Abstract
A commonly reported consequence of post-treatment nausea or vomiting is the development of anticipatory nausea and vomiting (ANV). In most published work, nausea is reported to occur before chemotherapy drugs are administered by approximately 20% of patients at any one chemotherapy cycle and by 25–30% of patients by their fourth chemotherapy cycle. Most studies in adult patients strongly support the view that the development of ANV involves elements of classical conditioning. The best method to avoid development of ANV is to adequately prevent both vomiting and nausea from the first exposure to chemotherapy. If anticipatory side effects develop, behavioral treatment techniques, such as systematic desensitization, have been shown effective. Benzodiazepines used in combination with behavioral techniques or antiemetics may also be useful. The evidence on which these conclusions are based is reviewed in this article.
Keywords: Anticipatory nausea, vomiting
Introduction
Anticipatory nausea and vomiting (ANV), also referred to as conditioned, learned or psychological nausea and vomiting, is widely believed to be a learned response to chemotherapy that 25% of patients develop by the fourth treatment cycle [34, 35]. It appears to link psychological, neurological and physiological systems[8]. The risk of ANV tends to increase with the number of cycles received [30] and the symptoms may persist long after the completion of chemotherapy [20]. ANV is difficult to control by pharmacological means, whereas behavioral therapies, most notably systematic desensitization, can be used to effectively treat it.
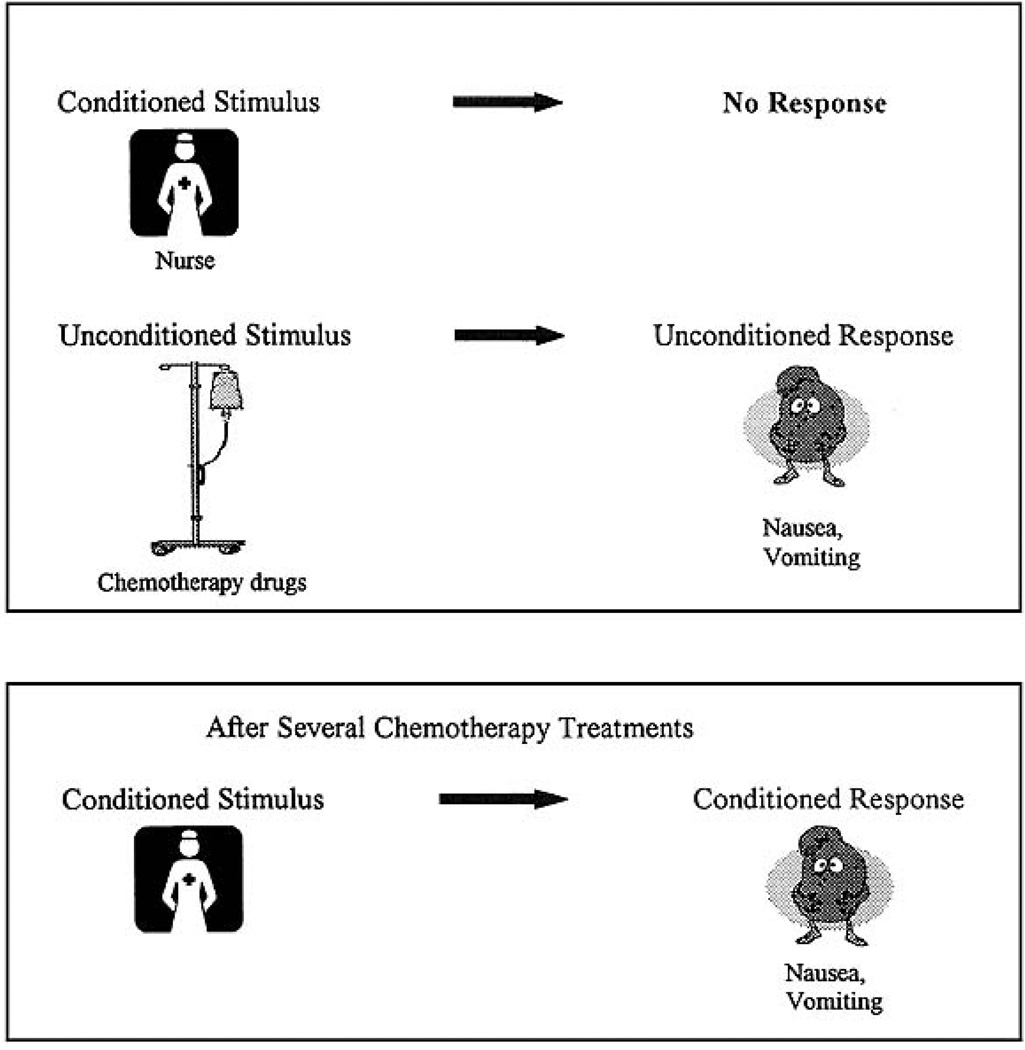
The development of ANV best fits a Pavlovian conditioning model [16, 30, 48] shown below as Figure 1. There are no data about the development, clinical course or treatment of anticipatory side effects that are at variance with this model. In this conditioning model, a conditioned stimulus (CS) (e.g., the sight of a nurse) is paired with an unconditioned stimulus (US) (e.g., chemotherapy), which reliably produces an unconditioned response (UR) (e.g., nausea). Following the conditioning period (repeated chemotherapy treatments), the CS is able, over time, to provoke a conditioned response (CR) identical to the UR. In the situation of a patient receiving chemotherapy, he/she finds him/herself at the treatment location surrounded by unfamiliar sights, sounds and smells. In addition, various psychological, cognitive and social factors are present during this experience. These stimuli become associated with the chemotherapy treatment and the subsequent NV that follow the chemotherapy infusion. After repeated chemotherapy cycles in which these stimuli are paired with the experience of subsequent nausea, they acquire the ability to trigger a response of nausea or vomiting even before the receipt of chemotherapy (i.e., ANV).
Fig 1.
Classical Conditioning of Nausea and Vomiting
Predicting ANV
Table 1 shows risk factors shown to be involved in the development of ANV. From a clinical standpoint, younger patients who have experienced severe and frequent nausea/vomiting after their prior treatments are at particularly high risk for the development of ANV. While the conditioning model is well accepted, cognitive factors, such as anxiety, self absorption, and response expectancies, can be involved in ANV development [4, 33, 35, 55, 60]. Anxiety may affect the development of NV at least in part through negative expectancies [4, 5, 22, 56], since expectancies have been shown to affect the generation of conditioning effects [24, 44, 53].
Table 1.
Risk factors for ANV
|
Hickok et al. [19] evaluated the role of patients’ expectations of nausea in the development of ANV in female cancer patients receiving their first course of chemotherapy. Of a total of 63 patients, 20 (32%) expected to experience nausea and 12 (19%) reported ANV before the third cycle. Pretreatment expectations predicted ANV at cycle three (Spearman’s r=0.41, P=0.001). Anticipatory nausea (AN) developed in 40% of patients who expected nausea; 13% of those who were uncertain whether they would develop it, and none of those who did not expect nausea. Logistic regression indicated that expecting nausea was the strongest predictor (χ2=13.15, P<0.001) of actually developing nausea.
In another study, the effects of changes in family relationships (cohesion, expression, and conflict) on patients’ physical adjustment to chemotherapy were examined. A total of 233 married cancer patients completed questionnaires consisting of measures of family relationships and chemotherapy-related nausea symptoms, at two assessments. An increase in family conflict was associated with an increased duration of PTN and greater severity of AN for younger adult patients but not for older adult patients. An increase in family conflict was also associated with a greater severity of AN for female patients but not for male patients. These findings suggest that intervention programs to help reduce family conflict and anxiety may be beneficial for younger adult and female patients [23].
Anticipatory emesis and the experimental setting
While there is no completely satisfactory laboratory model for ANV, some translational research on ANV has been conducted using a body rotation model as a nausea-inducing stimulus in humans and a conditioned gaping response in rats [18, 26] in an attempt to develop better prevention and treatment interventions in addition to preventing post-treatment nausea and vomiting. Studies using the rotation model suggest that an overshadowing procedure could be helpful in reducing the development of ANV [48]. Overshadowing is a technique whereby the subject is conditioned in an adverse experimental setting to respond to a strong stimulus, and then the stimulus is withdrawn at the next exposure to the adverse experience. Overshadowing has also been examined in a small study of cancer patients. In that study, 16 cancer patients were assigned to one of two groups: with overshadowing (OV+) and without overshadowing (OV−). At the start of all infusions of two consecutive chemotherapy cycles A and B (acquisition), OV+ subjects drank a distasteful saline beverage (the overshadowing CS), whereas group OV− drank water. All patients received water in cycle C (test). As expected, in cycle C (test), no patient of group OV+ showed AN; whereas, two patients of group OV− developed AN[50]. In the experimental animal settings examining the gaping response in rats, certain conditioning techniques, including: overshadowing [18, 47, 51], systemic treatment with lipopolysaccharide [10], tetrahydrocannabinol [38], manipulation of the endocannabinoid (EC) system [46] have been examined with inconsistent results. Tetrahydrocannabinol and cannabidiol have also been effective in reducing conditioned retching in a Suncus murinus model (musk shrew) [39]. Conditioning procedures in other animal models have been successfully used to alleviate nausea and vomiting [12, 25].
Appropriate control of acute and delayed emesis reduces ANV
One of the largest observational series evaluating ANV comprises data from 574 chemotherapy patients who received granisetron as their antiemetic treatment during repeat cycle chemotherapy. Per treatment cycle, fewer than 10% of patients displayed symptoms of AN and 2% or fewer had symptoms of anticipatory vomiting [2].
This implies that the rate of ANV is much less than observed in older studies, which used less satisfactory antiemetic programs. Two examples of conditions leading to ANV are given to illustrate the issue. One such example is a report by Wilcox et al. in the early 1980s. The authors studied 52 women treated with cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) adjuvant chemotherapy for breast carcinoma. Among the 52 patients, ANV occurred in 17 (33%), while acute and delayed emesis was experienced by 46 (88%). Of the 52 patients, 10 (19%) discontinued CMF adjuvant chemotherapy because of nausea and vomiting; 7 of the 10 (70%) had experienced anticipatory vomiting[57].
Another example of poor acute control leading to a high prevalence of ANV is a report of women receiving CMF or 5-fluorouracil/doxorubicin/cyclophosphamide (FAC). Antiemetic therapy included one corticoid plus ondansetron (in the FAC regimen), or one corticoid plus thiethylperazine (in the CMF regimen). For at least one cycle of chemotherapy, 86.1% and 91.7% patients in the FAC protocol experienced vomiting and nausea, respectively, and 11.1% had anticipatory vomiting, and 30.6% had AN. In the CMF protocol, 79.6% had post chemotherapy vomiting, and 71.7% had post chemotherapy nausea associated with at least one cycle. In this group, 7.4% had anticipatory vomiting, and 16.6% had AN. A high proportion of patients suffered anticipatory anxiety in both groups (75% in FAC, 74.1% in CMF). The stimuli most frequently associated with the appearance of anticipatory emesis were olfactory stimuli and cognitive stimuli [15].
There is some preliminary data that the relationship between ANV and post treatment nausea may be bi-directional as indicated by findings from 40 early stage breast cancer patients who developed AN. A significant correlation between the intensity of AN in the clinic prior to their treatment infusion and subsequent pos-treatment nausea during the 24 hours after the infusion was found in 40 early stage breast cancer patients who had developed AN showing that, once established, conditioned nausea may contribute to the severity of subsequent post-treatment nausea in patients receiving repeated cycles of chemotherapy for cancer [7]. It is also of interest that in adult patients, anticipatory immunomodulation (AIM) has also been observed and that some results suggest that ANV and AIM also occur in pediatric cancer patients and show features of a conditioned response [49].
Treatment of ANV
Psychological intervention and ANV
Behavioral interventions are especially appropriate to address ANV because it is a conditioned response, and they are best implemented prior to the complete/full development of the undesired conditioned response [16]. Evidence suggests that behavioral intervention can reduce ANV, decrease levels of anxiety and distress, and to a lesser extent decrease cancer-related pain and nausea [36]. The techniques have varied, including hypnosis [29, 42] and biofeedback [9], yoga [40] and many variations of relaxation methods [16]. It is of interest that even if anxiety levels of the patients are not always influenced, these techniques can control ANV [52]. As a learned phenomenon, ANV is treatable by means of behavioral approaches based on learning principles. Research on the behavioral treatment of conditioned adverse effects of chemotherapy has centered on three principal approaches: progressive muscle relaxation training (PRMT), systematic desensitization (SD), and hypnosis. PMRT appears to exert its greatest effects against adverse events that develop after administration of chemotherapy [32], although when combined with guided imagery, it has shown efficacy in reducing ANV [58].
SD is commonly used to treat learning-based difficulties, such as fears and phobias, and is particularly effective for ANV. One way in which phobias may develop is by means of the classical conditioning mechanism described previously. In many respects, anticipatory side effects display characteristics of phobic behaviors, although the match is far from perfect. SD involves the counter conditioning of a response incompatible with those stimuli that typically elicit a maladaptive reaction. In terms of ANV, the theory predicts that these symptoms would be reduced if patients could be taught an incompatible response (such as progressive muscle relaxation), rather than the conditioned response of NV, in response to the conditioned stimuli (the clinic; the nurse). This treatment has been effective in over half the patients to whom it is administered [13, 34].
Hypnosis/suggestion has been used successfully to prevent AN related to chemotherapy[29, 42] and to reduce nausea following chemotherapy [21, 45, 54, 61]. Although hypnosis was the first psychological technique used to control ANV, few controlled studies have been done. It has most often been used with children and adolescents, which may be because children are more readily hypnotized than adults [16, 27, 34, 37, 45].
Acupuncture/Acupressure
According to the NIH Consensus Development Panel (NIHCDP), acupuncture is effective for the treatment of postoperative and chemotherapy-related nausea and vomiting[1]. Several studies, including a systematic review have shown efficacy of acupuncture and acupressure in reducing chemotherapy-related nausea[11, 14, 17, 31, 43]. No studies, however, have found any definitive evidence supporting the use of acupuncture and acupressure in alleviating ANV. One potentially related study reported benefit for the use of acupuncture in treatment of “nervous vomiting” in a dental setting [59].
Benzodiazepines and ANV
Razavi et al. [41] conducted a double-blind, placebo-controlled study designed to assess the usefulness of adding low-dose alprazolam (0.5 mg to 2 mg per day) to a psychological support program including progressive relaxation training designed to prevent ANV in 57 women undergoing adjuvant chemotherapy for stage II primary breast cancer. At the second evaluation, the results showed a higher rate of AN (18% vs 0%) in the placebo compared with the alprazolam arm (P=0.038). These differences were no more significant at each of the further assessments. Significant differences were found for the intake of hypnotics at each assessment visit, with the rate of hypnotic users being significantly higher in the placebo (19%) compared with the alprazolam (0%) arm at the fourth assessment (P<0.05). The authors concluded that the adjunct of alprazolam to a psychological support program delays the occurrence of AN and controls sleeping problems secondary to adjunct chemotherapy.
Malik et al. [28] conducted a randomized trial to evaluate the efficacy of lorazepam in managing anticipatory, acute, and delayed emesis induced by high doses of cisplatin. A total of 180 events involving cisplatin administration (100 mg/m2 as a 24-h continuous infusion) were randomized to receive metoclopramide along with dexamethasone and clemastine with or without lorazepam. Lorazepam significantly reduced the incidence of AN and vomiting (P<0.05) as well as acute emesis (P=0.05) induced by cisplatin. Mild sedation and amnesia were significantly more common in patients receiving lorazepam (P<0.001). The authors concluded that lorazepam increases the efficacy of metoclopramide against cisplatin-induced anticipatory, acute, and delayed nausea and vomiting.
Conclusions
This review updates work published in 2005.[3] In 1998 and again in 2005, the Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC) stated [3, 6] that the best treatment for anticipatory emesis is the control of acute and delayed emesis so that ANV does not develop. Based on the above review of the literature, the 2009 panel reaffirms that earlier recommendations add the adjunctive suggestions shown in Table 2. Unfortunately, the use of behavioral interventions will remain difficult to implement, as most patients are treated in settings where the needed expertise is not available.
Table 2.
Guideline for managing anticipatory nausea and vomiting in patients receiving chemotherapy or radiation therapy
| Anticipatory nausea and vomiting should be managed by psychological techniques. |
| MASCC level of confidence: High |
| MASCC level of consensus: High |
| Use of benzodiazepines may be useful in preventing the development of ANV when used in conjunction with antiemetics (no new data since 2003) |
| MASCC level of confidence: Moderate |
| MASCC level of consensus: High |
Reference List
- 1.NIH Consensus Conference. Acupuncture. JAMA. 1998;280:1518–1524. [PubMed] [Google Scholar]
- 2.Aapro MS, Kirchner V, Terrey JP. The incidence of anticipatory nausea and vomiting after repeat cycle chemotherapy: The effect of granisetron. Br J Cancer. 1994;69:957–960. doi: 10.1038/bjc.1994.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aapro MS, Molassiotis A, Olver I. Anticipatory nausea and vomiting. Support Care Cancer. 2005;13:117–121. doi: 10.1007/s00520-004-0745-8. [DOI] [PubMed] [Google Scholar]
- 4.Andrykowski MA. The role of anxiety in the development of anticipatory nausea in cancer chemotherapy: A review and synthesis. Psychosom Med. 1990;52:458–475. doi: 10.1097/00006842-199007000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Andrykowski MA, Redd WH, Hatfield AK. Development of anticipatory nausea: a prospective analysis. J Consult Clin Psychol. 1985;53:447–454. doi: 10.1037//0022-006x.53.4.447. [DOI] [PubMed] [Google Scholar]
- 6.Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer. Prevention of chemotherapy- and radiotherapy-induced emesis: Results of Perugia Consensus Conference. Ann Oncol. 1998;9:811–819. [PubMed] [Google Scholar]
- 7.Bovbjerg DH. The continuing problem of post chemotherapy nausea and vomiting: contributions of classical conditioning. Auton Neurosci. 2006;129:92–98. doi: 10.1016/j.autneu.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Burish TG, Carey MP. Conditioned aversive responses in cancer chemotherapy patients: Theoretical and developmental analysis. J Consult Clin Psychol. 1986;54:593–600. doi: 10.1037//0022-006x.54.5.593. [DOI] [PubMed] [Google Scholar]
- 9.Burish TG, Shartner CD, Lyles JN. Effectiveness of multiple muscle-site EMG biofeedback and relaxation training in reducing the aversiveness of cancer chemotherapy. Biofeedback Self Regul. 1981;6:523–535. doi: 10.1007/BF00998737. [DOI] [PubMed] [Google Scholar]
- 10.Chan MY, Cross-Mellor SK, Kavaliers M, Ossenkopp KP. Lipopolysaccharide (LPS) blocks the acquisition of LiCl-induced gaping in a rodent model of anticipatory nausea. Neurosci Lett. 2009;450:301–305. doi: 10.1016/j.neulet.2008.11.052. [DOI] [PubMed] [Google Scholar]
- 11.Collins KB, Thomas DJ, Collins KB, Thomas DJ. Acupuncture and acupressure for the management of chemotherapy-induced nausea and vomiting. Journal of the American Academy of Nurse Practitioners. 2004;16:76–80. doi: 10.1111/j.1745-7599.2004.tb00376.x. [Review] [29 refs] [DOI] [PubMed] [Google Scholar]
- 12.Davey VA, Biederman GB. Conditioned antisickness: indirect evidence from rats and direct evidence from ferrets that conditioning alleviates drug-induced nausea and emesis. J Exp Psychol Anim Behav Process. 1998;24:483–491. [PubMed] [Google Scholar]
- 13.Elam CL, Andrykowski MA. Admission interview ratings: Relationship to applicant academic and demographic variables and interviewer characteristics. Acad Med. 1991;66:S13–S15. [PubMed] [Google Scholar]
- 14.Ezzo J, Vickers A, Richardson MA, Allen C, Dibble SL, Issell B, Lao L, Pearl M, Ramirez G, Roscoe JA, Shen J, Shivnan J, Streitberger K, Treish I, Zhang G. Acupuncture-point stimulation for chemotherapy-induced nausea and vomiting. J Clin Oncol. 2005;23:7188–7198. doi: 10.1200/JCO.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Marcos A, Martin M, Sanchez JJ, Rodriguez-Lescure A, Casado, Lopez MJ, Diaz-Rubio E. Acute and anticipatory emesis in breast cancer patients. Support Care Cancer. 1996;4:370–377. doi: 10.1007/BF01788844. [DOI] [PubMed] [Google Scholar]
- 16.Figueroa-Moseley C, Jean-Pierre P, Roscoe JA, Ryan JL, Kohli S, Palesh OG, Ryan EP, Carroll J, Morrow GR. Behavioral interventions in treating anticipatory nausea and vomiting. Journal of the National Comprehensive Cancer Network. 2007;5:44–50. doi: 10.6004/jnccn.2007.0006. [DOI] [PubMed] [Google Scholar]
- 17.Gardani G, Cerrone R, Biella C, Galbiati G, Proserpio E, Casiraghi M, Arnoffi J, Meregalli M, Trabattoni P, Dapretto E, Giani L, Messina G, Lissoni P. A progress study of 100 cancer patients treated by acupressure for chemotherapy-induced vomiting after failure with the pharmacological approach. Minerva Med. 2007;98:665–668. [PubMed] [Google Scholar]
- 18.Hall G, Symonds M. Overshadowing and latent inhibition of context aversion conditioning in the rat. Auton Neurosci. 2006;129:42–49. doi: 10.1016/j.autneu.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Hickok JT, Roscoe JA, Morrow GR. The role of patients' expectations in the development of anticipatory nausea related to chemotherapy for cancer. J Pain Symptom Manage. 2001;22:843–850. doi: 10.1016/s0885-3924(01)00317-7. [DOI] [PubMed] [Google Scholar]
- 20.Hursti T, Fredikson M, et al. Association between personality characteristics and the prevalence and extinction of conditioned nausea after chemotherapy. J Psychosoc Oncol. 1992;10:59–77. [Google Scholar]
- 21.Jacknow DS, Tschann JM, Link MP, Boyce WT. Hypnosis in the prevention of chemotherapy-related nausea and vomiting in children: a prospective study. Journal of Developmental & Behavioral Pediatrics. 1994;15:258–264. [PubMed] [Google Scholar]
- 22.Jacobsen PB, Andrykowski MA, Redd WH, Die-Trill M, Hakes TB, Kaufman RJ, Currie VE, Holland JC. Nonpharmacologic factors in the development of posttreatment nausea with adjuvant chemotherapy for breast cancer. Cancer. 1988;61:379–385. doi: 10.1002/1097-0142(19880115)61:2<379::aid-cncr2820610230>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Morrow GR. Changes in family relationships affect the development of chemotherapy-related nausea symptoms. Support Care Cancer. 2003;11:171–177. doi: 10.1007/s00520-002-0416-6. [DOI] [PubMed] [Google Scholar]
- 24.Kirsch I. Specifying nonspecifics: Psychological mechanisms of placebo effects. In: Harrington A, editor. The placebo effect: An interdisciplinary exploration. Cambridge, MA: Harvard University Press; 1997. pp. 166–186. [Google Scholar]
- 25.Lett BT. Pavlovian drug-sickness pairings result in the conditioning of an antisickness response. Behav Neurosci. 1983;97:779–784. doi: 10.1037//0735-7044.97.5.779. [DOI] [PubMed] [Google Scholar]
- 26.Limebeer CL, Krohn JP, Cross-Mellor S, Litt DE, Ossenkopp KP, Parker LA. Exposure to a context previously associated with nausea elicits conditioned gaping in rats: a model of anticipatory nausea. Behav Brain Res. 2008;187:33–40. doi: 10.1016/j.bbr.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Mackenzie A, Frawley GP. Preoperative hypnotherapy in the management of a child with anticipatory nausea and vomiting. Anaesth Intensive Care. 2007;35:784–787. doi: 10.1177/0310057X0703500522. [DOI] [PubMed] [Google Scholar]
- 28.Malik IA, Khan WA, Qazilbash M, Ata E, Butt A, Khan MA. Clinical efficacy of lorazepam in prophylaxis of anticipatory, acute, and delayed nausea and vomiting induced by high doses of cisplatin. A prospective randomized trial. Am J Clin Oncol. 1995;18:170–175. doi: 10.1097/00000421-199504000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Marchioro G, Azzarello G, Viviani F, Barbato F, Pavanetto M, Rosetti F, Pappagallo GL, Vinante O. Hypnosis in the treatment of anticipatory nausea and vomiting in patients receiving cancer chemotherapy. Oncology (Huntingt) 2000;59:100–104. doi: 10.1159/000012144. [DOI] [PubMed] [Google Scholar]
- 30.Matteson S, Roscoe JA, Hickok J, Morrow GR. The role of behavioral conditioning in the development of nausea. Am J Obstet Gynecol. 2002;185:S239–S243. doi: 10.1067/mob.2002.122597. [DOI] [PubMed] [Google Scholar]
- 31.Molassiotis A, Helin AM, Dabbour R, Hummerston S. The effects of P6 acupressure in the prophylaxis of chemotherapy-related nausea and vomiting in breast cancer patients. Complementary Therapies in Medicine. 2007;15:3–12. doi: 10.1016/j.ctim.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Molassiotis A, Yung HP, Yam BMC, Chan FYS, Mok TS. The effectiveness of progressive muscle relaxation training in managing chemotherapy-induced nausea and vomiting in Chinese breast cancer patients: A randomized controlled trial. Support Care Cancer. 2002;10:237–246. doi: 10.1007/s00520-001-0329-9. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery GH, Tomoyasu N, Bovbjerg DH, Andrykowski MA, Currie VE, Jacobsen PB, Redd WH. Patients' pretreatment expectations of chemotherapy-related nausea are an independent predictor of anticipatory nausea. Annals of Behavioral Medicine. 1998;20:104–109. doi: 10.1007/BF02884456. [DOI] [PubMed] [Google Scholar]
- 34.Morrow GR, Roscoe JA. Anticipatory nausea and vomiting: Models, mechanisms and management. In: Dicato M, editor. Medical management of cancer treatment induced emesis. London: Martin Dunitz; 1997. pp. 149–166. [Google Scholar]
- 35.Morrow GR, Roscoe JA, Kirshner JJ, Hynes HE, Rosenbluth RJ. Anticipatory nausea and vomiting in the era of 5-HT3 antiemetics. Support Care Cancer. 1998;6:244–247. doi: 10.1007/s005200050161. [DOI] [PubMed] [Google Scholar]
- 36.Mundy EA, DuHamel KN, Montgomery GH. The efficacy of behavioral interventions for cancer treatment-related side effects. Semin Clin Neuropsychiatry. 2003;8:253–275. [PubMed] [Google Scholar]
- 37.Olness K. Imagery (self-hypnosis) as adjunct therapy in childhood cancer: Clinical experience with 25 patients. Am J Pediatr Hematol /Oncol. 1981;3 313321. [PubMed] [Google Scholar]
- 38.Parker LA, Kemp SW. Tetrahydrocannabinol (THC) interferes with conditioned retching in Suncus murinus: an animal model of anticipatory nausea and vomiting (ANV) Neuroreport. 2001;12:749–751. doi: 10.1097/00001756-200103260-00027. [DOI] [PubMed] [Google Scholar]
- 39.Parker LA, Kwiatkowska M, Mechoulam R. Delta-9-tetrahydrocannabinol and cannabidiol, but not ondansetron, interfere with conditioned retching reactions elicited by a lithium-paired context in Suncus murinus: An animal model of anticipatory nausea and vomiting. Physiol Behav. 2006;87:66–71. doi: 10.1016/j.physbeh.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 40.Raghavendra RM, Nagarathna R, Nagendra HR, Gopinath KS, Srinath BS, Ravi BD, Patil S, Ramesh BS, Nalini R. Effects of an integrated yoga programme on chemotherapy-induced nausea and emesis in breast cancer patients. Eur J Cancer Care (Engl) 2007;16:462–474. doi: 10.1111/j.1365-2354.2006.00739.x. [DOI] [PubMed] [Google Scholar]
- 41.Razavi D, Delvaux N, Farvacques C, De Brier F, Van Heer C, Kaufman L, Derde M-P, Piccart M. Prevention of adjustment disorders and anticipatory nausea secondary to adjuvant chemotherapy: A double-blind, placebo- controlled study assessing the usefulness of alprazolam. J Clin Oncol. 1993;11:1384–1390. doi: 10.1200/JCO.1993.11.7.1384. [DOI] [PubMed] [Google Scholar]
- 42.Redd WH, Andresen GV, Minagawa RY. Hypnotic control of anticipatory emesis in patients receiving cancer chemotherapy. J Consult Clin Psychol. 1982;50:14–19. doi: 10.1037//0022-006x.50.1.14. [DOI] [PubMed] [Google Scholar]
- 43.Reindl TK, Geilen W, Hartmann R, Wiebelitz KR, Kan G, Wilhelm I, Lugauer S, Behrens C, Weiberlenn T, Hasan C, Gottschling S, Wild-Bergner T, Henze G, Driever PH. Acupuncture against chemotherapy-induced nausea and vomiting in pediatric oncology. Interim results of a multicenter crossover study. Support Care Cancer. 2006;14:172–176. doi: 10.1007/s00520-005-0846-z. [DOI] [PubMed] [Google Scholar]
- 44.Rescorla RA. Pavlovian Conditioning: It's not what you think. Am Psychol. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- 45.Richardson J, Smith JE, McCall G, Richardson A, Pilkington K, Kirsch I. Hypnosis for nausea and vomiting in cancer chemotherapy: A systematic review of the research evidence. Eur J Cancer Care (Engl) 2007;16:402–412. doi: 10.1111/j.1365-2354.2006.00736.x. [DOI] [PubMed] [Google Scholar]
- 46.Rock EM, Limebeer CL, Mechoulam R, Piomelli D, Parker LA. The effect of cannabidiol and URB597 on conditioned gaping (a model of nausea) elicited by a lithium-paired context in the rat. Psychopharmacology (Berl) 2008;196:389–395. doi: 10.1007/s00213-007-0970-1. [DOI] [PubMed] [Google Scholar]
- 47.Sansa J, Artigas AA, Prados J. Overshadowing and potentiation of illness-based contextconditioning. Psicológica. 2007;28:193–214. [Google Scholar]
- 48.Stockhorst U, Enck P, Klosterhalfen S. Role of classical conditioning in learning gastrointestinal symptoms. World J Gastroenterol. 2007;13:3430–3437. doi: 10.3748/wjg.v13.i25.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stockhorst U, Spennes-Saleh S, Korholz D, Gobel U, Schneider ME, Steingruber HJ, Klosterhalfen S. Anticipatory symptoms and anticipatory immune responses in pediatric cancer patients receiving chemotherapy: features of a classically conditioned response? Brain, Behavior, & Immunity. 2000;14(3):198–218. doi: 10.1006/brbi.1999.0581. [DOI] [PubMed] [Google Scholar]
- 50.Stockhorst U, Wiener JA, Klosterhalfen S, Klosterhalfen W, Aul C, Steingruber HJ. Effects of overshadowing on conditioned nausea in cancer patients: an experimental study. Physiol Behav. 1998;64:743–753. doi: 10.1016/s0031-9384(98)00135-8. [DOI] [PubMed] [Google Scholar]
- 51.Symonds M, Hall G. Overshadowing not potentiation of illness-based contextual conditioning by a novel taste. Anim Learn Behav. 1999;27:379–390. [Google Scholar]
- 52.Vasterling J, Jenkins RA, Tope DM, Burish TG. Cognitive distraction and relaxation training for the control of side effects due to cancer chemotherapy. J Behav Med. 1993;16:65–80. doi: 10.1007/BF00844755. [DOI] [PubMed] [Google Scholar]
- 53.Voudouris NJ, Peck CL, Coleman G. The role of conditioning and verbal expectancy in the placebo response. Pain. 1990;43:121–128. doi: 10.1016/0304-3959(90)90057-K. [DOI] [PubMed] [Google Scholar]
- 54.Walker LG, Dawson AA, Pollet SM, Ratcliffe MA, Hamilton L. Hypnotherapy for chemotherapy side effects. British Journal of Experimental and Clinical Hypnosis. 1988;5:79–82. [Google Scholar]
- 55.Watson M. Anticipatory nausea and vomiting: broadening the scope of psychological treatments. Support Care Cancer. 1993;1:171–177. doi: 10.1007/BF00366442. [DOI] [PubMed] [Google Scholar]
- 56.Watson M, McCarron J, Law M. Anticipatory nausea and emesis, and psychological morbidity: Assessment of prevalence among out-patients on mild to moderate chemotherapy regimens. Br J Cancer. 1992;66:862–866. doi: 10.1038/bjc.1992.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilcox PM, Fetting JH, Nettesheim KM, Abeloff MD. Anticipatory vomiting in women receiving cyclophosphamide, methotrexate, and 5-FU (CMF) adjuvant chemotherapy for breast carcinoma. Cancer Treat Rep. 1982;66:1601–1604. [PubMed] [Google Scholar]
- 58.Yoo HJ, Ahn SH, Kim SB, Kim WK, Han OS. Efficacy of progressive muscle relaxation training and guided imagery in reducing chemotherapy side effects in patients with breast cancer and in improving their quality of life. Support Care Cancer. 2005;13:826–833. doi: 10.1007/s00520-005-0806-7. [DOI] [PubMed] [Google Scholar]
- 59.Yugin L, Yugin L. Treating patients with nervous vomiting in the dental office by point-stimulating therapy. Special Care in Dentistry. 1989;9:27–28. doi: 10.1111/j.1754-4505.1989.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 60.Zachariae R, Paulsen K, Mehlsen M, Jensen AB, Johansson A, von der MH. Anticipatory nausea: the role of individual differences related to sensory perception and autonomic reactivity. Ann Behav Med. 2007;33:69–79. doi: 10.1207/s15324796abm3301_8. [DOI] [PubMed] [Google Scholar]
- 61.Zeltzer LK, Dolgin MJ, LeBaron S, LeBaron C. A randomized, controlled study of behavioral intervention for chemotherapy distress in children with cancer. Pediatrics. 1991;88:34–42. [PubMed] [Google Scholar]