Abstract
Objective
Recently vitamin D deficiency has been associated with increased risks for preeclampsia and diagnosis of early-onset, severe preeclampsia (EOSPE). The purpose of this investigation was to examine the association between vitamin D levels and small for gestational age (SGA) in patients with EOSPE.
Study Design
Patients with EOSPE were recruited and demographics, outcomes, and plasma were collected. 25-hydroxy-vitamin D (25-OH-D) was assessed by radioimmunoassay and reported in ng/mL. Results were analyzed by Mann Whitney U test and Spearman correlation and reported as median (Q1–Q3).
Results
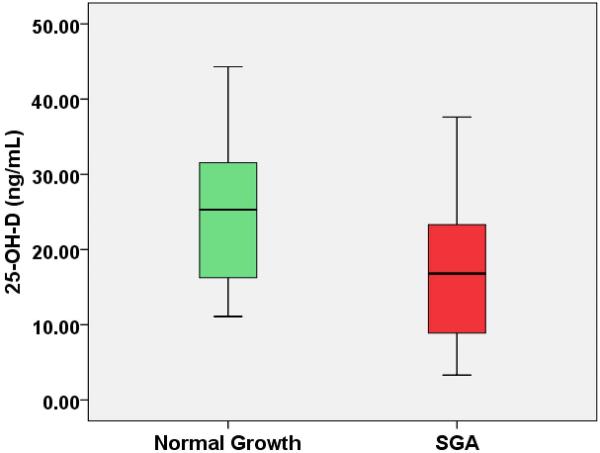
In patients with EOSPE (n=56), 25-OH-D was lower in patients with SGA (16.8 ng/mL [8.9–23]) verses normal fetal growth (25.3 ng/mL [16–33]) (p=0.02). 25-OH-D was significantly correlated with percentile growth at delivery (ρ = 0.31, p=0.02).
Conclusions
Vitamin D is lower among patients with SGA in EOSPE than those without growth retardation. We suspect that vitamin D may impact fetal growth through placental mechanisms.
Keywords: 25-hydroxyvitamin D, fetal growth restriction, preeclampsia, Vitamin D, small for gestational age (SGA)
Introduction
Vitamin D is a secosterol hormone recognized primarily for its role in calcium homeostasis.1 25-hydroxyvitamin D (25-OH-D) is an important measure of physiologic vitamin D status and has a half-life of about 2 weeks. The active form of vitamin D, 1,25-(OH)2D3 is produced through hydroxylation of 25-OH-D in the kidney or placenta and has a very short half life measured in minutes.2–3 Thus, most studies have focused on assessment of vitamin D influence upon disease using 25-OH-D levels. However, recent evidence suggests that vitamin D and especially a deficiency of vitamin D, which is common in the United States, may be involved in adverse pregnancy outcomes.4–6 We recently reported an association between low maternal 25-OH-D and early-onset, severe preeclampsia (EOSPE).7 Women with a diagnosis of EOSPE had significantly lower 25-OH-D levels and the data suggested a potential reduction of the risk of preeclampsia with only a 10ng/mL increase in maternal 25-OH-D. Bodnar, et.al. found that vitamin D deficiency in the second trimester measured by 25-OH-D, was also associated with an increased risk for preeclampsia.8 Epidemiologic evidence has also linked insufficient vitamin D intake and preeclampsia risk among nulliparious pregnancies.9
Early onset, severe preeclampsia (EOSPE) contributes 15% of the preterm births in the United States per annum and likely contributes to increased risk for vascular disease in later life.10–12 These women and their fetuses are also recognized to be at great risk for adverse pregnancy outcome with a 20-fold increased risk for maternal mortality and several-fold higher risk for neonatal morbidity or mortality dependent upon gestational age at delivery and presence of growth restriction in the fetus.12 Thus, this group may serve as a target population for improving outcomes associated with preeclampsia.
The purpose of this investigation was to examine the maternal plasma level of 25-OH-D in cases of EOSPE that resulted in the delivery of a small for gestational age (SGA) infant relative to pregnancies with EOSPE and normal fetal growth.
Methods
The Institutional Review Board at the Medical University of South Carolina (MUSC) approved this nested case control investigation from a prospective study of EOSPE patients enrolled at MUSC from 2007 to 2010.7 Patients included in this investigation consented to collection of demographic and outcome data as well as venipuncture for collection of plasma used for 25-OH-D analysis. Subjects were recruited from the inpatient Labor and Delivery unit at MUSC after confirmation of a diagnosis of EOSPE. EOSPE was defined as meeting the American College of Obstetrics and Gynecology criteria for severe preeclampsia and presenting with this diagnosis prior to 34 weeks completed gestational age.13 Patients with EOSPE or healthy controls were excluded if they also had a diagnosis of chronic hypertension, pregestational diabetes, renal disease, lupus, or tobacco use. Demographic data were collected on each EOSPE case at the time of plasma collection which included gestational age, maternal age, maternal prepregnancy body mass index (BMI), maternal systolic and diastolic blood pressure, and urine protein. Plasma was collected in the BD P100 v1.1, EDTA vacutainer tube (Becton Dickinson, Franklin Lakes, NJ) which contains a protease inhibitor cocktail from EOSPE cases at the time of diagnosis. Samples were processed and frozen in aliquots within thirty minutes of collection from each subject. The antepartum plasma sample collected at the time of diagnosis in EOSPE was assessed for total 25-OH-D in ng/mL using double antibody radioimmunoassay (DiaSorin, Stillwater, MN). In our lab, this assay has a less than 10% inter-assay and intra-assay reliability. Following delivery, outcome data were collected on EOSPE cases that included birth weight, gestational age at delivery, and an assessment of birth weight percentile with SGA defined as birth weight less than 10th%ile as assessed by gestational age at delivery.14 Cases were defined as patients with EOSPE and SGA verses controls who were patients with EOSPE and normal fetal growth.
Results of continuous and categorical variables were reported as median (25th percentile to 75th percentile) and percentage by case or control group, respectively. Bivariable analysis was conducted using the Mann Whitney U test for examination of continuous variables (maternal age, prepregnancy BMI, gestational age at plasma sample collection, gestational age at delivery, mean arterial pressure at sample collection, birth weight, and plasma 25-OH-D levels) by case or control group. Proportions were compared by case or control group using chi-square test. The distribution of data points was assessed by Shapiro-Wilk W test. Correlation between maternal 25-OH-D at diagnosis of EOSPE and percentile growth was determined by Spearman rank correlation (ρ). Simple linear regression was conducted between percentile growth and maternal 25-OH-D for examination of slope of regression line. F test and r2 values were calculated to assess the fit of the regression model. All statistical tests were two-sided with alpha set at 0.05 to control for Type I error. Data analysis was performed with SAS v.9.2 (SAS, Cary, NC).
Results
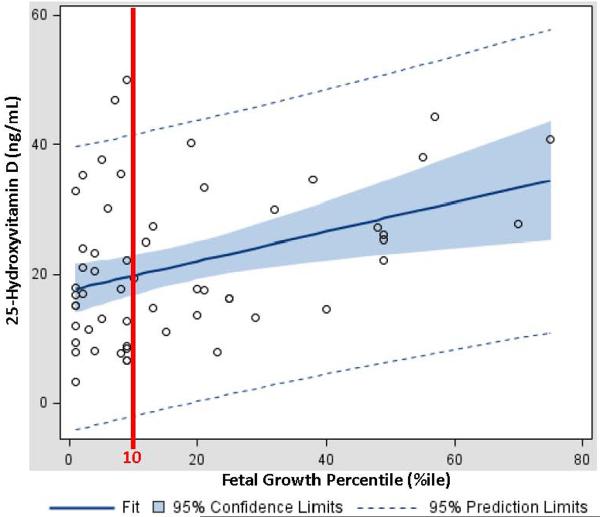
Fifty six patients with EOSPE were consented and included in this investigation. Pregnancy demographics and outcomes are summarized by EOSPE patients with SGA (cases) or EOSPE with normal growth (controls) in Table 1. (Table 1) Patients with SGA were noted to be similar to those with normal growth at delivery with respect to all demographics except for the gestational age at delivery and plasma 25-OH-D levels. The median plasma 25-OH-D level among patients with SGA was 33% less than those patients who exhibited normal growth at delivery (SGA 16.8 ng/mL vs. normal 25.3 ng/mL, p=0.02). Since SGA patients were delivered at a higher gestational age, we converted all birth weights to percentile growth for gestational age as a means of controlling for different gestational ages at delivery.14 A positive correlation was demonstrated (ρ = 0.31, p=0.02) between percentile growth and maternal 25-OH-D levels. In order to examine the relationship between percentile growth and maternal 25-OH-D levels, a simple linear regression was performed. The simple linear regression model determined the slope of the fitted regression line as y = 17.5 + 0.22x where x is the percentile birth weight and y is the maternal 25-OH-D level. The model, as shown in Figure 2, had excellent goodness of fit as assessed by F test (p = 0.004) and an r2 value of 0.14. (Figure 2)
Table I.
Demographics and Outcomes
| SGA (n=33) | Normal Growth (n=23) | P value | |
|---|---|---|---|
| Age (years) | 24 (21–29) | 24 (21–30) | NS* |
| Nulliparious (%) | 25% | 36% | NS |
| Gestation at Delivery (wks) | 31 (29–33) | 29 (27–30) | 0.01* |
| African American Race (%) | 34% | 13% | NS |
| Mean Arterial Pressure (mmHg) | 123 (120–130) | 129 (120–130) | NS* |
| BMI (kg/m2) | 32 (25–37) | 34 (29–38) | NS* |
| 25-Hydroxyvitamin D (ng/mL) | 16.8 (8.9–23) | 25.3 (16–33) | 0.02* |
Values reported as median (interquartile range)
Mann Whitney U test
Fisher Exact test
SGA = Small for Gestational Age
BMI = Body Mass Index (prior to pregnancy)
Figure 2. Simple Linear Regression between Percentile Growth and Maternal 25-Hydroxyvitamin D Levels.
There was a significant relationship between growth percentile at birth and maternal 25-OH-D: as improved birthweight (by percentile growth) was associated with higher plasma 25-OH-D levels.
Comment
In this investigation, we examined plasma 25-OH-D among patients diagnosed with EOSPE who either had SGA or normal growth. EOSPE is one of the most serious forms of hypertensive disease in pregnancy.12 This study found significantly lower maternal levels of 25-OH-D among patients with an SGA fetus relative to those with normal growth. Furthermore, there was a significant correlation between 25-OH-D and percentile growth suggesting a possible underlying mechanistic role for vitamin D in fetal growth that is likely mediated through actions in the placenta.5 This is the first report of a significant relationship between maternal vitamin D level and fetal growth. As this investigation focused on the most severely affected patients with preeclampsia, future clinical trials may focus on the possible impact of Vitamin D deficiency or supplementation upon fetal growth outcomes in preeclamptic and normal pregnancies.10, 12 Understanding the mechanisms behind fetal growth are complex and interventions to improve growth in conditions known to be at high risk for SGA are poorly understood.15–17 Vitamin D has been implicated in providing critical signals in gene regulation and expression in early placental development among placental trophoblast models.18–22 As the placenta has all of the necessary molecular machinery to convert 25-OH-D to the active form of vitamin D (1,25-OH2-D3), it has been suggested that the placenta may convert the storage form (25-OH-D) to the active form of vitamin D for local or paracrine utilization.5, 18 1,25-OH2-D3 has been shown to improve endothelial function among patients on renal hemodialysis through a possible paracrine action.23–24 In endothelial cells, 1,25-OH2-D3 has been demonstrated to increase expression of vascular endothelial growth factor (VEGF), a potent proangiogenic protein known to be diminished in preeclampsia) through binding to vitamin D receptor and co-localization to a vitamin D responsive element in the VEGF promoter.25 If this mechanism is also demonstrated in the placental trophoblast, it is possible that inadequate vitamin D levels may affect both preeclampsia and fetal growth through alterations in VEGF activity.
We have previously published data associating low maternal 25-OH-D levels with the diagnosis of EOSPE.7 In vitamin D deficiency, it is possible that the lack of these signals may play a critical role in Stage I of placental development that leads to the ultimate recognition of Stage II and a diagnosis of preeclampsia.19, 22, 26 It is not precisely known how these signals might lead to the ultimate diagnosis of preeclampsia, and thus, epidemiological observations of the incidence of preeclampsia associated with vitamin D deficiency currently lack fully defined pathways through which biomolecular mechanisms explain this relationship. However, based on the observations of this and other studies linking vitamin D deficiency and preeclampsia, vitamin D supplementation remains a possible intervention and possible improved pregnancy outcomes through prevention or delay of preeclampsia or improved fetal growth in pregnancies at high risk for preeclampsia.7–9
This study has important limitations that should be noted and addressed in future investigations. Since these patients were enrolled at the time of diagnosis of EOSPE, an assessment of preconception and early pregnancy dietary intake, sun exposure, or maternal baseline 25-OH-D was not available to help understand the impact of these factors on the development of EOSPE. It is also important to recognize that this investigation cannot stand alone to assert a role for vitamin D supplementation in pregnancy to improve pregnancy outcomes. A recent investigation noted that maternal, first trimester, free 25-OH-D levels were not associated with first trimester blood pressure or later development of preeclampsia among 170 patients where 39 subsequently developed preeclampsia. However, this investigation was limited by a small sample size and primary analysis of term preeclampsia. As EOSPE is known to have a more significant impact on maternal and fetal outcomes, this investigation may not be comparable to those where term preeclampsia is considered in possible association with maternal 25-OH-D.27 However, this study does provide additional evidence suggesting the need for prospective longitudinal studies or randomized controlled trials of vitamin D supplementation examining the potential for impact on adverse pregnancy outcomes.
Figure 1. 25-Hydroxyvitamin D Levels in IUGR vs. Normal Birthweight in EOSPE.
56 patients were enrolled with 59% of these exhibiting evidence of IUGR at birth. Maternal plasma 25-OH-D, a storage form of vitamin D, was found to be 33% reduced among those pregnancies with IUGR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 73rd Annual Meeting of the South Atlantic Association of Obstetricians and Gynecologists; January 2011, Hot Springs, Virginia.
References
- 1.Finberg L. Vitamin D deficiency and rickets. J Pediatr Endocrinol Metab. 2006 Mar;19(3):203. doi: 10.1515/jpem.2006.19.3.203. [DOI] [PubMed] [Google Scholar]
- 2.Hollis BW, Wagner CL, Drezner MK, Binkley NC. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status. J Steroid Biochem Mol Biol. 2007 Mar;103(3–5):631–634. doi: 10.1016/j.jsbmb.2006.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005 Feb;135(2):317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 4.Dror DK, Allen LH. Vitamin D inadequacy in pregnancy: biology, outcomes, and interventions. Nutr Rev. 2010 Aug;68(8):465–477. doi: 10.1111/j.1753-4887.2010.00306.x. [DOI] [PubMed] [Google Scholar]
- 5.Shin JS, Choi MY, Longtine MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta. 2010 Sep 21; doi: 10.1016/j.placenta.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D Deficiency and Insufficiency is Common during Pregnancy. Am J Perinatol. 2010 Jul 16; doi: 10.1055/s-0030-1262505. [DOI] [PubMed] [Google Scholar]
- 7.Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol. 2010 Oct;203(4):366, e361–366. doi: 10.1016/j.ajog.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007 Sep;92(9):3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haugen M, Brantsaeter AL, Trogstad L, et al. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology. 2009 Sep;20(5):720–726. doi: 10.1097/EDE.0b013e3181a70f08. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg RL, Rouse DJ. Prevention of premature birth. N Engl J Med. 1998 Jul 30;339(5):313–320. doi: 10.1056/NEJM199807303390506. [DOI] [PubMed] [Google Scholar]
- 11.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001 Nov 24;323(7323):1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 13.ACOG practice bulletin Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002 Jan;99(1):159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 14.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996 Feb;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 15.Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010 Aug;116(2 Pt 1):402–414. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- 16.Satterfield MC, Bazer FW, Spencer TE, Wu G. Sildenafil citrate treatment enhances amino acid availability in the conceptus and fetal growth in an ovine model of intrauterine growth restriction. J Nutr. 2010 Feb;140(2):251–258. doi: 10.3945/jn.109.114678. [DOI] [PubMed] [Google Scholar]
- 17.Vardavas CI, Chatzi L, Patelarou E, et al. Smoking and smoking cessation during early pregnancy and its effect on adverse pregnancy outcomes and fetal growth. Eur J Pediatr. 2010 Jun;169(6):741–748. doi: 10.1007/s00431-009-1107-9. [DOI] [PubMed] [Google Scholar]
- 18.Fischer D, Schroer A, Ludders D, et al. Metabolism of vitamin D3 in the placental tissue of normal and preeclampsia complicated pregnancies and premature births. Clin Exp Obstet Gynecol. 2007;34(2):80–84. [PubMed] [Google Scholar]
- 19.Diaz L, Noyola-Martinez N, Barrera D, et al. Calcitriol inhibits TNF-alpha-induced inflammatory cytokines in human trophoblasts. J Reprod Immunol. 2009 Jul;81(1):17–24. doi: 10.1016/j.jri.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Novakovic B, Sibson M, Ng HK, et al. Placenta-specific methylation of the vitamin D 24-hydroxylase gene: implications for feedback autoregulation of active vitamin D levels at the fetomaternal interface. J Biol Chem. 2009 May 29;284(22):14838–14848. doi: 10.1074/jbc.M809542200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halhali A, Tovar AR, Torres N, Bourges H, Garabedian M, Larrea F. Preeclampsia is associated with low circulating levels of insulin-like growth factor I and 1,25-dihydroxyvitamin D in maternal and umbilical cord compartments. J Clin Endocrinol Metab. 2000 May;85(5):1828–1833. doi: 10.1210/jcem.85.5.6528. [DOI] [PubMed] [Google Scholar]
- 22.Diaz L, Arranz C, Avila E, Halhali A, Vilchis F, Larrea F. Expression and activity of 25-hydroxyvitamin D-1 alpha-hydroxylase are restricted in cultures of human syncytiotrophoblast cells from preeclamptic pregnancies. J Clin Endocrinol Metab. 2002 Aug;87(8):3876–3882. doi: 10.1210/jcem.87.8.8730. [DOI] [PubMed] [Google Scholar]
- 23.Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004 Jun;65(6):2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 24.Cardus A, Parisi E, Gallego C, Aldea M, Fernandez E, Valdivielso JM. 1,25-Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF-mediated pathway. Kidney Int. 2006 Apr;69(8):1377–1384. doi: 10.1038/sj.ki.5000304. [DOI] [PubMed] [Google Scholar]
- 25.Cardus A, Panizo S, Encinas M, et al. 1,25-dihydroxyvitamin D3 regulates VEGF production through a vitamin D response element in the VEGF promoter. Atherosclerosis. 2009 May;204(1):85–89. doi: 10.1016/j.atherosclerosis.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Roberts JM, Gammill HS. Preeclampsia: Recent insights. Hypertension. 2005;46(6):1243–1249. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 27.Powe CE, Seely EW, Rana S, Bhan I, Ecker J, Karumanchi SA, Thadhani R. First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension. 2010;56(4):758–63. doi: 10.1161/HYPERTENSIONAHA.110.158238. [DOI] [PMC free article] [PubMed] [Google Scholar]