Abstract
BACKGROUND
Large multinucleated cells (MNC) commonly exist in tumorigenic cancer cell lines widely used in research, but their contributions to tumorigenesis are unknown.
METHODS
In this study, we characterized MNCs in the murine fibrosarcoma cell line UV-2237 in vitro and in vivo at a single cell level.
RESULTS
We observed that MNCs originated from a rare subpopulation of mononuclear cells; MNCs were positive for a senescent marker, β-galacosidase (SA-β-Gal); MNCs were responsible for the majority of clonogenic activity when cultured in hard agar; MNCs were more resistant to chemotherapeutic agents than were mononuclear cells; MNCs could undergo asymmetric division (producing mononuclear cells) and self-renewal in vitro and in vivo; and, most importantly a single MNC produced orthotopic subcutaneous tumors (composed mainly of mononuclear cells) that gave rise to spontaneous lung metastases in nude mice.
CONCLUSIONS
MNCs can be growth-arrested under stress, are highly resistant to chemotherapy, and can generate clonal orthotopic metastatic tumors
Keywords: multinucleated cells, senescent-like cancer cells, cancer-initiating cells
INTRODUCTION
Cancer cell lines have been widely used as major cancer research tools for decades. As heterogeneous as cells in a tumor tissue, cells of cancer cell lines are diverse in their tumorigenic potentials.1 The heterogeneity of tumor cells is a frequent cause of therapeutic failure. Tumor cells with a high degree of resistance to therapeutic agents and high capacity of tumorigenicity may serve as the source of cells for tumor relapse and metastasis. The origin of these drug resistant and tumorigenic cells remains unclear, and the known cancer stem cells (CSC) might be contributors.2, 3
Large multinucleated cells (MNC) commonly exist in cancer cell lines as well as in human cancer tissues.4–14 It is known that MNCs often survive after drug treatment of cancer cells cultured in vitro and MNCs of cancer may derive from cell fusion or cell division.15, 16 MNC’s distinct morphological features, enlarged cell size, multiple nuclei, and long-time survival in culture without multiplication, suggest that they are senescent cells17 with a programmed growth arrest at G1 despite a persistent active metabolism.18 Whether the senescent phenotype of MNCs is permanent or reversible is also an open question. The contribution of senescent cells to the biology of a given cancer cell line has not been evaluated.
In the present study, we investigated the origin of the MNCs in UV-2237 cells by long-term live cell imaging and determined the clonogenicity in agar culture, drug sensitivity, ability of self-renewal and growth in vitro and in vivo at the level of one cell.
MATERIALS AND METHODS
Cells and reagents
Murine fibrosarcoma UV-2237 cells were recovered from the frozen stock maintained in our laboratories. Cells were cultured in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), sodium pyruvate, nonessential amino acids, L-glutamine, and a twofold vitamin solution in 5% CO2-95% air at 37°C. GFP-fused histone 2B (GFP-H2B) expression vector (Cat #559241) was purchased from BD Biosciences (San Jose, CA). GFP expression vector was from Clontech (Mountain View, CA). The GeneJuice transfection reagent (Cat #70967-3) was from Novagen (San Diego, CA). Becto Agar (Cat #214010) was from Sigma-Aldrich (St. Louis, MO). Nylon meshes for 10 µm pore size (Cat #145939) and for 21 µm pore size (Cat #145940) were from Spectrum Laboratories (Racho, Dominquez, CA). Growth factor-reduced Matrigel (Cat #354230) and green fluorescent membrane staining dye Alexa Fluor 488WGA (Cat# W11261) were from Invitrogen (San Diego, CA). TdT-mediated dUTP Nick-End Labeling (TUNEL) kit (Cat #G3250) was from Promega (San Luis Obispo, CA).
Cell culture
Prior to any treatment, all cells were cultured in MEM supplemented with 10% FBS, sodium pyruvate, nonessential amino acids, L-glutamine, and a twofold vitamin solution in 5% CO2-95% air at 37°C. For hard agar culture, we used Becto-Agar at 2.0% dissolved in distilled water autoclaved for sterilization. Base layers of DMEM with 10% FBS and 0.4% agar were set in each well of 6-well culture plates. Over this bottom layer, a second layer of medium containing 0.6% agar and a suspension of single tumor cells (5,000 cells for mononuclear cells) were laid. After the top layer (with suspended tumor cells) gelled, 1–2 ml of Dulbecco’s MEM with 20% FBS was added. Culture plates were incubated at 37°C in an incubator with 5% CO2 and 95% air for 4 weeks. Colonies with diameters exceeding 50 µm were counted. Cells stably expressing GFP or GFP-H2B were established by transfecting cDNA encoding GFP and GFP-H2B followed by 3 weeks of selection in medium containing 500µg/ml Geneticin. For drug treatment, cells cultured in 6-well culture plates (n=6) at about 80% confluence were treated with 10 µM doxorubicin for 48 h with medium refreshment at 24 h. The control cells were treated with vehicle (same volume of DMSO). After treatment for 24 h, the cells were imaged with an inverted phase-contrast microscope for the presence of live cells and TUNEL assay. Images were captured with a cooled charged coupled device, Hamamatsu C5810 camera (Hamamatsu Photonics K.K., Bridgewater, NJ). At 72 h after drug treatment, detached dead cells were washed off and the remaining living cells were imaged and counted.
Long-time live cell imaging
Long-time live cell imaging was performed using a Biostation IM cell culture system (Nikon, Melville, NY). Time lapse phase-contrast and fluorescent images were automatically taken at 10-min intervals for 72 h.
Separation of MNCs from mononuclear cells
Adherently cultured or agar cultured cells were trypsinized to produce single cell suspensions. Based on the size difference between mononuclear cells and MNCs (the diameter of mononuclear cells is less than 10 µm while the diameter of MNCs is more than 21 µm), MNCs were separated from mononuclear cells by passing the cell mixture through a nylon mesh with 21µm pores. This method allowed mononuclear cells to pass through the mesh while retaining larger MNCs. Filtered cells were harvested with culture medium prior further analyses.
Animals
Male athymic nude mice (NCI-nu), 10-weeks of age were obtained from the Animal Production Area of the National Cancer Institute’s Frederick Cancer Research and Development Center (Frederick, MD). The mice were maintained under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the U. S. Department of Agriculture, U. S. Department of Health and Human Services, and the NIH.
One-cell tumorigenic assay
Cells stably expressing GFP (about 80% confluent) were harvested in trypsin (0.25%)/EDTA solution and then resuspended in fresh medium to generate single-cell suspensions. Large MNCs were then separated from small mononuclear cells as described previously. One single cell was picked up with a 20 µl pipette tip under a fluorescent microscope and then transferred to a well of a 96-well plate that contained 100 µl Hanks’ balanced salt solution (HBSS) with 50% growth factor-reduced Matrigel (Invitrogen, Carlsbad, CA). The single-cell status of each well of the 96-well plate was confirmed under a fluorescent microscope. The content of each well that contained one tumor cell was aspirated into a 1 ml syringe for subcutaneous injection. Four injection sites were made on the back of each nude mouse (n=15). Tumor growth was monitored for 8 weeks.
Recycling tumor cells from orthotopic–subcutaneous tumor tissues was carried out as follows: Tumors were aseptically dissected out from mice and minced into ~1-mm3 pieces in MEM containing 5% FBS, then rinsed in the same medium twice. Tumor tissues were enzymetically dissociated in a solution containing collagenase (200 units/ml) and DNase (1,500 units/ml) for 30 min at room temperature. Tumor cell suspensions were obtained by filtering the supernatants through a wire sieve. The cell suspensions were then cultured in fresh medium for 48 h. After the unattached cells were washed off, the adherent cells containing both MNC and mononuclear cells were separated.19 Single cells were harvested for implantation into the subcutis of nude mice (n=8).
Immunocytochemistry
Tumor cells plated onto four-chamber slides at a density of 1×104 cells/well in 10% MEM were incubated overnight. The cells were then washed once and then fixed in acetone for 15 min. The slides were blocked in phosphate-buffered saline (PBS) containing 5% normal horse serum and 1% normal goat serum. The slides were incubated in primary antibody (1:100 dilution) overnight at 4�C, rinsed three times with PBS, and then incubated for 10 min in protein blocking solution. Slides were then washed twice in PBS and the cell nuclei stained with Hoescht 33342 (Polysciences, Inc., Warrington, PA) for 2 min followed by washing in PBS. For unconjugated primary antibodies, the slides were then incubated in a biotin tyramide solution for 10 min, washed twice, and incubated for another 45 min in streptavidin-conjugated Alexa fluorescent 594. For the fluorescence conjugated primary antibodies, the step of incubation with a secondary antibody was omitted. Fluorescence bleaching was minimized by mounting the slides with glycerol/PBS medium containing 0.1 M propyl gallate (Sigma, St. Louis, MO). Immunofluorescence microscopy was performed using a Zeiss Axioplan fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with a 100-W Hg-lamp and narrow band pass excitation filters (Chroma Technology Corp., Brattleboro, VT). Images were captured with a cooled charged coupled device Hamamatsu C5810 camera (Hamamatsu Photonics K.K.) and ImagePro software (Media Cybernetics Inc., Silver Spring, MD). Composite photographs were made using PhotoShop software (Adobe Systems, Mountain View, CA).
Cell senescence assay
Cell senescence assay kit was purchased from Chemicon Intl (Temecula, CA) (Cat# KAA002) and assay was carried out according to the protocol provided by the manufacturer.
Electronic transmission microscopy
Transmission electron microscopy was conducted in the High Resolution Electron Microscopy Facility at M. D. Anderson Cancer Center (Houston, TX) (www.mdanderson.org/HREMF).
Statistical analysis
The Student’s T-test with normal distribution and equal variance was used to assess the difference of colonies formed by mononuclear cells and MNCs, TUNEL positivity of mononuclear cells and MNCs, and of tumor formation by mononuclear cells and MNCs. P<0.05 was defined as the statistical significance.
RESULTS AND DISCUSSION
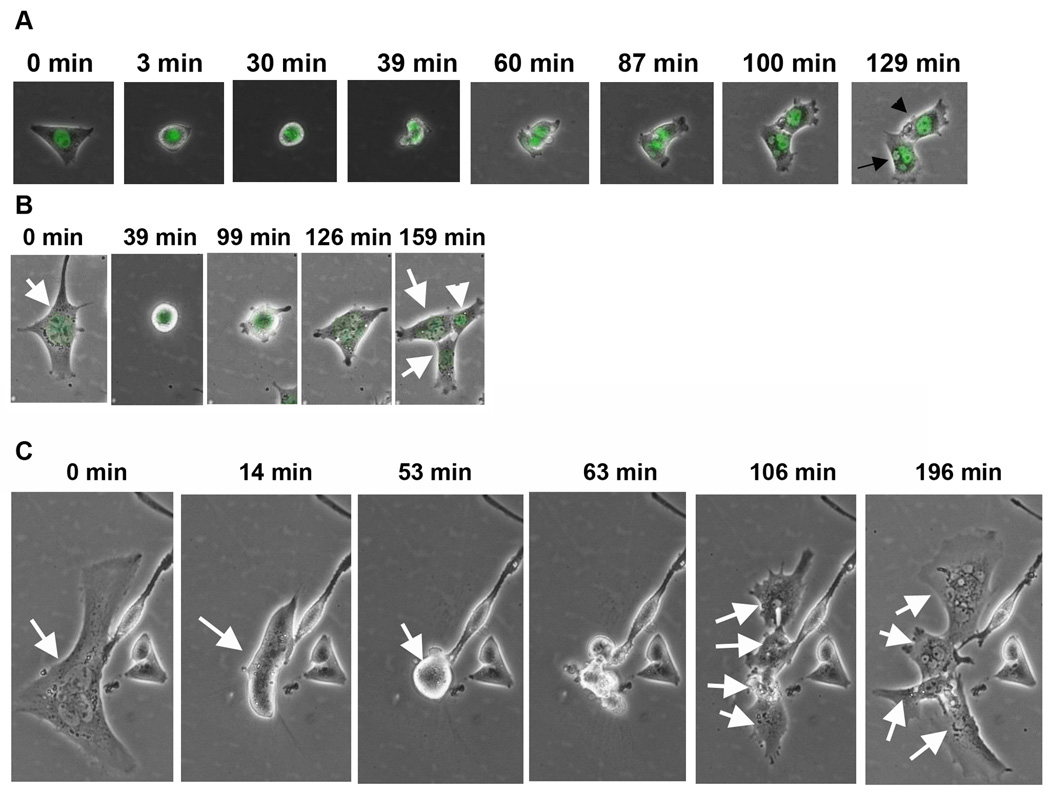
MNCs exist in most of the commonly used cancer cell lines. Whether MNCs are derived from cell-cell fusion has been a debate.15, 16 Taking advantage of current live cell imaging technology, we were able to observe a complete process of MNC formation. To monitor the dynamics karyokenesis, we stably expressed a green fluorescent protein fused nuclear protein histone 2B (GFP-H2B) in the cells for live imaging. We then combined phase-contrast and green fluorescence imaging to observe the morphological changes and karyokinesis. As shown in Figure 1A, a single mononuclear cell could undergo multinucleation due to an absence of cytokinesis. Because the majority of cells in colonies formed by a single MNC are mononuclear, it appears that MNCs can divide asymmetrically, yielding proliferative mononuclear cells. The asymmetric division of MNCs was observed by live-imaging of adhesive cultured cells. As shown in Figure 1B, MNC produced mononuclear cells by asymmetric cell division. The mononuclear cells derived from MNC are rapidly proliferating. The ability of self-renewal of MNCs was also detected by live cell monitoring. As shown in Figure 1C, one MNC produced 4 MNCs by one round of cell division.
Figure 1. Live cell imaging of multinucleation by mononuclear cells, asymmetric division by MNCs, and self-renewal by MNC.
A, a mononuclear UV-2237 cell divided into one mononuclear (arrowhead) and one MNC (arrow) cell. B, an MNC divided into one mononuclear (arrow head) and two MNCs (arrows). C, an MNC divided into four MNCs (arrows).
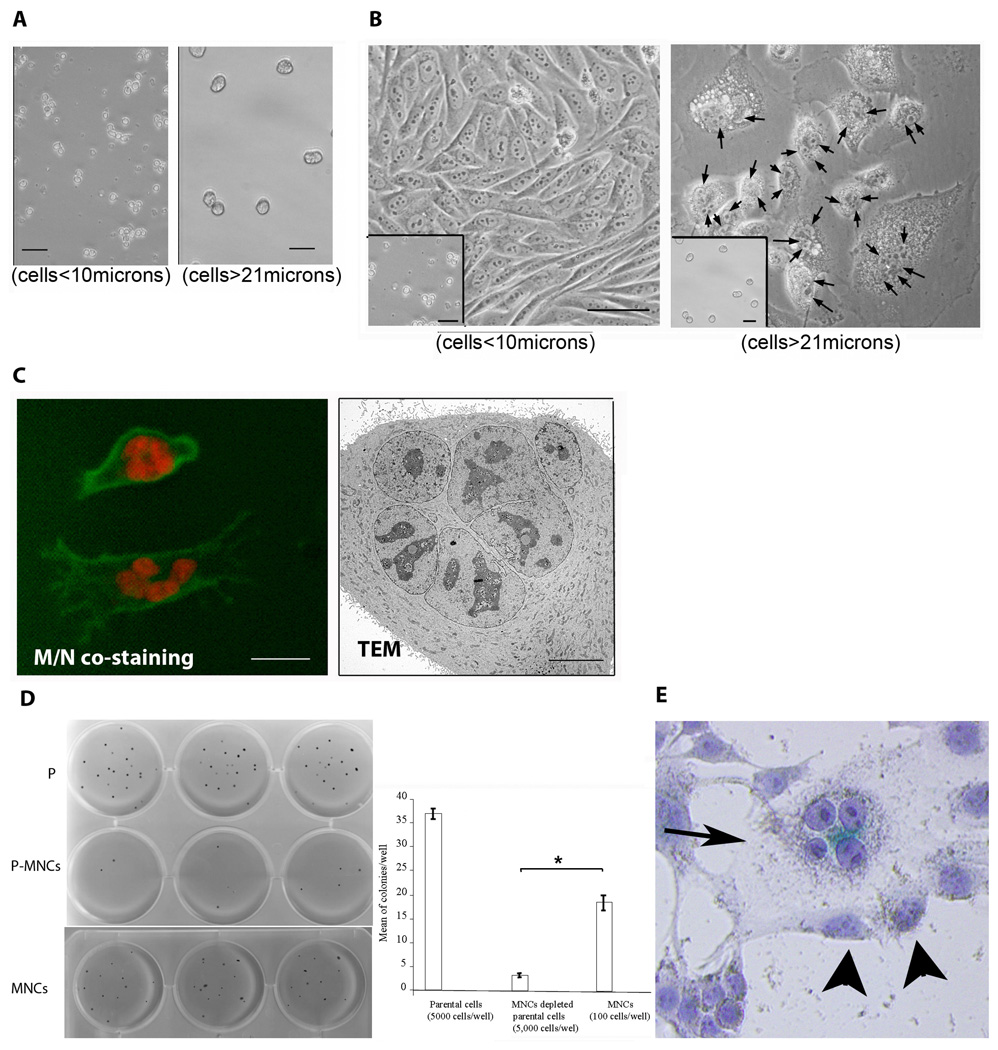
The clonogenicity of MNCs was measured using hard agar (0.6% agar) culture,20 in which we separated the large MNCs from the small cells by a sequential passage through nylon meshes with 10 and 21 µm pore size (Fig. 2A). We then seeded the separated MNCs and small cells into liquid medium and observed that the large cells were exclusively MNCs and the small cells were exclusively mononuclear (Fig. 2B). We further confirmed the multinuclear status of the large cells by fluorescent co-staining of the nucleus with propidium iodide and of cell membrane with Alexa Fluor 488WGA dye viewed under a fluorescent light microscope and by transmission electronic microscopy (Fig. 2C).
Figure 2. MNCs account for the majority of clonogenic potency of cancer cells and MNCs are β-gal positive.
A, large MNC cells (>21µm) were separated from the small cells (<10µm) and reseeded back to culture with liquid medium (B), the large cells were found to be exclusively MNCs revealed by costaining of nucleus (propodium iodide, red) and cytoplasm membrane (Alexa Fluor 488 WGA, green) and transmission electronic microcope (TEM) (C). D, colony formation by parental UV-2237 cells (Ps) and MNC-depleted cells (P-MNCs) and isolated MNCs (MNCs). E, β-gal staining of UV-2237 cells, of note is that the MNC (arrow) is positive for β-gal (blue color). (Error bar = mean ± S.D.). *P<0.05. Bar = 50 µm.
Surprisingly, when these isolated MNCs and mononuclear cells were seeded in hard agar, the clonogenicity of MNCs was significantly higher than the mononuclear cells (Fig. 2D). The colony formation by MNCs was unexpected, because MNCs of cancer cell lines are thought to be senescent. To determine whether the MNCs were senescent cells, we performed β-galactosidase senescence assay.17 As expected, the MNCs were indeed β-galactosidase-positive (Fig. 2E). Assay of β-galactosidase has been widely used in assessing and identifying senescent cells; however, thorough evaluation of growth arrest of β-galactosidase-positive cells under physiological or pathological conditions has not often been rare, i.e., the possibility remains that β-galactosidase-positive cells can re-enter growth. It is suggested that it is more appropriate to name cells with senescent-cell morphology and β-galactosidase activity as “senenscent-like cells” rather than “senescent cells”.21 Evidence suggests that some types of cellular senescence are reversible.22
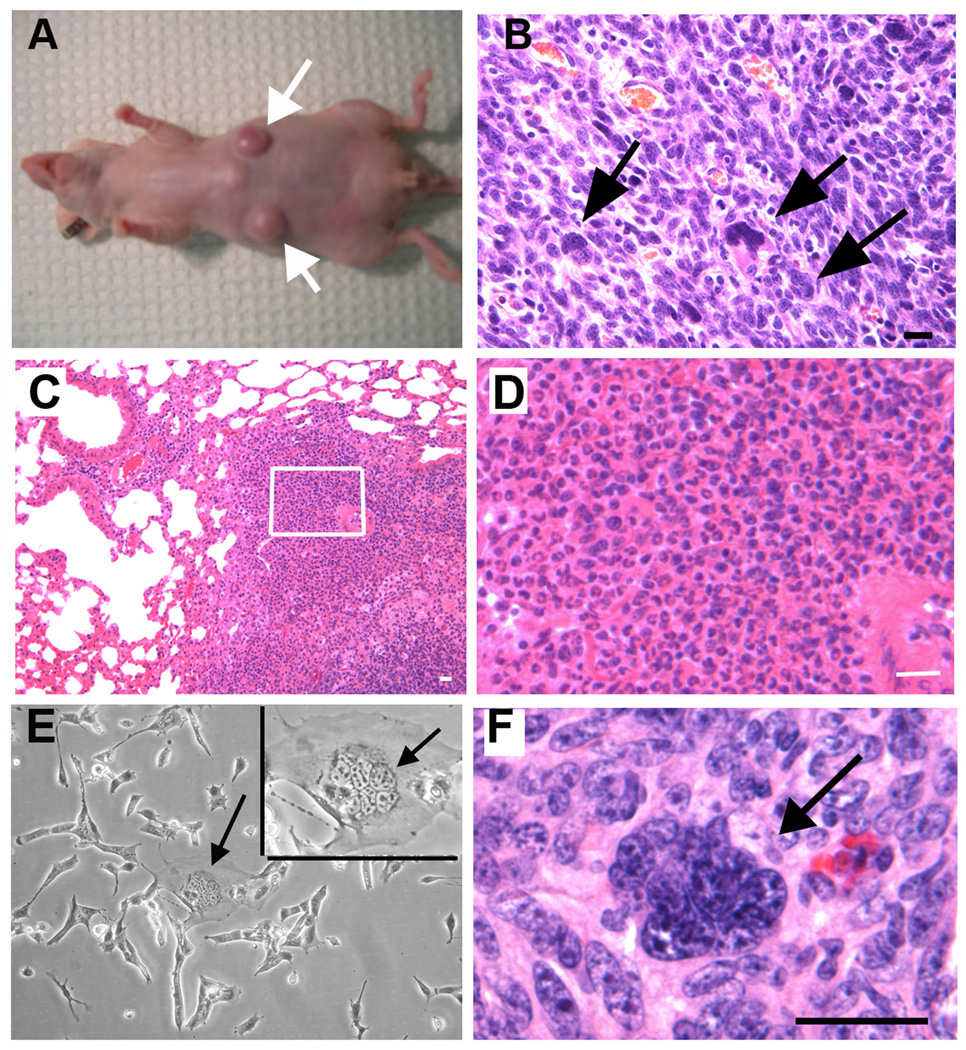
The clonogenic activity, the asymmetric division, and the ability of self-renewal exhibited by MNCs suggested that MNCs might have the ability to initiate tumors in vivo. To test this possibility, we performed one-cell tumorigenic assays in nude mice using UV-2237 cells. UV-2237 fibrosarcoma cells are ideal for this in vivo assay for two reasons: (1) tissue microenvironment, and (2) histocompatibility. Since the UV-2237 is a fibrosarcoma cell line, the subcutis represents the orthotopic microenvironment for growth of those cells. Previous work showed that pre-treatment of immuno-incompetent mice with the immunosuppressive drug VP-16 enhanced the rate of human tumor engraftment,23 suggesting that immuno-incompetent mice may be capable of rejecting human cells. UV-2237 is of the C3H/HeN mouse origin,24 decreasing the possibility of tissue rejection by the host. To perform one-cell inoculation, we first established a UV-2237 cell line stably transfected with GFP. We separated multinuclear UV-2237-GFP cells from mononuclear GFP cells based on size. We then isolated single cells using an inverted fluorescence microscope with a 20-μl pipette tip and transferred single cells each into well of a 96-well plate containing HBSS with 50% growth factor-reduced Matrigel. We used a fluorescence microscope to examine the wells for a GFP signal to confirm that each well only contained a single cell. A single cell in a volume of 100 μl was injected into the subcutis of a nude mouse (to prevent immune rejection). Four separate and distant injection sites were made in each mouse, with 15 mice injected with MNCs (total of 60 sites) and 10 mice injected with mononuclear cells (total of 40 sites). After tumor cell injection, the 96-well plate was reexamined for the presence of GFP to ensure that the cells had been transplanted. Two months after tumor cell injection, the mice injected with a single MNC per site had 13 subcutaneous tumors (13 of 60 injections) (Figs. 3A and 3B), while the mice injected with a single mononuclear cell had 1 tumor (1 of 40 injections). In addition, multiple lung metastases were found in one of the mice injected with a single MNC (Figs. 3C and 3D). To test whether MNCs can self-renew in vivo, we isolated MNCs from an MNC-induced tumor. As shown in Figure 3E, MNCs were found in the recycled tumor cells. We then inoculated nude mice with a single recycled MNC. Within 8 weeks, 5 of 32 recycled MNCs formed subcutaneous tumors (Fig. 3F), suggesting that MNCs can self-renew in vivo. Collectively, these results suggest that MNCs have a high probability of initiating tumors.
Figure 3. Tumor formation by a single MNC in nude mice.
A, Two tumors were produced in a nude mouse injected subcutaneously at each injected site with one MNC (4 injection sites/mouse). B, H&E staining of tumor tissues of (A) (MNCs are indicated by arrows). (Bar = 50 µm). C and D, a lung metastasis formed by a MNC derived tumor. (Bar = 20 µm). E, MNCs (arrows) in tumor cells recycled from tumor formed by one MNC. (Bar = 50 µm). F, hematoxylin and eosin staining of a tumor formed by one MNC recycled from a single MNC-produced tumor (MNCs indicated by arrows). Bar = 50 µm.
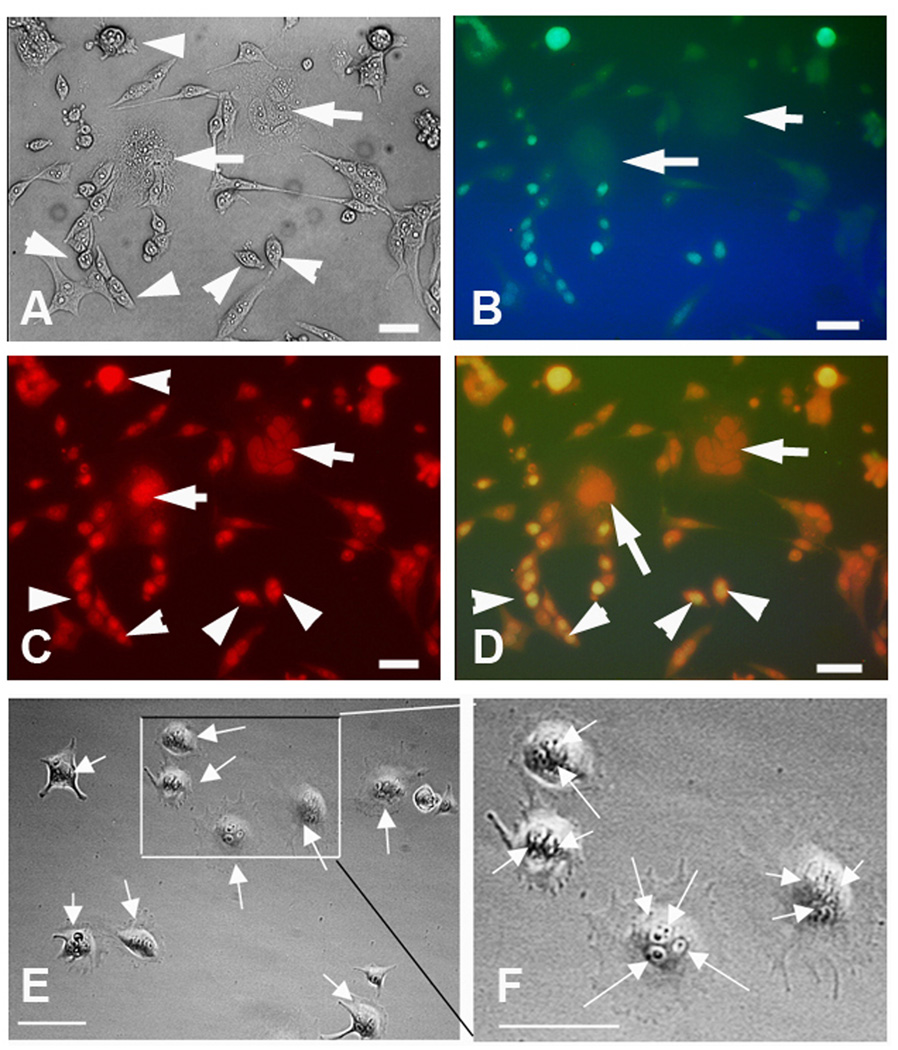
In addition to asymmetric cell division, self-renewal, and tumor formation by one cell, MNCs were also more resistant to chemotherapeutic agents than mononuclear tumor cells. As shown in Figure 4, treatment of parental cell lines which contain both single nucleated cells and MNCs with high doses of adriamycin (10 µM for 24 h) led to massive apoptotic cell death of mononuclear cells evidenced by TUNEL assay. In contrast, the MNCs were negative for TUNEL. At 72 h after treatment with adriamycin when a majority of the cells were dead, the remaining live adherent cells were exclusively MNCs.
Figure 4. MNCs are more resistant to adriamycin than are mononuclear cells.
A–D, TUNEL assay of UV-2237 cells treated with adriamycin (10 µM for 24 h). MNCs are indicated by arrows, mononuclear cells are indicated by arrowheads. TUNEL positive signals are in green and propidium iodide (PI) counterstained nuclei are in red. The percentage of positive TUNEL cells within mononuclear cells were 74.4+/-8.1%, and none TUNEL positive cells were fund in the MNCs. Bar = 50µm. E and F, At the 72 hr after adriamycin treatment, when the dead cells were washed off from the culture dish, all the remaining live cells were found to be MNCs (nucleus indicated by arrows).Bar=50µm.
The features of MNCs found in this study suggest a dormant nature, growth arrest under stress, and ability to re-enter growth phase, which are also features of cancer stemloids (cancer stem cell like cells).25 The cancer stemloids are both self-renewable and proliferative, unlike cancer stem cells that are not active in proliferation.2,3 Cancer stem cells can become cancer stemloids by gaining the functions of proliferation, and non-cancer stem cells can also become cancer stemloids by gaining the property of self-renewal.25
Human cancer tissues also contain MNCs. As a rare population of cancer cells, multinucleated bizarre tumor cells (with identical morphologies of the large multinucleated senescent-like cells shown in our preliminary data) exist in many types of neoplasms of different organs, including breast,5 uterus,7 lung,6 kidney,13 prostate,10 thyroid,14 liver,12 salivary glands,9 testicles,4 and central nervous system,8 and are correlated with aggressiveness and poor prognosis, especially for tumors with pleomorphism.13 Virus infection was speculated to be a cause of MNC of tumor tissues; however, studies found no evidence of virus in MNCs containing tumor tissues.26 In addition, chemotherapeutic treatments indeed increase the numbers of large multinucleated cells in cancer tissues.11 The drug resistance and senescence-like features of MNCs found in this study indicate that similar mechanisms might be used by some cancer cells of human cancer tissues exposed to chemotherapy to survive. The clonogenic and single-cell tumorigenic features of MNCs bear therapeutic potential, i.e. MNCs might contribute to the development of tumor dormancy and relapse and identify targetable signaling pathways that are important for the survival/growth of MNCs.
Clonogenicity and multinucleation might be features of cells of stem cell origin given that normal tissue stem cells are clonogenic in semi-solid medium27–29 and that bone marrow-derived stem cells could undergo multinucleation.30 It is possible that the mononuclear cells that can undergo multinucleation may have features of cancer stem cells; however, thus far, molecular tools of isolating these mononuclear cells from cancer cell lines are not available.
Our present data support the possibility that solid tumors can originate from a single cell. Moreover, we demonstrated the self-renewal and asymmetric division processes of MNCs that are resistant to chemotherapy. Further investigation into the features of the mononuclear cells that can give rise to MNCs is required to enrich our understanding of cancer stem cells.
Acknowledgement
The authors thank Rachel Tsan for her excellent technical support, Kenneth Dunner, Jr., for conducting expert transmission electron microscopy, and Arminda Martinez for expert assistance in the preparation of this manuscript..
This study was supported in part by the Center of Nuclear Receptor and Cell Signaling of the University of Houston, Cancer Center Support Core Grant CA16672 and Grant 1U54-CA143837-01 from the National Cancer Institute of the National Institutes of Health.
Footnotes
CONLFLICT OF INTEREST DISCLOSURES
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- 2.Diehn M, Cho RW, Clarke MF. Therapeutic implications of the cancer stem cell hypothesis. Semin Radiat Oncol. 2009;19:78–86. doi: 10.1016/j.semradonc.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 4.Albores-Saavedra J, Huffman H, Alvarado-Cabrero I, Ayala AG. Anaplastic variant of spermatocytic seminoma. Hum Patho.l. 1996;27:650–655. doi: 10.1016/s0046-8177(96)90393-7. [DOI] [PubMed] [Google Scholar]
- 5.Douglas-Jones AG, Barr WT. Breast carcinoma with tumor giant cells. Report of a case with fine needle aspiration cytology. Acta Cytol. 1989;33:109–114. [PubMed] [Google Scholar]
- 6.Jackson MD, Albrecht R, Roggli VL, Shelburne JD. Pulmonary blastoma: an ultrastructural and histochemical study. Ultrastruct Pathol. 1984;7:259–268. doi: 10.3109/01913128409141486. [DOI] [PubMed] [Google Scholar]
- 7.Jones MA, Young RH, Scully RE. Endometrial adenocarcinoma with a component of giant cell carcinoma. Int J Gynecol Pathol. 1991;10:260–270. doi: 10.1097/00004347-199107000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Kawano H, Kubota T, Sato K, Goya T, Arikawa S, Wakisaka S. Immunohistochemical study of giant cell in glioblastoma. Clin Neuropathol. 1995;14:118–123. [PubMed] [Google Scholar]
- 9.q. Moore JG, Bocklage T. Fine-needle aspiration biopsy of large-cell undifferentiated carcinoma of the salivary glands: presentation of two cases, literature review, and differential cytodiagnosis of high-grade salivary gland malignancies. Diagn Cytopathol. 1998;19:44–50. doi: 10.1002/(sici)1097-0339(199807)19:1<44::aid-dc9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Parwani AV, Herawi M, Epstein JI. Pleomorphic giant cell adenocarcinoma of the prostate: report of 6 cases. Am J Surg Pathol. 2006;30:1254–1259. doi: 10.1097/01.pas.0000209841.77595.4b. [DOI] [PubMed] [Google Scholar]
- 11.Sneige N, Kemp B, Pusztai L, Asmar L, Hortobagyi GN. Chemotherapy-induced histologic changes in mastectomy specimens and their potential significance. Breast. 2001;10:492–500. doi: 10.1054/brst.2001.0310. [DOI] [PubMed] [Google Scholar]
- 12.Sola-Perez J, Perez-Guillermo M, Gimenez-Bascunana A, Garre-Sanchez C. Cytopathology of undifferentiated (embryonal) sarcoma of the liver. Diagn Cytopathol. 1995;13:44–51. doi: 10.1002/dc.2840130110. [DOI] [PubMed] [Google Scholar]
- 13.Staelens L, Van Poppel H, Vanuytsel L, Van Oosterom A, Van Damme B, Baert L. Sarcomatoid renal cell carcinoma: case report and review of the literature. Acta Urol Belg. 1997;65:39–42. [PubMed] [Google Scholar]
- 14.Walter P, Pusel J, Rousselot P. Multinucleated giant cell tumor of the thyroid: an unusual anaplastic carcinoma (author's transl) Pathol Res Pract. 1980;167:402–409. doi: 10.1016/s0344-0338(80)80070-7. [DOI] [PubMed] [Google Scholar]
- 15.Ariizumi T, Ogose A, Kawashima H, Hotta T, Umezu H, Endo N. Multinucleation followed by an acytokinetic cell division in myxofibrosarcoma with giant cell proliferation. J Exp Clin Cancer Res. 2009;28:44. doi: 10.1186/1756-9966-28-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosaka M, Hatori M, Smith R, Kokubun S. Giant cell formation through fusion of cells derived from a human giant cell tumor of tendon sheath. J Orthop Sci. 2004;9:581–584. doi: 10.1007/s00776-004-0825-0. [DOI] [PubMed] [Google Scholar]
- 17.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 18.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci. U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morikawa K, Walker SM, Jessup JM, Fidler IJ. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 1988;48:1943–1948. [PubMed] [Google Scholar]
- 20.Li L, Price JE, Fan D, Zhang RD, Bucana CD, Fidler IJ. Correlation of growth capacity of human tumor cells in hard agarose with their in vivo proliferative capacity at specific metastatic sites. J Natl Cancer Inst. 1989;81:1406–1412. doi: 10.1093/jnci/81.18.1406. [DOI] [PubMed] [Google Scholar]
- 21.Hwang ES. Replicative senescence and senescence-like state induced in cancer-derived cells. Mech Ageing Dev. 2002;123:1681–1694. doi: 10.1016/s0047-6374(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 22.Park SC, Cho KA, Jang IS, Kim KT, Ryu SJ. Functional efficiency of the senescent cells: replace or restore? Ann N Y Acad Sci. 2004;1019:309–316. doi: 10.1196/annals.1297.052. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 24.Kripke ML. Latency, histology, and antigenicity of tumors induced by ultraviolet light in three inbred mouse strains. Cancer Res. 1977;37:1395–1400. [PubMed] [Google Scholar]
- 25.Blagosklonny MV. Cancer stem cell and cancer stemloids: from biology to therapy. Cancer Biol Ther. 2007;6:1684–1690. doi: 10.4161/cbt.6.11.5167. [DOI] [PubMed] [Google Scholar]
- 26.Kambham N, Troxell M, Longacre TA. Multinucleated epithelial giant cells in colorectal polyps: a potential mimic of viropathic and/or dysplastic changes. Am J Surg Pathol. 2005;29:912–919. doi: 10.1097/01.pas.0000164614.30576.da. [DOI] [PubMed] [Google Scholar]
- 27.Lin TM, Chang HW, Wang KH, et al. Isolation and identification of mesenchymal stem cells from human lipoma tissue. Biochem Biophys Res Commun. 2007;361:883–889. doi: 10.1016/j.bbrc.2007.07.116. [DOI] [PubMed] [Google Scholar]
- 28.Spence SE, Keller JR, Ruscetti FW, et al. Engraftment of ex vivo expanded and cycling human cord blood hematopoietic progenitor cells in SCID mice. Exp Hematol. 1998;26:507–514. [PubMed] [Google Scholar]
- 29.Yang YC, Wang SW, Hung HY, et al. Isolation and characterization of human gastric cell lines with stem cell phenotypes. J Gastroenterol Hepatol. 2007;22:1460–1468. doi: 10.1111/j.1440-1746.2007.05031.x. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida K, Obata S, Ono M, Esaki M, Maejima T, Sawada H. TPA-induced multinucleation of a mesenchymal stem cell-like clone is mediated primarily by karyokinesis without cytokinesis, although cell-cell fusion also occurs. Eur J Cell Biol. 2007;86:461–471. doi: 10.1016/j.ejcb.2007.04.003. [DOI] [PubMed] [Google Scholar]