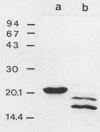
Abstract
Binding characteristics of the two major fimbrial hemagglutinin types of uropathogenic Proteus mirabilis were determined in frozen sections of human kidney and in exfoliated uroepithelial cells. P. mirabilis 3087, which expresses the MR/P fimbriae, adhered avidly to the tubular epithelial cells of the kidney and also to the epithelial cells of urinary sediment. No adhesion to glomerular or peritubular elements of the kidney was detected. Indirect immunogold silver staining also showed that the purified MR/P fimbriae recognized the same kidney domains. Adhesion of strain 3087 to uroepithelial cells was completely inhibited by Fab fragments of antibodies against the purified MR/P fimbriae. A completely different tissue-binding pattern was exhibited by the MR/K fimbriae of P. mirabilis 2456. In the kidney, the MR/K fimbriae bound strongly to the Bowman's capsule of the glomeruli and to the tubular basement membranes. A weak binding to glomerular mesangium and tubular epithelial cells was also seen. Strain 2456 did not adhere to epithelial cells of urinary sediment. Analysis of normal human urine showed that it contains low-molecular-weight molecules capable of inhibiting the binding of the MR/P fimbriae; no urinary inhibitors could be detected for the MR/K fimbriae. Poor in vivo binding capacity to intact human uroepithelial cells may be an important factor in explaining the relatively low pathogenicity of P. mirabilis in healthy hosts.
Full text
PDF

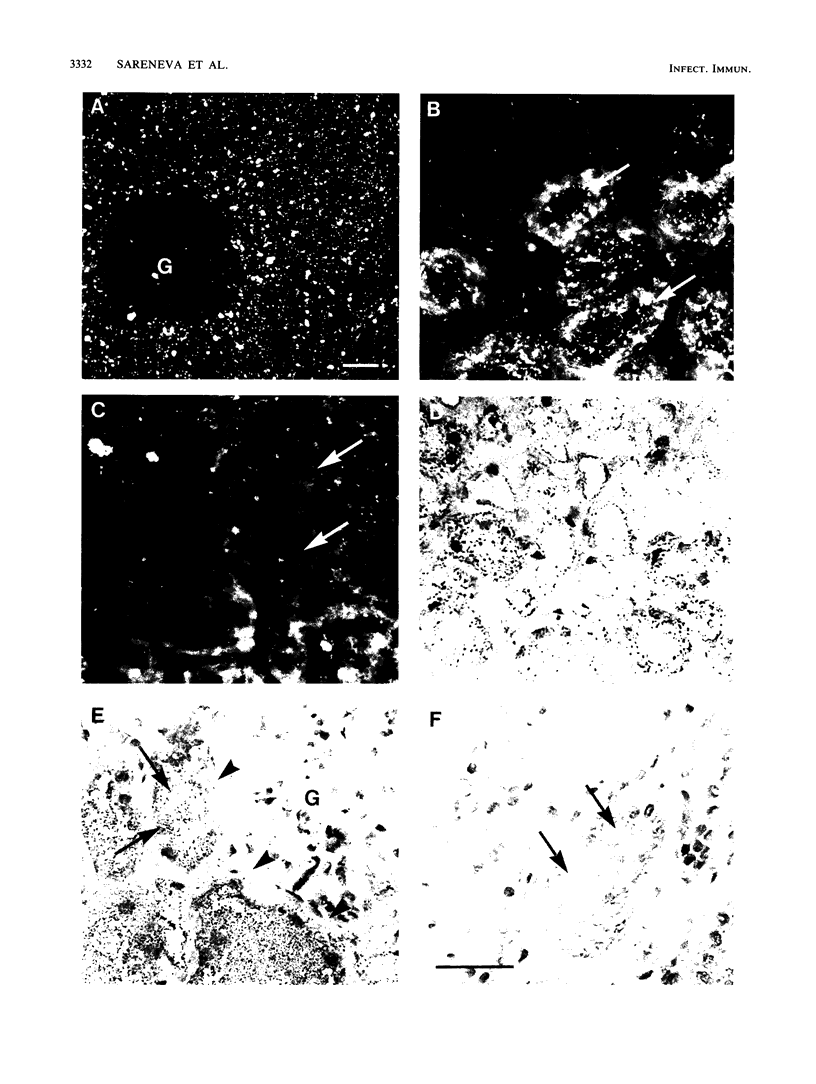




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adegbola R. A., Old D. C., Senior B. W. The adhesins and fimbriae of Proteus mirabilis strains associated with high and low affinity for the urinary tract. J Med Microbiol. 1983 Nov;16(4):427–431. doi: 10.1099/00222615-16-4-427. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P. Fimbriae and adhesive properties in Klebsiella strains. J Gen Microbiol. 1959 Aug;21:271–286. doi: 10.1099/00221287-21-1-271. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hanson L. A., Jodal U., Lindberg U., Akerlund A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet. 1976 Sep 4;1(7984):490–492. [PubMed] [Google Scholar]
- Edén C. S., Larsson P., Lomberg H. Attachment of Proteus mirabilis to human urinary sediment epithelial cells in vitro is different from that of Escherichia coli. Infect Immun. 1980 Mar;27(3):804–807. doi: 10.1128/iai.27.3.804-807.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach G. F., Clegg S., Allen B. L. Identification and characterization of the genes encoding the type 3 and type 1 fimbrial adhesins of Klebsiella pneumoniae. J Bacteriol. 1989 Mar;171(3):1262–1270. doi: 10.1128/jb.171.3.1262-1270.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976 Mar;13(5):346–350. [PubMed] [Google Scholar]
- Hull S., Clegg S., Sanborg Eden C., Hull R. Multiple forms of genes in pyelonephritogenic Escherichia coli encoding adhesins binding globoseries glycolipid receptors. Infect Immun. 1985 Jan;47(1):80–83. doi: 10.1128/iai.47.1.80-83.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. New Method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980 Feb;27(2):569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Parkkinen J., Hacker J., Finne J., Pere A., Rhen M., Holthöfer H. Binding of Escherichia coli S fimbriae to human kidney epithelium. Infect Immun. 1986 Nov;54(2):322–327. doi: 10.1128/iai.54.2.322-327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Virkola R., Westurlund B., Holthöfer H., Parkkinen J. Tissue tropism of Escherichia coli adhesins in human extraintestinal infections. Curr Top Microbiol Immunol. 1990;151:115–127. doi: 10.1007/978-3-642-74703-8_6. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen-Rhen V., Rhen M., Pere A., Parkkinen J., Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984 Aug;159(2):762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsson P., Fasth A., Jodal U., Akerlund A. S., Edén C. S. Urinary tract infections caused by Proteus mirabilis in children. The antibody response to O and H antigens and Tamm-Horsfall protein and bacterial adherence to uro-epithelium. Acta Paediatr Scand. 1978 Sep;67(5):591–596. doi: 10.1111/j.1651-2227.1978.tb17807.x. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R., Tenney J. H., Mayrer A. R., Crisp L. J., Penner J. L., Warren J. W. MR/K hemagglutination of Providencia stuartii correlates with adherence to catheters and with persistence in catheter-associated bacteriuria. J Infect Dis. 1988 Feb;157(2):264–271. doi: 10.1093/infdis/157.2.264. [DOI] [PubMed] [Google Scholar]
- Nowicki B., Holthöfer H., Saraneva T., Rhen M., Väisänen-Rhen V., Korhonen T. K. Location of adhesion sites for P-fimbriated and for 075X-positive Escherichia coli in the human kidney. Microb Pathog. 1986 Apr;1(2):169–180. doi: 10.1016/0882-4010(86)90019-7. [DOI] [PubMed] [Google Scholar]
- Nowicki B., Moulds J., Hull R., Hull S. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect Immun. 1988 May;56(5):1057–1060. doi: 10.1128/iai.56.5.1057-1060.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Adegbola R. A. Haemagglutinins and fimbriae of Morganella, Proteus and Providencia. J Med Microbiol. 1982 Nov;15(4):551–564. doi: 10.1099/00222615-15-4-551. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Finne J. Isolation and structural characterization of five major sialyloligosaccharides and a sialylglycopeptide from normal human urine. Eur J Biochem. 1983 Nov 2;136(2):355–361. doi: 10.1111/j.1432-1033.1983.tb07749.x. [DOI] [PubMed] [Google Scholar]
- Pazin G. J., Braude A. I. Immobilizing antibodies in urine. II. Prevention of ascending spread of Proteus mirabilis. Invest Urol. 1974 Sep;12(2):129–133. [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Investigation of the haemolytic activity of Proteus mirabilis strains. Antonie Van Leeuwenhoek. 1983 Apr;49(1):1–11. doi: 10.1007/BF00457874. [DOI] [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Vero cell invasiveness of Proteus mirabilis. Infect Immun. 1984 Mar;43(3):1068–1071. doi: 10.1128/iai.43.3.1068-1071.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pere A., Väisänen-Rhen V., Rhen M., Tenhunen J., Korhonen T. K. Analysis of P fimbriae on Escherichia coli O2, O4, and O6 strains by immunoprecipitation. Infect Immun. 1986 Feb;51(2):618–625. doi: 10.1128/iai.51.2.618-625.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoia D., Martinetto P., Achino A., Pugliese A. Adhesion of Proteus species to various cell types. Eur J Clin Microbiol. 1983 Dec;2(6):571–576. doi: 10.1007/BF02016568. [DOI] [PubMed] [Google Scholar]
- Senior B. W. The special affinity of particular types of Proteus mirabilis for the urinary tract. J Med Microbiol. 1979 Feb;12(1):1–8. doi: 10.1099/00222615-12-1-1. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Ofek I. Influence of pili on the virulence of Proteus mirabilis in experimental hematogenous pyelonephritis. J Infect Dis. 1978 Nov;138(5):664–667. doi: 10.1093/infdis/138.5.664. [DOI] [PubMed] [Google Scholar]
- TAMM I., HORSFALL F. L., Jr A mucoprotein derived from human urine which reacts with influenza, mumps, and Newcastle disease viruses. J Exp Med. 1952 Jan;95(1):71–97. doi: 10.1084/jem.95.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaschoff E. Die Okologie und Bedeutung der Proteusgruppe. Klin Wochenschr. 1969 Aug 15;47(16):837–844. doi: 10.1007/BF01879914. [DOI] [PubMed] [Google Scholar]
- Virkola R., Westerlund B., Holthöfer H., Parkkinen J., Kekomäki M., Korhonen T. K. Binding characteristics of Escherichia coli adhesins in human urinary bladder. Infect Immun. 1988 Oct;56(10):2615–2622. doi: 10.1128/iai.56.10.2615-2622.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund B., Kuusela P., Risteli J., Risteli L., Vartio T., Rauvala H., Virkola R., Korhonen T. K. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol Microbiol. 1989 Mar;3(3):329–337. doi: 10.1111/j.1365-2958.1989.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Wray S. K., Hull S. I., Cook R. G., Barrish J., Hull R. A. Identification and characterization of a uroepithelial cell adhesin from a uropathogenic isolate of Proteus mirabilis. Infect Immun. 1986 Oct;54(1):43–49. doi: 10.1128/iai.54.1.43-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]