Introduction
The plasma membrane of the erythrocyte accounts for all of this cell’s antigenic, transport, and mechanical characteristics, particularly its ability to undergo large passive deformations during repeated passage through the narrow capillaries of the microvasculature, throughout its 120-day life span. The determinant of normal membrane cohesion is the system of “vertical” linkages between the phospholipid bilayer and membrane skeleton, formed by the interactions of the cytoplasmic domains of various membrane proteins with the spectrin-based skeletal network. Band 3 and Rh-associated glycoprotein (RhAG) provide such links by interacting with ankyrin, which in turn binds to β-spectrin. Protein 4.2 binds to both band 3 and ankyrin and can regulate the avidity of the interaction between band 3 and ankyrin. Glycophorin C, band 3, XK, Rh, and Duffy all bind to protein 4.1R, the third member of the ternary junctional complex with β-spectrin and actin1–2.
Red cell membrane disorders are inherited diseases due to mutations in various membrane or skeletal proteins, resulting in decreased red cell deformability, reduced life span and premature removal of the erythrocytes from the circulation. The red cell membrane disorders include hereditary spherocytosis, hereditary elliptocytosis, hereditary ovalocytosis and hereditary stomatocytosis.
Hereditary spherocytosis
Hereditary spherocytosis is the most common congenital haemolytic anaemia in Caucasians, with an estimated prevalence ranging from 1:2,000 to 1:5,000. Approximately 75% of cases display an autosomal dominant pattern of inheritance, the remaining comprise recessive forms and de novo mutations. The main clinical features of hereditary spherocytosis are haemolytic anaemia which can range from compensated to severe, sometimes requiring exchange transfusion at birth and/or repeated blood transfusions, variable jaundice, splenomegaly and cholelythiasis. The molecular defect is highly heterogeneous, involving the genes encoding for spectrin, ankyrin, band 3 and protein 4.2. The deficiency or dysfunction of any of these proteins, which are involved in the attachment of the cytoskeleton to the membrane integral domain, results in a loss of surface area and leads to spheroid, osmotically fragile cells that are selectively trapped in the spleen. The defective protein can be detected by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) which allows the identification of different subsets of patients; some subjects with hereditary spherocytosis remain however unclassified by this technique.
Recently, we analysed a large database of 300 patients with hereditary spherocytosis grouped according to the results of SDS-PAGE, to ascertain whether the clinical and haematological features and the response to splenectomy are related to the type of molecular defect. We also compared the sensitivity of the most common laboratory screening tests for hereditary spherocytosis (NaCl osmotic fragility test on both fresh and incubated blood, standard glycerol lysis test, acidified glycerol lysis test [AGLT] and pink test) in various subsets of patient3. As regards the first point, we found that band 3 and spectrin deficiencies were the most common protein abnormalities (54% and 31%, respectively) and 11% of cases were not classified by SDS-PAGE analysis. Spectrin deficiency was more frequently diagnosed in childhood and band 3 deficiency in adulthood. Splenomegaly and gallstones were more frequent in band 3-deficient patients, whereas anaemia, neonatal jaundice and a need for transfusions were more common in spectrin/ankyrin deficiency. Consistently, haemoglobin concentration was slightly lower and spherocyte number and haemolysis markers higher in spectrin than in band 3 deficiency. Splenectomy corrected the anaemia in all cases but one with severe spectrin deficiency; in addition, after splenectomy spectrin-deficient patients showed a slightly lower median rise in haemoglobin, and a higher reticulocyte number than band 3-deficient patients, suggesting that the clinical pattern was more severe in the former.
Red cell osmotic fragility was assessed by a battery of tests, and their sensitivity ranged from 48% to 95% independently of the cytoskeletal abnormality and of the amount of protein deficiency3. The association of the AGLT and the NaCl test on incubated blood reached a sensitivity of 99%, even considering atypical patients, i.e. those with normal reticulocyte counts and/or no spherocytes in the peripheral blood. Interestingly, in splenectomised patients the percent positivity of all the osmotic fragility tests was increased, as compared with not splenectomised cases; in particular, both the AGLT and the NaCl test on incubated blood reached 100% sensitivity. Moreover, surgery allowed the identification of the membrane defect in all the previously unclassified cases (4 with spectrin deficiency, 3 with spectrin/ankyrin deficiency, and 1 with band 3 deficiency).
We concluded that the definition of the red cell membrane defect in hereditary spherocytosis has no major clinical implications; however, SDS-PAGE analysis may be useful for a differential diagnosis with other haematological disorders that mimic hereditary spherocytosis, such as congenital dyserythropoietic anaemia type II.
Hereditary elliptocytosis
Hereditary elliptocytosis is an autosomal dominant disorder, characterised by the presence of elliptically shaped red cells on peripheral blood smear, more common in malaria endemic regions in West Africa (prevalence 2%)1. Patients with hereditary elliptocytosis are generally asymptomatic but approximately 10% have moderate to severe anaemia including a few reported cases of hydrops foetalis and the severe variant hereditary pyropoikilocytosis, characterised by important membrane fragmentation and reduced membrane surface area. Hereditary elliptocytosis is caused by weakened “horizontal” linkages in membrane skeleton due either to a defective spectrin dimer-dimer interaction or a defective spectrin-actin-protein 4.1R junctional complex.
The severity of the disease is related to extent of decrease in membrane mechanical stability and resultant loss of membrane surface area. As in hereditary spherocytosis, splenectomy reduces the severity of anaemia by increasing the circulatory life span of fragmented red cells, although very few patients with hereditary elliptocytosis need surgery.
Hereditary stomatocytoses
Overhydrated hereditary stomatocytosis is an autosomal dominant disorder characterised by the presence of large numbers of stomatocytes on blood smears in association with moderately severe to severe anaemia1. The distinctive feature of red cells is their increased sphericity due to increased cell volume without a concomitant increase in membrane surface area. This is due to a marked increase of intracellular sodium as a consequence of the cell’s inability to regulate its cation homeostasis. Stomatocytes with increased sphericity are sequestered by the spleen. However, while splenectomy is highly beneficial in the management of patients with hereditary spherocytosis and hereditary elliptocytosis, it is contraindicated in overhydrated hereditary stomatocytosis, because it leads to an increased risk of venous thromboembolic complications. Molecular analysis showed two mutations associated with overhydrated hereditary stomatocytosis in RHAG (causing Ile61Arg and Ser65Phe), which were dominantly inherited and result in widening of the membrane pore and passage of cations4.
Dehydrated hereditary stomatocytosis (xerocytosis) is an autosomal dominant disorder characterised by decreased intracellular potassium content and loss of cell water, increased cytoplasmic viscosity and a typical increased mean cell haemoglobin concentration1. Cell dehydration has only a marginal effect on the survival of erythrocytes in dehydrated hereditary stomatocytosis, which is characterised by well-compensated anaemia, with only a mild to moderately enlarged spleen. The genetic basis of dehydrated hereditary stomatocytosis remains unknown, but linkage analysis suggests segregation with chromosome 16q23-q245.
Hereditary ovalocytosis
Hereditary ovalocytosis is an autosomal dominant disease characterised by the presence of oval-shaped red cells with one or two transverse ridges or a longitudinal slit on a blood smear, and is very common in Southeast Asia where its prevalence ranges from 5% to 25%1. The condition is due to decreased membrane deformability as assessed by ektacytometry. A genomic deletion of 27 bp encoding amino acids 400 to 408 of band 3 has been identified, however the mechanism that leads to a marked increase in membrane rigidity has yet to be established.
Diagnosis
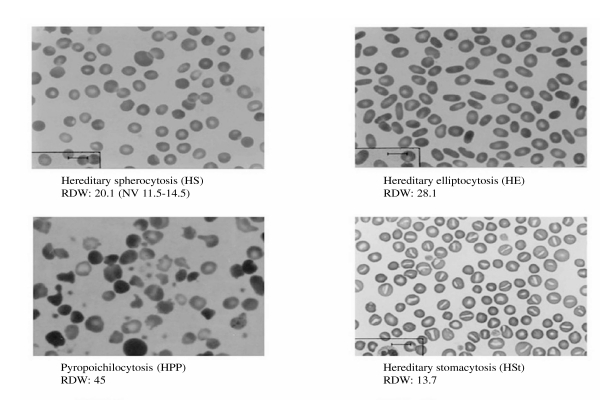
As regards the diagnostic pathway of the red cell membrane disorders, examination of a blood film is fundamental and gives important information (Figure 1), if performed by an expert (for example the presence of typical elements: spherocytes, stomatocytes, ovalocytes, elliptocytes), and should be performed in all individuals with a positive family history of haemolytic anaemia and a negative direct antiglobulin test. Suggested first step screening for red cell membrane defects includes the panel of osmotic fragility tests and eosin-5-maleimide (EMA)-binding6–7. As regards osmotic fragility (NaCl osmotic fragility test on both fresh and incubated blood, standard glycerol lysis test, AGLT and pink test) we have already shown that the association of the AGLT and the NaCl test on incubated blood reaches a sensitivity of 99%3; EMA-binding is a flow cytometric method based on the fluorescence of red blood cells after incubation with eosin-5-maleimide dye which binds specifically to band 3, and it has been reported to have a sensitivity of 89–96%8–11. We recently evaluated the sensitivity and specificity of the EMA-binding test on 108 consecutive patients with hereditary spherocytosis and 42 patients with other haemolytic anaemias12. The sensitivity of EMA-binding was 87%, and its specificity, determined by testing 400 normal subjects, was 98%. With regard to the patients without hereditary spherocytosis, the test was positive only in congenital dyserythropoietic anaemia type II. Consistently, although the definition of the red cell membrane defect has no major clinical implications and is not currently recommended in typical cases, it should be underlined that SDS-PAGE analysis may be useful for a differential diagnosis with other haematological disorders that mimic hereditary spherocytosis, such as congenital dyserythropoietic anaemia type II. Concomitant causes of haemolysis should been considered and ruled out in difficult cases (enzyme or haemoglobin defects)3.
Figure 1.
Blood morphology of red cell membrane defects.
Treatment
The treatment of red cell membrane defects is based on supportive measures: folate therapy is recommended in severe and moderate forms of haemolytic anaemia and red cell transfusions may be required in severely anaemic cases, particularly in the first years of life, and during pregnancy, aplastic crisis and infections. Clinically, hereditary spherocytosis has been divided in mild, moderate, and severe forms, depending on haemoglobin levels (respectively 11–15, 8–12 and 6–8 mg/dL), and haemolytic parameters (reticulocytes 3–6, >6, and >10% respectively, and bilirubin 17–34, >34, and >51 μg/L, respectively). Splenectomy is usually not required in mild hereditary spherocytosis, indicated before puberty in moderate forms and necessary in severe forms. Surgery should be delayed until the age of 6 years, with appropriate counselling about the risk of infections, which is not completely eliminated by the currently recommended pre-operative vaccinations and post-splenectomy antibiotic prophylaxis6. Laparoscopic surgery, when performed by experienced surgeons, can result in a shorter hospital stay and less pain13,14. The need for re-immunisation and its frequency are unclear as are the optimal duration of post-splenectomy antibiotic prophylaxis and choice of drug. Iron overload and gallstones should be carefully monitored and treated.
Footnotes
Presented in part at the XXXIX Convegno Nazionale di Studi di Medicina Trasfusionale (Milan, Italy, 9–12 June 2010).
References
- 1.Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112:3939–48. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet. 2008;372:1411–26. doi: 10.1016/S0140-6736(08)61588-3. [DOI] [PubMed] [Google Scholar]
- 3.Mariani M, Barcellini W, Vercellati C, et al. Clinical and hematologic features of 300 patients affected by hereditary spherocytosis grouped according to the type of the membrane protein defect. Haematologica. 2008;93:1310–7. doi: 10.3324/haematol.12546. [DOI] [PubMed] [Google Scholar]
- 4.Bruce LJ, Guizouarn H, Burton NM, et al. The monovalent cation leak in overhydrated stomatocytic red blood cells results from amino acid substitutions in the Rh-associated glycoprotein. Blood. 2009;113:1350–7. doi: 10.1182/blood-2008-07-171140. [DOI] [PubMed] [Google Scholar]
- 5.Bruce LJ. Hereditary stomatocytosis and cation leaky red cells - recent developments. Blood Cells Mol Dis. 2009;42:216–22. doi: 10.1016/j.bcmd.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Bolton-Maggs PH, Stevens RF, Dodd NJ, et al. General Haematology Task Force of the British Committee for Standards in Haematology. Guidelines for the diagnosis and management of hereditary spherocytosis. Br J Haematol. 2004;126:455–74. doi: 10.1111/j.1365-2141.2004.05052.x. [DOI] [PubMed] [Google Scholar]
- 7.King MJ, Behrens J, Rogers C, et al. Rapid flow cytometric test for the diagnosis of membrane cytoskeleton-associated haemolytic anaemia. Br J Haematol. 2000;111:924–33. [PubMed] [Google Scholar]
- 8.King MJ, Smythe JS, Mushens R. Eosin-5-maleimide binding to band 3 and Rh-related proteins forms the basis of a screening test for hereditary spherocytosis. Br J Haematol. 2004;124:106–13. doi: 10.1046/j.1365-2141.2003.04730.x. [DOI] [PubMed] [Google Scholar]
- 9.Kedar PS, Colah RB, Kulkarni S, et al. Experience with eosin-5′-maleimide as a diagnostic tool for red cell membrane cytoskeleton disorders. Clin Lab Haematol. 2003;25:373–6. doi: 10.1046/j.0141-9854.2003.00557.x. [DOI] [PubMed] [Google Scholar]
- 10.Stoya G, Gruhn B, Vogelsang H, et al. Flow cytometry as a diagnostic tool for hereditary spherocytosis. Acta Haematol. 2006;116:186–91. doi: 10.1159/000094679. [DOI] [PubMed] [Google Scholar]
- 11.Girodon F, Garçon L, Bergoin E, et al. Usefulness of the eosin-5′-maleimide cytometric method as a first-line screening test for the diagnosis of hereditary spherocytosis: comparison with ektacytometry and protein electrophoresis. Br J Haematol. 2008;140:468–70. doi: 10.1111/j.1365-2141.2007.06944.x. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi P, Fermo E, Vercellati C, et al. Comparison of the eosin-5-maleimide flow cytometric method with osmotic fragility tests used in diagnosis of hereditary spherocytosis. Haematologica. 2009;94 (suppl 4):132. [Google Scholar]
- 13.Abdullah F, Zhang Y, Camp M, et al. Splenectomy in hereditary spherocytosis: review of 1,657 patients and application of the pediatric quality indicators. Pediatr Blood Cancer. 2009;52:834–7. doi: 10.1002/pbc.21954. [DOI] [PubMed] [Google Scholar]
- 14.Morinis J, Dutta S, Blanchette V, et al. Laparoscopic partial vs total splenectomy in children with hereditary spherocytosis. J Pediatr Surg. 2008;43:1649–52. doi: 10.1016/j.jpedsurg.2008.02.012. [DOI] [PubMed] [Google Scholar]