blockade, suggesting a significant role for vascular smooth muscle cell β-Ad receptors in mediating the dilation to curcumin. We speculate therefore that the residual dilation at low curcumin concentrations with LNNA is via the vascular smooth muscle β-Ad, the magnitude of which differs by vessel class in this tissue.
We investigated alternate mechanisms that could explain a residual dilation with LNNA, and eliminated the possibility of an indirect process for curcumin-induced dilation through sympathetic nerve release of norepinephrine (6OHDA data). Lastly, based on the literature, we do not believe an alternate mechanism is responsible for these effects. For example, curcumin has a host of actions on cellular processes (Aggarwal and Sung, 2009), including induction of hemeoxygenase-1 (HO-1) that produces carbon monoxide (CO, Ryter, Jawed and Augustine, 2005). CO targets guanylyl cyclase to produce cGMP, causing vasodilation (e.g., Samora, Goodwill, Frisbee et al., 2010). However, the several hour timeline of curcumin-induced expression of HO-1 does not fit our vasoactive responses, which were acute and immediate.
Comparing our study to the paucity of literature examining vasoactive responses to curcumin, we speculate that three factors are important to account for differences in response: the curcuminoid itself; the toxic nature of micromolar curcumin; the vessel size and species tested. From the literature, constriction to curcumin (seen as increased force development in arterial rings) has only been shown for the polysaccharide extracted component of crude curcumin (Sasaki, Gogo, Tohda et al., 2003) or for non vascular tissue (Gilani, Shah, Ghayur et al., 2005). The highly purifed curcumin preparation that we used was solvent extracted followed by HPLC verification of curcumin I (>99%) (Singer, McClain, Romanov, et al., 2007), and thus constriction in our hands is not due to other curcuminoids. Previous findings by others using crude, solvent-extracted curcumin I, or synthesized curcumin I, indicated decreased tension (interpreted as vasodilation) in artery rings in the micromolar range, without evidence of increased tension (vasoconstriction) (Ahn, Park, Woo et al., 2009; Gilani, Shah, Ghayur et al., 2005; Sasaki, Gogo, Tohda et al., 2003; Xu, Long, Dai and Liu, 2007). The authors' interpretation of their data was that the continued decrease tension observed with increasing concentrations of curcumin was attributable to β-adrenergic receptors, part of which was prevented by propranolol (Xu, Long, Dai and Liu, 2007). However a return to control tension after curcumin removal was not shown for these studies, and in most cases the total time of curcumin exposure was not given (Foresti, Hoque, Monti et al., 2005; Gilani, Shah, Ghayur et al., 2005; Kunwar, Barik, Mishra et al., 2008). It is therefore unknown whether the total change in tension was an active process in these macrovessels, or whether high curcumin concentrations were cytotoxic over their incubation periods. In contrast, we directly observed small arterioles in situ with brief exposures and verified return to baseline. Curcumin induced a robust, sustained vasodilation at picomolar to low nanomolar levels that was completely recoverable with repeated 60 second exposures, as evidenced by no change in dilatory and constrictor capability before vs. after curcumin. To our knowledge the vasoactive response of curcumin in peripheral microvessels is previously unreported.
In conclusion, in the hamster cheek pouch model, curcumin is a potent vasodilator in the subnanomolar concentration range, shifting to constriction in the micromolar range. Both responses can be attributed to adrenergic receptors directly. Upon examination of the molecular structure of curcumin (Aggarwal and Sung, 2009) and its close similarity to norepinephrine, and α-Ad or β-Ad agonists and antagonists (Hardman and Limbird, 2001), our finding that curcumin acts through these receptors is not surprising. Expanding our interpretation of these findings to other microvascular tissue beds, curcumin is capable of inducing either constriction or dilation, and dilation can be endothelium-dependent or –independent. The precise adrenergic receptor distribution in each tissue will determine vasoactive responses to curcumin.
Importantly, the clinical relevance of this finding is directly related to our ongoing work that shows the beneficial effect of nano-molar curcumin in ameliorating thermal burn progression (Singer, McClain, Romanov, et al., 2007,Singer, Taira and Lin, 2010). In specific, curcumin administered i.v. 1 and 24 hrs after thermal injury reduced burn injury progression (Singer, Taira and Lin, 2010). A potential mechanism by which this occurs is via dilation of the blood vessels in the immediate vicinity of the burn, in the zone of potential ischemia.
Materials and Methods
Animal Model
With approval by the State University of New York at Stony Brook's Institutional Animal Care and Use Committee, male adult hamsters (120±12g, 116±34 days, N=60) were anesthetized with pentobarbital sodium (70 mg/kg i.p.) and tracheostomized. The left cheek pouch tissue was exteriorized and dissected for intravital microscopy observations of terminal arterioles coursing through loose areolar connective tissue on the adventitial side of the pouch. The thick keratinocyte layer that is normally open to the buccal cavity was placed face down on the dissection board and not directly observed. For additional histological orientation, please refer to figure 1 in reference (Murray, Kramer, Hesson et al., 2010). The control tissue bath solution was bicarbonate buffered saline (in mmol/L) 132 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, and 20 NaHCO3 (equilibrated with gas containing 5% CO2-95% N2 gas, pH 7.4 at 34°C, without proteins added: Frame and Mabanta, 2007; Frame, Rivers, Altland et al., 2007; Georgi, Dewar and Frame, 2010), which was flowed continuously over the tissue at 5ml/min. Adenosine (10−4mol/L) and phenylephrine (10−4mol/L) were dripped (10−4 L) onto the tissue and used to confirm dilator and constrictor tone, respectively. Thirty minutes later, microvascular responses (diameter change) were obtained according to Protocols 1–3, below.
Arteriolar Networks
The hamster cheek pouch is a 400–500 μm thick tissue composed of repeating terminal arteriolar networks arising from arcading arterioles. One terminal arteriolar network consists of a terminal feed arteriole providing nutrient flow to 3–5 terminal branches (Frame and Mabanta, 2007; Frame, Rivers, Altland et al., 2007; Georgi, Dewar and Frame, 2010). By definition the terminal branches only feed capillaries. The cheek pouch tissue is composed of both a muscular and mucosal region. The mucosal region is innervated by only sensory nerves while the muscular region (retractor muscle) is innervated by sensory, motor, and autonomic nerves (Grasby, Morris and Segal, 1999); here we examined the mucosal region only. In the present study curcumin was applied via micropipette to the entrance to the terminal network as the terminal arteriole arose from the arcade, thus defining the response to curcumin for two classes of blood vessels: conduit arcade arterioles and nutritive terminal arterioles (directly feeding capillaries).
Micropipette Drug Application
Micropipettes were made (Pipette Puller, Model 740 David Kopf Instruments Tujunga, CA) with 4–8 μm diameter tip openings over a distance of 100–200 μm. They were backfilled with test agents plus a flow tracer, 10−5mol/L FITC-BSA (fluorescein isothiocyanate conjugated to bovine serum albumin), and positioned ~25 μm from the vessel wall. Controlled delivery of test agents was achieved using a pressure ejection system (MPPI-2 Applied Scientific Instrumentation, INC Eugene, OR), where the balance pressure was set so that no FITC dye ejection was visible in the holding position, and the ejection pressure was the minimum that provided a steady outflow of FITC (typically 0.2 psi). Due to the position of the micropipette relative to the test arteriole and the constantly flowing superfusate, micropipette contents were washed over the test arteriole and then immediately removed from the observation site. Using this technique, the volume ejected in a typical exposure is on the order of picoliters, and the concentration of test agents at the wall of the vessel is estimated to be half of the concentration in the pipette (Georgi, Dewar and Frame, 2010). Micropipette drug exposure was preceded by a 30 second baseline, and the test agent was applied for a prescribed time, as noted for individual protocols.
Protocol 1- Locally applied curcumin
Curcumin obtained from Chromadex (Irvine, CA) was previously tested for purity; this ethanol extraction process followed by preparative HPLC yields curcumin I (>99%) as demonstrated by mass spectroscopy (Singer, Taira and Lin, 2010). The purified curcumin (10−2mol/L) was stored at −80°C in ethanol until used, and then diluted in control suffusate (10−12mol/L-10−4mol/L, n=7). In each animal, two to three networks were tested (minimum of 500μm apart), performing the complete concentration response at each site. We have previously shown that this distance assures independent observations in this tissue (Frame and Mabanta, 2007; Frame, Rivers, Altland et al., 2007; Georgi, Dewar and Frame, 2010); with curcumin, likewise, there was no difference in responses between sites. The highest dose of curcumin tested (10−4mol/L) contained 1% ethanol. An ethanol dose response was repeated here using 0.0001 – 1% ethanol, encompassing the range of 10−8 to 10−4mol/L curcumin (n=3). Curcumin, or ethanol alone, was applied for 60 seconds via micropipette to the junction where the arteriolar terminal feed arose from the arcade, exposing both vessel segments.
Protocol 2- Suffusate applied antagonists
Only one antagonist was tested per animal, and two to three sites that were more than 500 microns apart were tested per animal. Phentolamine (α-adrenergic receptor antagonist, 10−5mol/L, n=6), propranolol (β-adrenergic receptor antagonist, 10−5mol/L, n=6), atropine (muscarinic receptor antagonist, 10−7mol/L, n=7), or Nω-nitro-L-arginine (LNNA, nitric oxide synthase antagonist 10−5mol/L, n=5; and 10−4mol/L, n=7) were added to the flowing control suffusate for 5 minutes before and then continuously while Protocol 1 was performed. In five additional animals, phentolamine and propranolol were applied together. Blockade was confirmed with phenylephrine (α-adrenergic receptor agonist, 10−5mol/L), isoproterenol (adrenergic receptor agonist, 10−5mol/L), acetylcholine (muscarinic receptor agonist,10−4mol/L) and nitroprusside (cGMP mediated dilation agonist, 10−4mol/L).
The sympathetic nerve toxin, 6-hydroxy dopamine (6-OHDA, 10−3mol/L, n=5) was applied to a stationary tissue bath. Suffusate flow was stopped, and bone wax was used to create a pool encircling the cheek pouch tissue. The neurotoxin was added to the pool for 20 minutes. Then, control suffusate flow was returned, and Protocol 1 was performed.
Protocol 3- Micropipette applied antagonists
Only one antagonist was tested per animal, and two to three sites were tested per animal. PD142893 (endothelin receptor A and B antagonist, 10−5mol/L, n=4), was applied to the observation site via micropipette for 5 minutes prior to and then continuously during curcumin exposure. Curcumin (10−12–10−4mol/L) was applied in increasing concentrations, as per Protocol 1. Rp-8Br-cGMPS (cGMP antagonist, 10−4mol/L), or Rp-8Br-cAMPS (cAMP antagonist, 10−4mol/L), separately and together at separate sites within the same animal (n=5), were applied to the observation site via micropipette for 5 minutes prior to and then continuously during curcumin exposure to only 10−8 or 10−6mol/L curcumin. Blockade was confirmed with nitroprusside (cGMP mediated dilation agonist, 10−4mol/L) and adenosine (cAMP mediated dilation agonist, 10−4mol/L).
Microscopy and Videorecording
The vasculature was observed using a modified Nikon upright microscope with infinity optics. Data was obtained using a Nikon 50× LWD objective (NA 0.45) imaged with a Gen/Sys intensifier and CCD (charge coupled device) 72s (Dage MTI) camera, and recorded with a Panasonic AG-7350 SVHS videorecorder. Diameter was measured off-line using a videocaliper system (FOR-A) calibrated with a scale micrometer.
Statistical Analysis
Diameter changes were calculated as the fractional change from baseline, [(baseline-peak) / baseline]. The fractional change is reported to facilitate comparison of two sizes of arterioles. The level of dilatory and constrictor tone was similar for each class of arteriole. Concentration response relationships were analyzed by fitting a sigmoid shaped curve to the mean values and obtaining the EC50 and maximal values (Origin Laboratories Software, weighted by standard deviations). Comparisons were made between treatments using unpaired t-tests; when comparisons involved multiple sequential treatments (e.g., doses), analysis of variance for repeated measures was used. All experimental design employed a β-probability function of 0.6, a priori, and a statistical significance, α-probability function of 0.05, post hoc (Snedecor and Cochran, 1974).
Supplementary Material
Acknowledgements
None
Sources of Funding Funded by the NIH HL55492, NIH DK68401, AHA 0655908T, and the Armed Forces Institute of Regenerative Medicine, contract grant#W81XWH-08-2-0034.
Abbreviations used
- α-Ad
alpha adrenergic receptor
- β-Ad
beta adrenergic receptor
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- ET
endothelin
- LNNA
Nω-nitro-L-arginine
- EC50
effective concentration at half maximal response
- M3
muscarinic 3 receptor
- NO
nitric oxide
- NOS
nitric oxide synthase
- Rp-8br-cAMP
cAMP antagonist
- Rp-8Br-cGMP
cGMP antagonist
- SD
standard deviation
- SEM
standard error of the mean
Footnotes
Conflict of Interest MD Frame, RA Clark, AJ Singer. Patent Pending for use of curcumin in thermal burns identifies the mechanism of action by which curcumin ameliorates thermal burn injury progression. Title: Curcumin controls blood flow through adrenergic receptors.
References
- Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30(2):2–85. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Ahn CM, Park BG, Woo HB, et al. Synthesis of sulfonyl curcumin mimics exerting a vasodilatation effect on the basilar artery of rabbits. Bioorg Med Chem Lett. 2009:191481–1483. doi: 10.1016/j.bmcl.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Braverman IM. The Cutaneous Microcirculation: Ultrastructure and Microanatomical Organization (Review) Microcirculation. 1997;4:329–340. doi: 10.3109/10739689709146797. [DOI] [PubMed] [Google Scholar]
- Crassous PA, Flavahan S, Flavahan NA. Acute dilation to alpha(2)-adrenoceptor antagonists uncovers dual constriction and dilation mediated by arterial alpha(2)-adrenoceptors. Br J Pharm. 2009;158:1344–1355. doi: 10.1111/j.1476-5381.2009.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti R, Hoque M, Monti D, et al. Differential Activation of Heme Oxygenase-1 by Chalcones and Rosolic Acid in Endothelial Cells. J Pharm Exp Thera. 2005;312:686–693. doi: 10.1124/jpet.104.074153. [DOI] [PubMed] [Google Scholar]
- Frame MD, Fox R, Kim D, et al. Diminished arteriolar responses in nitrate tolerance involve ROS and angiotensin II. Am J Physiol Heart Circ. 2002;282:H2377–H2385. doi: 10.1152/ajpheart.00429.2001. [DOI] [PubMed] [Google Scholar]
- Frame MD, Mabanta L. Remote microvascular preconditioning alters specific vasoactive responses. Microcirc. 2007;14:739–754. doi: 10.1080/10739680701410074. [DOI] [PubMed] [Google Scholar]
- Frame MD, Rivers RJ, Altland O, et al. Mechanisms initiating integrin stimulated flow recruitment in arteriolar networks. J Appl Physiol. 2007;102:2279–2287. doi: 10.1152/japplphysiol.00537.2006. [DOI] [PubMed] [Google Scholar]
- Georgi MK, Dewar AM, Frame MD. Downstream Exposure to Growth Factors Causes Elevated Velocity and Dilation in Arteriolar Networks. J Vasc Res. 2010;48:11–22. doi: 10.1159/000317396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani AH, Shah AJ, Ghayur MN, et al. Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sciences. 2005;763:089–3105. doi: 10.1016/j.lfs.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Grasby DJ, Morris JL, Segal SS. Heterogeneity of vascular innervation in hamster cheek pouch and retractor muscle. J Vasc Res. 1999;36:465–76. doi: 10.1159/000025689. [DOI] [PubMed] [Google Scholar]
- Gyorfi A, Fazekas A, Rosivall L. Neurogenic inflammation and the oral mucosa. J Clin Periodontol. 1992;19:731–6. doi: 10.1111/j.1600-051x.1992.tb02162.x. Review. [DOI] [PubMed] [Google Scholar]
- Hardman JG, Limbird LE, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th Edition McGraw-Hill; New York: 2001. [Google Scholar]
- Koo A. Vasodilator effect of terbutaline: In vivo evidence for the existence of beta 2 adrenoceptors in the microcirculation of rats, hamsters and guinea pigs. J Cardiovas Pharm. 1984;6:897–901. [PubMed] [Google Scholar]
- Kunwar A, Barik A, Mishra B, et al. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and turmor cells. Biochim Biophys Acta. 2008;1780:673–679. doi: 10.1016/j.bbagen.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Moritz AR. Studies of thermal injury. III. The pathology and pathogenesis of cutaneous burns. An experimental study. Am J Pathol. 1947;23:915–941. [PMC free article] [PubMed] [Google Scholar]
- Murray LA, Kramer MS, Hesson DP, et al. Serum amyloid P ameliorates radiation-induced oral mucositis and fibrosis. Fibrogenesis & Tissue Repair. 2010;3:11. doi: 10.1186/1755-1536-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaklander AL, Siegel SM. Cutaneous innervation: Form and function. J Am Acad Dermatol. 2005;53:1027–37. doi: 10.1016/j.jaad.2005.08.049. Review. [DOI] [PubMed] [Google Scholar]
- Regas FC, Ehrlich HP. Elucidating the vascular response to burns with a new rat model. J Trauma. 1992;32:557–563. doi: 10.1097/00005373-199205000-00004. [DOI] [PubMed] [Google Scholar]
- Ryter S, Jawed A, Augustine C. Heme Oxygenase-1/Carbon Monoxide: From Basic Science to Therapeutic Applications. Physiol Rev. 2005;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Samora JB, Goodwill AG, Frisbee JC, et al. Growth-dependent changes in the contribution of carbon monoxide to arteriolar function. J Vasc Res. 2010;47:23–34. doi: 10.1159/000231718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Goto H, Tohda C, et al. Effects of Curcuma Drugs on Vasomotion in Isolated Rat Aorta. Biol Pharm Bull. 2003;26(8):8–1135. doi: 10.1248/bpb.26.1135. [DOI] [PubMed] [Google Scholar]
- Scardina GA, Ruggieri A, Messina P. Oral microcirculation observed in vivo by videocapillaroscopy: a review. J Oral Sci. 2009;51:1–10. doi: 10.2334/josnusd.51.1. [DOI] [PubMed] [Google Scholar]
- Singer AJ, McClain SA, Romanov A, et al. Curcumin reduces burn progression in rats. Academic Emerg Med. 2007;14:1125–1129. doi: 10.1197/j.aem.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Taira BR, Lin F, et al. Curcumin reduces injury progression in a rat comb burn model. J Burn Care Res. 2010 doi: 10.1097/BCR.0b013e318203337b. in press. DOI: 10.1097/BCR.0b013e318203337b. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. The Iowa State University Press; Ames: 1974. [Google Scholar]
- Sun Y, McGarrigle D, Huang X-Y. When a G-protein receptor does not couple to a G-protein. Mol. BioSyst. 2007;3:849–854. doi: 10.1039/b706343a. [DOI] [PubMed] [Google Scholar]
- Vo LT, Papword GD, Delaney PM, et al. A study of vascular response to thermal injury on hairless mice by fibre optic confocal imaging, laser doppler flowmetry and conventional histology. Burns. 1998;1998;24:319–324. doi: 10.1016/s0305-4179(98)00028-x. [DOI] [PubMed] [Google Scholar]
- Woo MS, Jung SH, Kim SY, et al. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochemical and Biophysical Research Communications. 2005;335:1017–1025. doi: 10.1016/j.bbrc.2005.07.174. [DOI] [PubMed] [Google Scholar]
- Xu PH, Long Y, Dai F, Liu ZL. The relaxant effect of curcumin on porcine coronary arterial ring segments. Vasc Pharm. 2007;47:25–30. doi: 10.1016/j.vph.2007.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.