Abstract
Background
The LIFE study is a two-phase randomized clinical trial comparing two approaches to maintaining weight loss following guided weight loss. Phase I provided a nonrandomized intensive 6-month behavioral weight loss intervention to 472 obese (BMI 30–50) adult participants. Phase II is the randomized weight-loss maintenance portion of the study. This paper focuses on Phase I measures of sleep, screen time, depression, and stress.
Methods
The Phase I intervention consisted of 22 group sessions led over 26 weeks by behavioral counselors. Recommendations included reducing dietary intake by 500 calories per day, adopting the DASH dietary pattern, and increasing physical exercise to at least 180 minutes per week. Measures reported here are sleep time, insomnia, screen time, depression, and stress at entry and post weight loss intervention follow up.
Results
The mean weight loss for all participants over the intensive Phase I weight loss intervention was 6.3 kg (SD 7.1). Sixty percent (N=285) of participants lost at least 4.5 kg (10 lbs) and were randomized into Phase II. Participants (N=472) attended a mean of 73.1 % (SD 26.7) of sessions, completed 5.1 (SD 1.9) daily food records/week, and reported 195.1 (SD 123.1) minutes of exercise per week. Using logistic regression, sleep time (quadratic trend, p=.030) and lower stress (p=.024) at entry predicted success in the weight loss program, and lower baseline stress predicted greater weight loss during Phase I (p=.021). In addition, weight loss was significantly correlated with declines in stress (p=.048) and depression (p=.035).
Conclusion
Results suggest that clinicians and investigators might consider targeting sleep, depression, and stress as part of a behavioral weight loss intervention.
Keywords: Obesity, Weight Loss, Stress, Depression, Sleep
INTRODUCTION
Two thirds of U.S. adults are either obese (body mass index [BMI] at or above 30) or overweight (BMI of 25.0 – 29.9). (1, 2)The reasons for the obesity epidemic seem to be multi-factorial, involving an interaction of genetic, environmental, and lifestyle factors.(3) Among lifestyle factors, disordered sleep patterns(4, 5) have been identified as one likely risk factor for obesity. A growing number of experimental studies in adults have observed an inverse relationship between sleep duration and mediators of weight gain. Possible hormonal mechanisms include decreased levels of leptin and increased levels of ghrelin. Leptin and ghrelin are positively associated with satiety and hunger, respectively.(6, 7)
The positive association between screen time and obesity in children is well known,(8–10) and recent studies have found a similar positive association in adults. Pettee(11) and colleagues found television-watching hours to be positively associated with BMI and percentage body fat, and negatively associated with cardiorespiratory fitness and moderate-to-high intensity physical activity. Similarly, individuals using computers for 11 or more hours per week were at higher risk for obesity(12). Screen time has also been positively associated with weight regain following substantial weight loss.(13)
In addition, both depression and stress have been associated with obesity. Cross-sectional and prospective studies have shown a significant positive association between obesity and depression(14–17) with a recent meta-analysis(18) suggesting a bidirectional relationship; obesity was found to increase risk for depression and depression was predictive of developing obesity. Similarly, both human and animal studies suggest that chronic stress increases the intake of energy- and nutrient-dense foods and consequently the development of visceral obesity.(19–21) Two recent reviews suggest that chronic stress activates the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS), resulting in the accumulation of visceral fat and related health problems (e.g., type 2 diabetes, cardiometabolic complications).(22, 23)
Previous studies have found strong associations between weight loss and intervention-session attendance and adherence to exercise and food diary recommendations (24). Little is known, however, about associations between sleep and screen time in the context of weight-loss intervention programs. This paper focuses on these behaviors in the context of an intensive weight-loss intervention for adults.
Our analyses addressed the following questions:
Do measures of sleep time, insomnia, and screen time at entry predict success in a weight loss program? Do measures of depression and stress add to the predictive model?
Do changes in sleep time, insomnia, screen time, depression, and stress correlate with weight loss?
Do associations between weight loss, session attendance, exercise minutes, and food records in our study population replicate those previously documented in the literature?
Methods and Procedures
Study Design
The LIFE study is an investigator-initiated trial sponsored by the National Center for Complementary and Alternative Medicine (NCCAM). The study was reviewed and approved by Kaiser Permanente Northwest's IRB, and all participants provided written informed consent. A complete description of the rationale, design, and methods for the LIFE study has been published elsewhere.(25)
Briefly, the LIFE study is a two-phase trial examining two alternative strategies for maintaining weight loss. Phase I provided a nonrandomized, 6-month intensive behavioral weight loss intervention to 472 adult participants. This intervention program included recommendations to reduce dietary intake by 500 calories per day, adopt an eating pattern consistent with the DASH dietary pattern,(26) and increase moderate intensity physical exercise to at least 180 minutes per week. The DASH diet emphasizes fruits, vegetables, and low-fat dairy products, includes whole grains, poultry, fish, and nuts, and is reduced in fats, red meat, sweets, and sugar-containing beverages. As such, the DASH diet is reduced in total fat, saturated fat, and cholesterol, and increased in potassium, calcium, magnesium, fiber, and protein
Participants who lost at least 4.5kg during Phase I were eligible for Phase II, the randomized weight-loss maintenance portion of the study. In this phase, participants are randomized to one of two weight loss maintenance interventions, with follow up through eighteen months post initial study entry. This paper focuses on results from the Phase I weight-loss intervention. Results from the second phase of the LIFE study will be presented separately when they become available.
Eligibility
Participants were men and women aged 30 years or more, with a BMI of 30–50 but weighing less than 400 pounds. Medical exclusions included recent (within the previous two years) cardiovascular event, cancer treatment, or inpatient psychiatric hospitalization. Diabetics injecting insulin, individuals taking weight loss medications, and those who had undergone bariatric surgery or liposuction in the previous year were excluded. Additional exclusions included previous experience with acupuncture or acupressure for weight management, plans to leave the metropolitan area prior to the end of the program, weight loss of more than 20 pounds over the prior 6 months, current pregnancy or breast feeding, and concurrent participation in another clinical trial.
Recruitment and Entry
We recruited participants from the membership of Kaiser Permanente Northwest (KPNW), a non-profit health maintenance organization that provides comprehensive medical care to approximately 470,000 members in the Portland, Oregon metropolitan area. Two outreach strategies were implemented. First, we identified potentially eligible participants electronically from KPNW administrative databases, and mailed an invitation to participate. Second, we used a variety of media to publicize the study broadly among KPNW members and employees. These included emails to broad distribution lists of KPNW employees, postings in employee newsletters, articles in member-directed health and wellness publications, and announcements on the member web site.
Individuals interested in the study contacted recruiters via telephone, email, or secure web site. Those who were interested and potentially eligible were scheduled for an information session to learn more about the study. At the information session, the principal investigator provided a general overview of the study and answered questions. Those interested in being considered for enrollment scheduled an entry visit to determine final eligibility. Participants who met eligibility criteria at the entry visit and attended the first weight loss session (G1) were considered to be enrolled.
Weight Loss Intervention
The weight-loss intervention consisted of 22 group sessions led by nutrition and behavioral counselors, over six months. Specific intervention goals are listed in Table 1.
Table 1.
Intervention participant targets
|
The intervention approach, based on social cognitive theory, behavioral self-management techniques, trans-theoretical, or stages-of-change, models, and motivation enhancement(27–31) was designed to be supportive, participant-centered and interactive. The intervention targets focused on weight loss. Each group (20–25 participants) met weekly for 90 minutes. Group leaders followed standardized session outlines. Each session began with a check-in focused on accomplishments and barriers with regard to each participant's individual weekly goals. Guided small-group activities followed the check-in and fostered problem-solving, social support and relapse prevention planning. Group activities included calorie awareness activities, record-keeping skill development, nutrition and physical activity demonstrations and goal-setting modules. At the end of each session, each participant developed a plan for the upcoming week by setting food and exercise goals, and a specific action plan for achieving each goal. Participants were provided with a weekly record-keeping diary and encouraged to track all foods consumed and all exercise completed. The intervention leaders reviewed the diaries each week and provided encouragement and support for keeping records. Participants were weighed at each group session.
Intervention Standardization, Training, and Quality Control
The group session curriculum was adapted from the first phase of the Weight Loss Maintenance Trial. Group leaders met together weekly to discuss the progress of each group and to assure the topics were covered in similar ways. To ensure that leaders conducted groups according to the session outlines, we used a system of random observations. Observers (lead interventionist, PI, co-investigators, and other outside experts) completed an observation checklist and discussed any findings with the group leader in a debrief session. The PI regularly reviewed the observation checklists.
Assessments
At the entry visit, trained and certified staff measured participant height to the nearest 0.1cm using a calibrated, wall-mounted stadiometer. Staff measured weight at entry, at G1, at each weight loss session attended, and again at the post-weight loss intervention (PWL) visit approximately 6 months later. The latter visit was used to determine eligibility for randomization into Phase II. Weight was measured to the nearest ¼ pound by a high-quality digital scale with the participant in light indoor clothes without shoes. Weight was measured in pounds and converted to kilograms for analysis and reporting. The Bureau of Weights and Standards certified the scale annually, and study personnel assessed the calibration at least quarterly. Change in weight was calculated as weight at the PWL visit (conclusion of the weight loss program) minus weight at G1, so change less than 0 indicates weight loss.
The following measures were collected at the entry and PWL visits. The Insomnia Severity Index (ISI)(32) is a 5-item questionnaire measuring a participant's perception of his or her sleep quality in the previous two weeks. Scores range from zero to 28, with a higher score indicating worse sleep quality. Participants indicated sleep time by selecting a response closest to their average amount of sleep in a typical 24-hour period. Response categories, in hours, were less than or equal to five, greater than five to six, greater than six to seven, greater than seven to eight, greater than eight to nine, and greater than nine.
Participants indicated screen time by selecting a response closest to their average amount of screen time per 24 hour period (computer use or television) during the previous 7 days, with separate values for work days and days off.
The Perceived Stress Scale (PSS) is a ten-item questionnaire with scores ranging from zero to 40; higher scores indicate greater stress in the previous month.(33, 34)
The Personal Health Questionnaire – Depression Subscale (PHQ-8) is an eight item instrument with scores ranging from zero to 24;, higher score indicates greater depression symptoms during the previous 2 weeks.(35)
Statistical Analysis
In preparation for analysis, the data were audited and missing values were replaced using multiple imputation (data augmentation with Markov Chain Monte Carlo generation of imputed values, SAS PROC MI). The inclusive strategy recommended by Collins(36) was used, with correlates of missing values as well as of missingness included in the MI model. Five copies of the imputed data were generated, followed by identical analyses across all copies and combination of results using Rubin's(37) rules.
As this is a two phase study, a main purpose of the PWL visit was to determine eligibility for the protocol's second phase. Participants who had fallen short of the 4.5kg weight loss requirement were less likely to show up for their PWL visit, and a total of 120 participants had weights imputed for this visit. However, because participant weights were recorded at each of the 22 group weight loss sessions attended, there were multiple weights documented across time for every participant, and the imputation thus calculated at extremely high efficiency (>99%)
We used a logistic regression model to evaluate whether sleep time, ISI score, and screen time at entry predict success in the weight loss program, where randomization into Phase II was the binary measure of success. Ordinal sleep and screen time measures were parameterized as orthogonal polynomials. Secondarily, PHQ-8 and PSS scores were added to the predictive model. In post hoc analysis, measures found to be not significant in the full model were stepped out. A multiple regression analysis with weight change (normally distributed) as the dependent variable examined the same predictors in the same series of steps.
Correlations between change in sleep time, insomnia, screen time, depression, and stress with weight loss were estimated, including graphical evaluation of potential non-linearity. Correlations were also estimated among adherence measures (session attendance, exercise minutes, food diaries) and between these and weight loss.
Sample Size
Power calculations projected a requirement of 288 participants for phase II of the project. Based upon experience from our prior studies, we targeted a total of 450 participants for phase 1, anticipating a 64% yield to phase II. Our final numbers showed 472 enrolled in phase I, with a 60% yield to phase II.
RESULTS
Recruitment and Baseline Characteristics
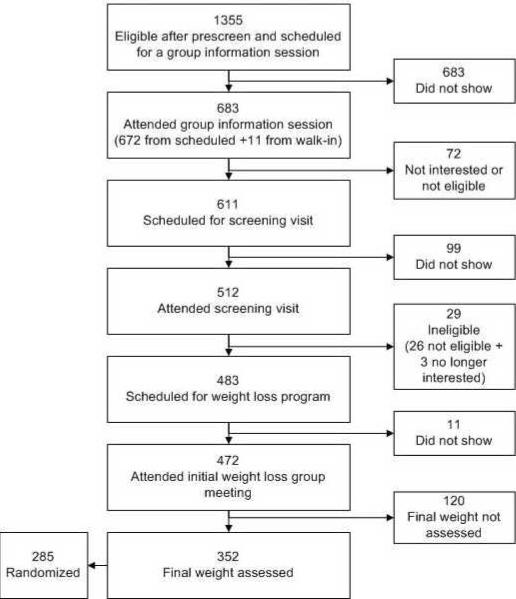
Of 1355 individuals scheduled for the informational sessions, 472 ultimately enrolled in the weight loss program (Figure 1). The baseline characteristics of those who enrolled are shown in Table 2. The mean participant age was 55 years, and the mean BMI was 37.7. Twenty five percent of participants were age 65 or over, and 83% were women. Consistent with demographics of the metropolitan Portland area,10% of participants were from minority populations.
Figure 1.
Flow of Study Participants
Table 2.
Entry characteristics (N=472)
| Characteristic | Mean (sd) or % |
|---|---|
| Age, years | 55.3 (11.7) |
|
| |
| Weight, kg (@ G1) | 104.3 (17.1) |
|
| |
| BMI | 37.7 (5.2) |
|
| |
| ISI (insomnia) | 7 (5.4) |
|
| |
| PHQ8 (depression) | 5.2 (4.2) |
|
| |
| PSS (stress) | 12.3 (6.3) |
|
| |
| Women (%) | 83 |
|
| |
| Minority participants (%) | 10 |
|
| |
| *Education, highest level (%) | |
| Grade/ Some high school | 1 |
| Completed high school | 8 |
| Some college | 37 |
| Completed college | 23 |
| Post college | 30 |
|
| |
| Household income, dollars (%) | |
| <30K | 10 |
| 30–59K | 37 |
| 60–89K | 31 |
| >=90K | 22 |
|
| |
| Screen time, work days, hours (%) | |
| NA and <=1 | 15 |
| >1 and <=3 | 39 |
| >3 and <=5 | 24 |
| >5 and <=7 | 12 |
| >7 | 10 |
|
| |
| Screen time, days off, hours (%) | |
| NA and <=1 | 9 |
| >1 and <=3 | 29 |
| >3 and <=5 | 41 |
| >5 and <=7 | 14 |
| >7 | 7 |
|
| |
| Sleep time, hours (%) | |
| <=5 | 3 |
| >5 and <=6 | 14 |
| >6 and <=7 | 34 |
| >7 and <=8 | 37 |
| >8 and <=9 | 11 |
| >9 | 1 |
total not equal to 100% due to rounding
sd=standard deviation NA=not applicable
Weight change
The mean weight loss at 6 months was 6.3 kg (SD 7.1). Sixty percent (N=285) of participants lost at least 4.5 kg (10 lbs) and were randomized into the second phase of the study.
Attendance, Adherence, and Follow-up
Participants attended a mean of 73.1 % (SD 26.7) of the 22 group sessions, completed 5.1 (SD 1.9) daily food records per week, and reported 195.1 (SD 123.1) minutes of exercise per week.
Baseline Predictors of Weight Loss Success: logistic regression (Question 1)
In the logistic regression on logit for probability of success, only the quadratic trend on sleep time reached significance (p=.036). When PSS and PHQ-8 were added, the pattern of significance did not change. However, after stepping out the terms with p > .05, the final odds ratios (OR) were 0.966 (CI 0.937, 0.995, p=.024) for 1-point change in PSS (as stress increases, weight loss is less), and OR = 0.797 (CI 0.649, 0.978, p=.030) for quadratic trend in sleep time.
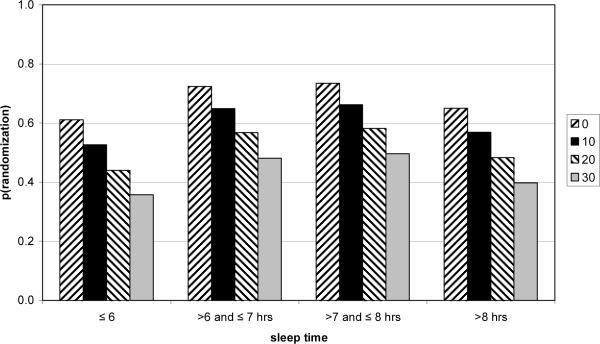
As Figure 2 illustrates, those who slept >6–7 or >7 to 8 hours daily had higher rates of eligibility for phase II, and baseline stress scores were negatively related to eligibility rate in each sleep category. Indeed, participants with the lowest stress scores in the mid range sleep categories had approximately double the phase II randomization rate of those with the highest stress scores sleeping less than or equal to 6 hours daily.
Figure 2.
Predicted Probability of Randomization into Phase II as a Function of Stress and Sleep Time at Entry. Each bar with the same pattern depicts the same rounded PSS score, at different levels of sleep time.
Baseline Predictors of Weight Loss Success: multiple regression (Question 1)
The first regression model for prediction of weight loss failed to show that sleep and screen time measures were associated with weight loss, although ISI showed a weak association (p=.066). When PSS and PHQ-8 were added to the model, only PSS showed an association better than p<.10. After stepping out predictors with p > .05, only stress level (PSS) was found to be associated with subsequent weight loss (as stress increases, weight change is less). (slope 0.132, SE 0.054 t=2.42 p=.021)
Changes in Baseline Predictors Associated with Weight Loss (Question 2)
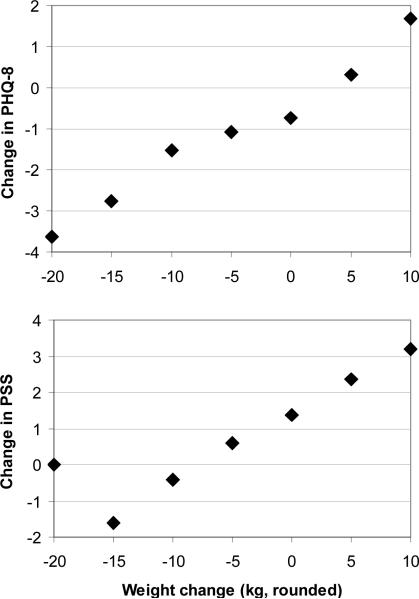
Weight loss correlated significantly with change from entry to PWL in stress (PSS) (r=.159, p=.048) and depression (PHQ-8)(r=.223, p=.035)(Figure 3). Change over the same period in sleep time, screen time, and insomnia (ISI) failed to show a significant linear correlation with weight loss.
Figure 3.
Association of weight change and change in depression (PHQ-8) and stress (PSS)
Association of Process Measures and Weight Loss (Question 3)
Attendance, exercise minutes, and number of daily food records were all positively correlated with one another (attendance/exercise r=.362, attendance/food records r=.539, exercise/food records r=.439, all p<.001), and with weight loss (weight/attendance r=−.621, weight/exercise r=−.361, weight/food records r=−.501, all p < .001)
Post Hoc Correlation Analysis
We also examined the associations among all of the change scores. Of 15 correlations examined, we found 5 significant correlations at α = .05 (Table 3). If we apply a Bonferroni correction (α = .05/15 = .003), just two of these are associations are strong enough to be considered significant; these are: change in PHQ-8 with change in ISI (r = 0.33, p < .001) and with change in PSS (r = 0.43, p<.001).
Table 3.
Significant correlations among hypothesized mediators of weight change
| Pair | r | s.e. | p < |
|---|---|---|---|
| Change in sleep vs. change in Screen time: work days | 0.03 | 0.06 | 0.5848 |
| Change in sleep vs. change in Screen time: off days | 0.09 | 0.06 | 0.1580 |
| Change in sleep vs. change in ISI | −0.14 | 0.06 | 0.0170 |
| Change in sleep vs. change in PHQ-8 | −0.02 | 0.06 | 0.7728 |
| Change in sleep vs. change in PSS | 0.00 | 0.07 | 0.9812 |
| Change in Screen time: work days vs. off days | 0.14 | 0.06 | 0.0190 |
| Change in Screen time: work days vs. change in ISI | 0.04 | 0.06 | 0.5533 |
| Change in Screen time: work days vs. change in PHQ-8 | 0.03 | 0.07 | 0.6728 |
| Change in Screen time: work days vs. change in PSS | 0.03 | 0.06 | 0.5620 |
| Change in Screen time: off days vs. change in ISI | 0.09 | 0.05 | 0.0960 |
| Change in Screen time: off days vs. change in PHQ-8 | 0.13 | 0.06 | 0.0408 |
| Change in Screen time: off days vs. change in PSS | 0.10 | 0.06 | 0.1431 |
| Change in ISI vs. change in PHQ-8 | 0.33 | 0.06 | <.0001 |
| Change in ISI vs. change in PSS | 0.18 | 0.08 | 0.0666 |
| Change in PHQ-8 vs. change in PSS | 0.43 | 0.06 | <.0001 |
DISCUSSION
We found that entry sleep time predicted success in the weight loss program. Specifically, participants sleeping 6 hours or less or more than 8 hours daily were less likely to achieve eligibility for phase II than those sleeping from 6 to 8 hours a day. In addition, entry stress (PSS) score predicted both eligibility for phase II and actual weight loss. As inspection of Figure 2 shows, those sleeping less than or equal to 6 hours and reporting the highest stress score at baseline were approximately only half as likely to achieve eligibility for phase II as those sleeping greater than 6 to 8 hours with a low stress score. These results suggest that early evaluation of sleep and stress levels in long term weight management studies could potentially identify which participants might benefit from additional counseling and resources.
In addition to association with success in the weight loss program, change in PSS score correlated with change in weight during the intensive Phase I behavioral weight loss intervention. These findings are of potential significance for both clinicians and investigators. Recent research suggests that chronic stress results in an increased intake of energy- and nutrient-dense foods and that hormonal reactions to stressors may be tightly intertwined with the endocrine regulation of appetite.(22,23) Further, there is accumulating evidence that highly palatable food can activate a neurologic reward system (opioid, dopamine, and endocannabanoid signaling in the limbic system(38)) producing powerful behavioral reinforcement to food similar to that seen to drugs of abuse. Thus, if stress becomes chronic and eating is learned to be an effective coping behavior, highly palatable food may appear to be `addictive'. Given these potential mechanisms, helping people to both reduce stress in their lives and to better understand the possible link between stress and their appetite for energy- and nutrient-dense palatable foods may bolster their efforts at weight management. Further, stress management tools could help individuals manage stress-related appetite cues, supporting their weight reduction efforts.
In our analyses, screen time neither predicted success in the weight loss program nor changed in association with weight loss. Exercise minutes, on the other hand, were highly correlated with weight loss. It would seem that for successful weight loss, sedentary habits of whatever type need to be reduced. As a first premise, computers and television could be expected to interfere with weight management in contexts where they directly compete with, replace, or discourage physical activity. It is unclear, however, whether passive television watching and computer use have similar weight-control consequences in this context. In addition, many people use computers at work, further complicating the assessment. Future research in this area should include attention not only to developing straightforward measures to accurately assess screen time, but also to delineating the impact of various subsets of screen exposure on levels of physical activity.
For participants in this study, mean “exercise minutes per week” was 195.1 (SD 123.1), while the median was 175. 1 (range 0–950.3). These relatively high figures may be attributable in part to our methodology, as the weekly exercises minutes were tabulated from participants' self report diaries.
In summary, our results are consistent with previously observed findings that adherence to each of the key intervention components (attendance, physical activity, keeping food diaries) is highly correlated with adherence to the others, and that adherence to each of these is highly correlated with weight loss(39) In addition, we found that baseline stress (PSS) predicted weight loss, baseline stress and sleep time predicted eligibility for continuing into Phase 2 of the trial, and that changes in weight during the weight loss program were linearly associated with changes in both depression (PHQ-8) and stress. Because our data are collected from a well educated cohort of motivated study participants, it warrants emphasis that these results may be of only limited generalizability. Nevertheless, further research is warranted to determine whether directly targeting stress, sleep, and/or depression as an adjunct to established behavioral weight loss interventions could improve weight loss.
ACKNOWLEDGMENTS
This work was funded by a grant (5R01AT003928) from The National Center for Complementary and Alternative Medicine, National Institutes of Health
Footnotes
DISCLOSURES There are no competing financial interests to disclose.
ClinicalTrials.gov Identifier: NCT00526565
Reference List
- 1.Ogden CL, Carroll MD, McDowell MA, Flegal KM, Division of Health and Nutrition Examination Surveys Obesity among adults in the United States - No statistically significant change since 2003–2004. NCHS Data Brief. 2007. 2007 Jan; [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 4.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28(10):1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 5.Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165(1):25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 6.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiegel K, Tasali E, Penev P, Van CE. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 8.Crespo CJ, Smit E, Troiano RP, Bartlett SJ, Macera CA, Andersen RE. Television watching, energy intake, and obesity in US children: results from the third National Health and Nutrition Examination Survey, 1988–1994. Archives of Pediatrics & Adolescent Medicine. 2001;155(3):360–5. doi: 10.1001/archpedi.155.3.360. [DOI] [PubMed] [Google Scholar]
- 9.Janssen I, Katzmarzyk PT, Boyce WF, King MA, Pickett W. Overweight and obesity in Canadian adolescents and their associations with dietary habits and physical activity patterns. Journal of Adolescent Health. 2004;35(5):360–7. doi: 10.1016/j.jadohealth.2003.11.095. [DOI] [PubMed] [Google Scholar]
- 10.Tremblay MS, Willms JD. Is the Canadian childhood obesity epidemic related to physical inactivity? International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2003;27(9):1100–5. doi: 10.1038/sj.ijo.0802376. [DOI] [PubMed] [Google Scholar]
- 11.Pettee KK, Ham SA, Macera CA, Ainsworth BE. The reliability of a survey question on television viewing and associations with health risk factors in US adults. Obesity (Silver Spring) 2009;17(3):487–93. doi: 10.1038/oby.2008.554. [DOI] [PubMed] [Google Scholar]
- 12.Shields M, Tremblay MS. Screen time among Canadian adults: a profile. Health Rep. 2008;19(2):31–43. [PubMed] [Google Scholar]
- 13.Weiss EC, Galuska DA, Kettel KL, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007;33(1):34–40. doi: 10.1016/j.amepre.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 14.Needham BL, Epel ES, Adler NE, Kiefe C. Trajectories of change in obesity and symptoms of depression: the CARDIA study. Am J Public Health. 2010;100(6):1040–6. doi: 10.2105/AJPH.2009.172809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158(12):1139–47. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 16.Strine TW, Mokdad AH, Dube SR, et al. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen Hosp Psychiatry. 2008;30(2):127–37. doi: 10.1016/j.genhosppsych.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Herva A, Laitinen J, Miettunen J, et al. Obesity and depression: results from the longitudinal Northern Finland 1966 Birth Cohort Study. Int J Obes (Lond) 2006;30(3):520–7. doi: 10.1038/sj.ijo.0803174. [DOI] [PubMed] [Google Scholar]
- 18.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–9. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 19.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26(1):37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 20.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23(11–12):887–94. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Gluck ME. Stress response and binge eating disorder. Appetite. 2006;46(1):26–30. doi: 10.1016/j.appet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91(4):449–58. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Kyrou I, Tsigos C. Stress hormones: physiological stress and regulation of metabolism. Curr Opin Pharmacol. 2009;9(6):787–93. doi: 10.1016/j.coph.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Hollis JF, Gullion CM, Stevens VJ, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35(2):118–26. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elder C, Gallison C, Lindberg N, Debar L, Funk K, Ritenbaugh CK, Stevens V. Randomized Trial of Tapas Acupressure for Weight Loss Maintenance: Rationale and Study Design. Journal of Alternative & Complementary Medicine. 2010 doi: 10.1089/acm.2009.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Funk KL, Elmer PJ, Stevens VJ, et al. PREMIER--A Trial of Lifestyle Interventions for Blood Pressure Control: Intervention Design and Rationale. Health Promot Pract. 2008;9(3):271–80. doi: 10.1177/1524839906289035. [DOI] [PubMed] [Google Scholar]
- 27.Bandura A. Social foundations of thought and action: A social cognitive theory. Prentice Hall; Englewood Cliffs, NJ: 1986. [Google Scholar]
- 28.Watson DLTR. Self-directed behavior: Self-modification for personal adjustment. Brooks/Cole; Pacific Grove, CA: 1989. [Google Scholar]
- 29.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–5. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 30.Miller WR, Rollnick S. Motivational interviewing: preparing people to change addictive behavior. The Guildford Press; New York: 1991. [Google Scholar]
- 31.Bandura A, Adams N. Analysis of self-efficacy theory of behavioral change. Cognitive Therapy and Research. 1977;1(4):287–310. [Google Scholar]
- 32.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 33.Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health. Sage; Newbury Park, CA: 1988. [Google Scholar]
- 34.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 35.Kroanke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals. 2002;32:1–7. [Google Scholar]
- 36.Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 2001;6(4):330–51. [PubMed] [Google Scholar]
- 37.Little RJ, Rubin DB. Statistical analysis with missing data. John Wiley & Sons; New York: 2002. [Google Scholar]
- 38.Cota D, Tschop MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Rev. 2006;51(1):85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132(6):2226–38. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]