Summary
tert-Butyl hydroperoxide (tBHP), an organic peroxide, has been shown to cause irreversible damage to keratinocytes in vitro with prolonged administration at high concentrations, and reversible damage with short-term administration at low concentrations. To investigate the effects of tBHP on keratinocytes in vivo, we analysed hair growth in tBHP-treated neonatal rats. Sprague–Dawley and Long–Evans rat pups were injected subcutaneously with tBHP or vehicle once daily for 6 days, and hair growth was monitored. The tBHP-treated rats had a significant delay in hair growth. However, this delay reversed within days, and the hair coats, including hair pigmentation, of tBHP-treated and sham-treated rats were indistinguishable 2 weeks later. Histological analysis and BrdU labelling of S phase cells confirmed the delay in hair-follicle growth and its reversal in tBHP-treated rats. Our results indicated that the changes incurred in hair follicles by short-term use of high-dose oxidants in vivo are temporary and reversible.
The skin is constantly exposed to relatively high levels of ultraviolet light and high oxygen tension, and to oxidizing chemicals present in cosmetic and pharmaceutical products. Exposure to these environmental challenges, in addition to oxidants produced during normal cellular processes, can result in oxidative stress that causes tissue and cellular damage.1–3 In the skin, oxidative stress has been associated with the development of vitiligo and rosacea.4,5 In addition, susceptibility of melanocytes to oxidative stress may be responsible for senile hair greying.6–8
It is important to understand the effects of oxidants on skin keratinocytes because these cells encounter the highest levels of environmental oxidants. Previous studies have shown that cultured keratinocytes suffer irreversible damages with long-term, high-dose treatment of tert-butyl hydroperoxide (tBHP), an organic peroxide, although the damage was reversible in keratinocytes given short-term, low-dose treatment.9 In addition, topical application of linolein hydroperoxides (lipid peroxides) in adult mice was shown to induce apoptosis of hair follicle (HF) keratinocytes and subsequent early onset of catagen (HF regression phase).10 However, it is not known whether such oxidant-induced HF damage is permanent.
In this study, we investigated the effects of tBHP on HF growth in neonatal Sprague–Dawley (SD) and Long–Evans (LE) rat pups. We treated the pups daily for 6 days and monitored hair growth for an additional 2 weeks. Use of neonatal rats facilitated the analysis of oxidative damage on visible hair-shaft growth during the synchronized postnatal HF morphogenesis. Additionally, use of the pigmented LE rats also allowed us to examine the effects of tBHP on hair-shaft pigmentation.
Report
All animal care and use procedures were approved by the University of Miami Institutional Animal Care and Use Committee.
Nursing SD and LE rat pups (Charles River Laboratories, Wilmington, MA, USA) were randomly assigned to receive subcutaneous injections in the nape of the neck, either tBHP (Sigma-Aldrich, St. Louis, MO, USA) 100 μL of 20 μL/mL diluted in phosphate-buffered saline (PBS) or PBS (vehicle) alone, once daily from day 5 to day 10 after birth, and monitored for hair growth. This treatment regimen was based on our previous experience. After treatment cessation, tBHP-treated and sham-treated pups were killed every 2 days for 13 days, and skin samples were collected. There were three pups per treatment group per time point. and the experiments were replicated a total of seven times between the SD and LE rats. For each time point, one tBHP-injected and one PBS-injected pup were injected intraperitoneally with 5-bromo-2′-deoxyuridine (BrdU) labelling reagent 10 μL/g (Invitrogen Corp. Carlsbad, CA, USA), 2 h before euthanasia. Tissues were embedded in paraffin wax, sectioned, and stained with haematoxylin and eosin for histolopathological examination, or stained for BrdU-positive (S phase) cells using immunohistochemistry (Invitrogen Corp.).
Hair growth in the untreated areas was indistinguishable between tBHP-treated and sham-treated SD (albino) or LE (pigmented) rat pups, and between untreated and sham-treated areas of the same rat. However, tBHP-treated areas began to show a delay in hair growth by day 11 after birth (day 11), 1 day after the last oxidant treatment (Fig. 1a). This delay caused a drastic difference in skin appearance between the tBHP-treated and sham-treated areas in pups on days 13, 17 and 19 (Fig. 1b–d,f–h, and data not shown). Whereas control rats had a smooth hair coat, the skin in the tBHP-treated areas was bare at these stages, so that it appeared pink in the albino rats (Fig. 1b–d) and grey in the pigmented ‘hood’ of the LE rats (Fig. 1f–h). This delay in hair growth persisted until day 21 (Fig. 1i), when the tBHP-treated areas became plush with elongating hair shafts (Figs 1i,2i). By day 23, no gross differences in the hair coat, including hair pigmentation, were seen between the tBHP-treated and sham-treated areas, or between the tBHP-treated and surrounding areas (Fig. 1e, and data not shown), indicating that the effects of tBHP on hair-shaft growth were temporary and reversible. Similar delays in hair growth were seen in treated areas in both SD and LE pups (Fig. 1).
Figure 1.
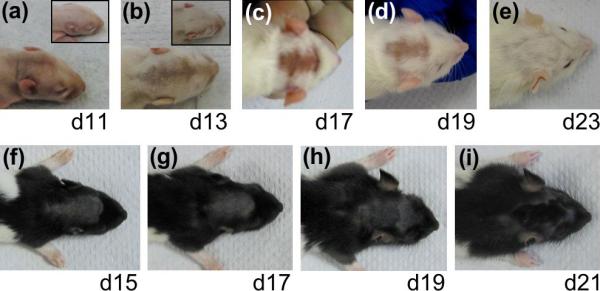
Delay in hair growth in (a–e) Sprague-Dawley and (f–i) Long-Evans rats upon treatment with tert-butyl hydroperoxide (tBHP). The ages of the rats are indicated. (Insets a,b) Sham-treated control rats. Delay in hair growth began to show on (a) day 11 and persisted until (i) day 21. Similar delays in hair growth were seen in the treated areas in both strains of rats.
Figure 2.
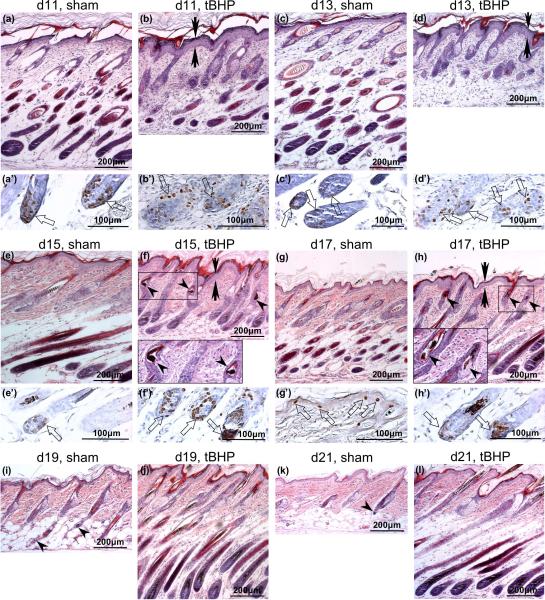
Formalin-fixed, paraffin wax-embedded sections of skin from (a–d, a′–d′′ Sprague-Dawley pup skin and (e–l, e′–h′) Long-Evans pup skin treated with (a,c,e,g,i,k) phosphate-buffered saline (PBS, sham) and (b,d,f,h,j,l) tert-butyl hydroperoxide (tBHP). (a–l) Haematoxylin and eosin, original magnification × 100; (a′–h′) 5-bromo-2′-deoxyuridine (BrdU) (brown nuclei), original magnification × 320. Images of both (a,c,e,g,i,k) sham-treated and (b,d,f,h,j,l) tBHP-treated sections are at the same magnification, with the epidermis aligned, to show the transitions between hair-follicle growth, regression and resting phases, reflected in the thickness of the skin and the length of the hair follicles. The ages of the rats are indicated. (b,d,f,h) Epidermal hyperplasia (arrows); (f,h and insets) distorted hair follicles with melanin clumps (arrowheads)(original magnification × 200); catagen hair follicles (i, arrowheads); telogen hair follicle (k, arrowhead). (a′–h′) BrdU labelling, counterstained with haematoxylin, of S phase cells in the same skin samples shown in panels (a–h), enlarged to show BrdU-positive nuclei in the hair-follicle bulbs.
Histological analysis was carried out on formalin-fixed, paraffin wax-embedded skin samples of tBHP-treated and sham-treated areas from day 11 to day 21 (Fig. 2). The thickness of rat skin and the length of the HFs reflect HF phases during the hair cycle, with thicker skin and longer HFs during late anagen (growth phase) (Figure. 2c, 2h,j) and thinner skin and shorter HFs during catagen (regression phase) (Fig. 2i) and telogen (resting phase) (Fig. 2k). Histological examination confirmed the delay in HF growth in tBHP-treated areas (Fig. 2). Whereas HFs in the sham-treated skin reached the peak of follicular length on day 13 (Fig. 2c), HFs in tBHP-treated skin did not do so until day 19 (Fig. 2j), when the sham-treated skin had already entered catagen (Fig. 2i, arrowheads). Other effects of tBHP treatment, such as epidermal hyperplasia (Fig. 2b,d, arrows), HF distortion and presence of melanin clumps in the hair canal (Fig. 2f,h, arrowheads), diminished with time (Fig. 2h,j). In the LE rats, we did not observe any abnormal pigmentation in the hair shafts or HFs in tBHP-treated rats after day 17 (Fig. 2j,l).
BrdU-positive keratinocytes were detected in all the skin sections we examined with anagen HF morphology, confirming the delay in HF growth in tBHP-treated skin (Fig. 2a′–2 h′, thick arrows). At day 11, one day after treatment cessation, abundant BrdU labelling was seen in the HFs in tBHP-treated skin (Fig. 2b′), even though they were still much shorter than those in sham-treated skin (Fig. 2a,b), indicating recovery in keratinocyte proliferation. BrdU incorporation in tBHP-treated skin persisted for at least another 8 days (Fig. 2d′,f′,h′ and data not shown), when sham-treated skin was already in catagen (Fig. 2i). By day 17 in sham-treated skin and day 21 in tBHP-treated skin, there was little BrdU labelling in the hair follicles; however, we detected BrdU-positive nuclei in the expanding epidermis (Fig. 2g′ and data not shown) of these young rats.
Taken together, these results provide both gross and histological evidence of hair growth arrest in neonatal SD and LE rats treated short-term with high doses of tBHP. All the damage incurred was reversible. To our knowledge, this is the first study to investigate the effects of oxidants on HF growth during postnatal HF morphogenesis. tBHP probably has similar effects on synchronized anagen hair follicles in adults, although this remains to be tested.
Unlike in adult rodents, HFs in our neonatal model undergo spontaneously synchronous hair growth, and do not require induction by depilation. In addition, the use of pigmented LE pups allowed monitoring of hair pigmentation after treatment. These characteristics make our neonatal rat model a useful tool for future studies aimed at delineating the mechanisms of oxidative stress-induced growth arrest in HFs.
Learning points
Rat pups treated with tBHP, an organic peroxide, on days 5–10 after birth, had a significant delay in hair growth.
This delay was seen in both albino SD and pigmented LE pups.
The delay was rapidly reversible, and the hair coats, including hair pigmentation, of tBHP-treated and sham-treated rats were indistinguishable two weeks later.
Histological analysis and BrdU labelling of S-phase cells confirmed the delay in hair-follicle growth and its reversal in tBHP-treated rats.
The changes incurred in hair follicles by short-term use of high-dose oxidants in vivo are temporary and reversible.
Acknowledgements
We are grateful for the support of the Locks of Love Foundation (J.J.J.) and the Brian V. Jegasothy M.D. Basic Science Research Award (T.C.W.) in this investigation. T.C.W. is supported by a Career Development Award from NIH/NIAMS (AR050487). We thank S. B. Koncal for technical input.
Footnotes
Conflict of interest: none declared.
References
- 1.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–95. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Benz CC, Yau C. Ageing, oxidative stress and cancer: paradigms in parallax. Nat Rev Cancer. 2008;8:875–9. doi: 10.1038/nrc2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rees MD, Kennett EC, Whitelock JM, Davies MJ. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic Biol Med. 2008;44:1973–2001. doi: 10.1016/j.freeradbiomed.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Spencer JD, Gibbons NC, Rokos H, et al. Oxidative stress via hydrogen peroxide affects proopiomelanocortin peptides directly in the epidermis of patients with vitiligo. J Invest Dermatol. 2007;127:411–20. doi: 10.1038/sj.jid.5700538. [DOI] [PubMed] [Google Scholar]
- 5.Tisma VS, Basta-Juzbasic A, Jaganjac M, et al. Oxidative stress and ferritin expression in the skin of patients with rosacea. J Am Acad Dermatol. 2009;60:270–6. doi: 10.1016/j.jaad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Tobin DJ, Paus R. Graying: gerontobiology of the hair follicle pigmentary unit. Exp Gerontol. 2001;36:29–54. doi: 10.1016/s0531-5565(00)00210-2. [DOI] [PubMed] [Google Scholar]
- 7.Arck PC, Overall R, Spatz K, et al. Towards a ‘free radical theory of graying’: melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006;20:1567–9. doi: 10.1096/fj.05-4039fje. [DOI] [PubMed] [Google Scholar]
- 8.Wood JM, Decker H, Hartmann H, et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 2009;23:2065–75. doi: 10.1096/fj.08-125435. [DOI] [PubMed] [Google Scholar]
- 9.Vessey DA, Lee KH, Blacker KL. Characterization of the oxidative stress initiated in cultured human keratinocytes by treatment with peroxides. J Invest Dermatol. 1992;99:859–63. doi: 10.1111/1523-1747.ep12614831. [DOI] [PubMed] [Google Scholar]
- 10.Naito A, Midorikawa T, Yoshino T, Ohdera M. Lipid peroxides induce early onset of catagen phase in murine hair cycles. Int J Mol Med. 2008;22:725–9. [PubMed] [Google Scholar]