Abstract
The high sensitivity, extended mass range, and fast data acquisition/processing of mass spectrometry and its coupling with native electrospray ionization (ESI) make the combination complementary to other biophysical methods of protein analysis. Protein assemblies with molecular masses up to MDa are now accessible by this approach. Most current approaches have used quadrupole/time-of-flight tandem mass spectrometry, sometimes coupled with ion mobility, to reveal stoichiometry, shape, and dissociation of protein assemblies. The amino-acid sequence of the subunits, however, still relies heavily on independent bottom-up proteomics. We describe here an approach to study protein assemblies that integrates electron-capture dissociation (ECD), native ESI, and FTICR mass spectrometry (12 Tesla). Flexible regions of assembly subunits of yeast alcohol dehydrogenase (147 kDa), concanavalin A (103 kDa), and photosynthetic Fenna-Matthews-Olson antenna protein complex (140 kDa) can be sequenced by ECD or “activated-ion” ECD. Furthermore, non-covalent metal-binding sites can also be determined for the ConA assembly. Most importantly, the regions that undergo fragmentation, either from one of the termini by ECD or from the middle of a protein, as initiated by CID, correlate well with the B-factor from X-ray crystallography of that protein. This factor is a measure of the extent an atom can move from its coordinated position as a function of temperature or crystal imperfections. The approach provides not only top-down proteomics information of the complex subunits but also structural insights complementary to those obtained by ion mobility.
INTRODUCTION
Determination of protein structure and function is important in areas ranging from human health to photosynthesis.1, 2, 3 That most proteins carry out their functions as a complex or protein assembly presents a serious challenge for analytical chemistry research. Protein assemblies can have molecular masses from kDa to MDa and contain many components.4, 5 For example, in the yeast proteome, approximately 70% of the more than 4000 proteins identified by mass spectrometry (MS) are involved in protein-protein interactions. One estimate is that more than 500 protein complexes are formed, each containing five subunits on average.6 To understand the structure and function of these high-order assemblies, especially those that are difficult to study by using traditional techniques, complementary methods are needed to provide structural information.
Protein structures are usually investigated by electron microscopy, X-ray crystallography, NMR spectroscopy, and small-angle scattering,7, 5 and those of protein assemblies are particularly challenging. One rapidly growing area is MS of protein assemblies.8 The major approach for introducing assemblies to the gas phase is native electrospray (ESI) from aqueous solutions under conditions that are highly aqueous and close to physiological.9 The studies afford information about stoichiometry, structure, and subunit interactions. Although MS cannot provide atomic-level resolution, as can X-ray crystallography and NMR, its advantages for interrogating near-native-state protein assemblies are small sample consumption, high throughput, and unique specificity to sample size heterogeneity; these combined advantages make it complementary to traditional structural biology techniques.10
Since Ganem and co-workers11 first demonstrated the application of MS for protein complexes, both the instrumentation and methods of MS have improved, increasing its potential in structural biology10; progress was reviewed recently.12, 13, 14 Evidence that complexes can be kept near their native conformations in the gas phase is also emerging.15, 16, 17 The analysis of membrane protein complexes18, 19 and large protein machines up to MDa,20 for example, are now being undertaken.
Two mass analyzers, time-of-flight (TOF) and Fourier transform ion cyclotron resonance (FTICR), are the appropriate and commonly used tools for measuring the high m/z protein-assembly ions introduced via native ESI. The principal instrumentation used thus far is a quadrupole/time-of-flight (QToF)21 owing to its high upper-mass limit and compatibility as an interface with ion mobility (IM). A small number of groups are using FTICR MS coupled with native ESI.22, 23 Although QToF instruments provide accurate mass, dissociation chemistry, and when coupled with IM, assembly-shape information, identification of the subunit via sequence information still relies on standard bottom-up sequencing.8 The disadvantage is that the dynamics, assembly shape and structural layout are lost in any bottom-up analysis.
A top-down approach that integrates those two independent experiments into one platform would have significant advantages for sample preparation and data interpretation.24 Different fragmentation methods, CID, ECD, BIRD, IRMPD and SID, are available for the top-down approach.25, 26, 27, 28, 29, 30, 31 For example, Robinson and co-workers32 have used a modified QTOF mass spectrometer and IM to study the unfolding of protein assemblies. Some fragment ions from backbone cleavages can be seen, showing the potential of tandem MS in structural studies of protein assemblies. A recent study on human transthyretin demonstrated that peptide fragments are generated by a charge-state-dependent decay.33 Charge reduction can make CID experiments successful for breaking the backbone of protein complexes, because more charges make complexes less stable upon CID such that subunit release is preferred, whereas fewer charges stabilize the complex such that backbone cleavage competes with subunit disassembly.
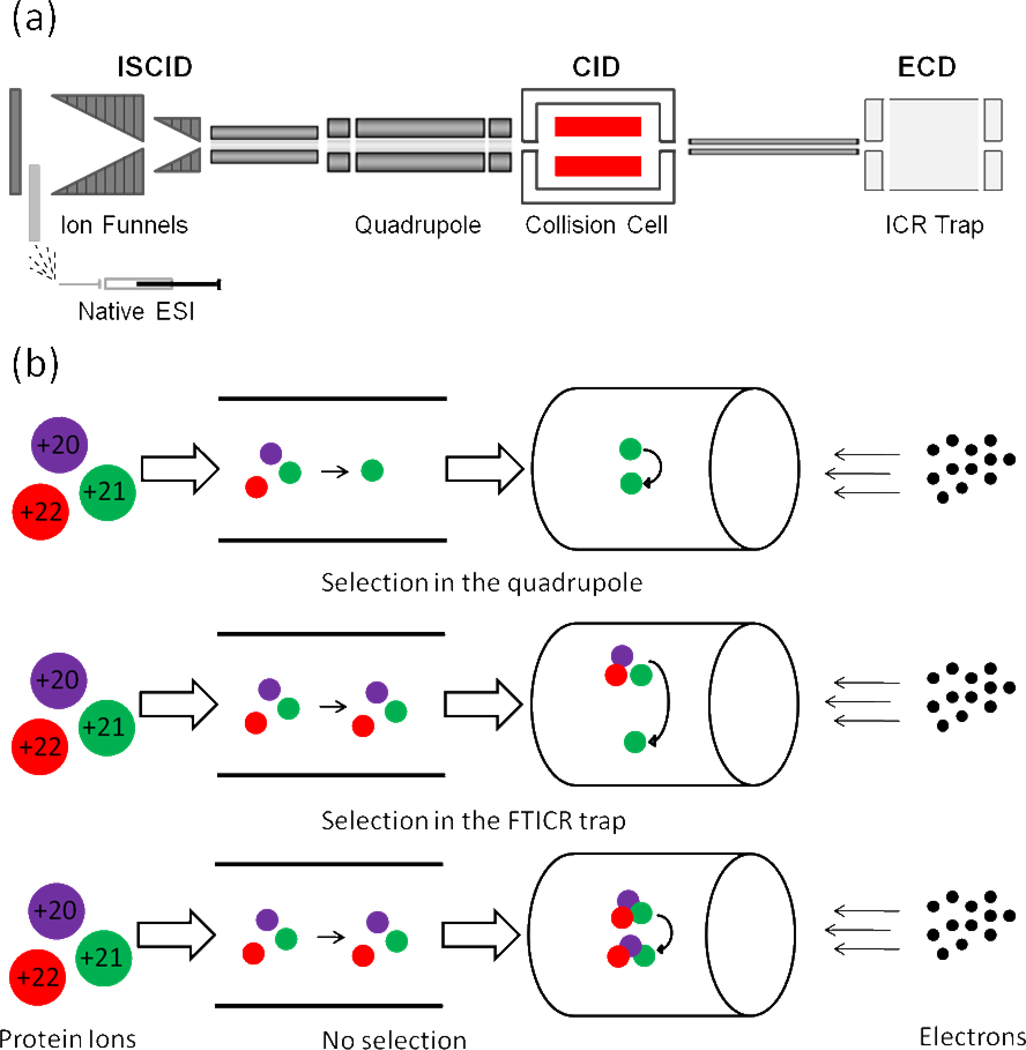
The advantages of hybrid Qq-FTICR MS are its versatile fragmentation methods for top-down purposes (Figure 1a). Using hybrid Qq-FTICR, one can fragment protein assemblies by ECD or IRMPD in the ICR trap or by CID or ISCID (in-source fragmentation) in the front end of the instrument to form highly charged sub-complexes or monomeric subunits. Additionally, ECD fragmentation to generate sequence ions of a subunit is facilitated when the subunit bears a large number of charges while they are stored in the FTICR trap. The high mass resolving power of FTICR allows the resolution of isotope peaks, making feasible the analysis of some complicated product-ion spectra. Heeren and co-workers34 first reported the application of ECD and FTICR MS in the study of non-covalent protein assemblies. They selected each charge state of the assembly in the FTICR trap before ECD, and observed no ECD-induced backbone cleavage product ions (i.e., sequence ions), possibly because the dynamic range was insufficient.
Figure 1.
(a) The layout of Hybrid Qq-FTICR instrument. (b) ECD top-down sequencing by FTICR MS: (1) after selection of one charge state in the quadrupole region and fragmentation in the ICR trap (top); (2) after selection and fragmentation in the FTICR trap (middle); and (3) with no pre-selection by quadrupole or in the ICR trap (bottom).
We reported recently that a large number of consecutive backbone cleavages occur for the 147 kDa yeast alcohol dehydrogenase tetramer upon ECD in a 12 tesla FTICR mass spectrometer.35 We now follow up this observation with a report on a more detailed and general development and application of ECD and CID to several different homogeneous protein assemblies. We show that the masses, subunit identities, metal-ion binding sites, and some structural information can be obtained in one experiment. The ECD fragmentation patterns of the protein complexes activated at different collision energies show preferred fragmentations of one terminus over the other, or of the middle region, indicating that the fragmentations are structurally significant. We also seek an explanation of those regions undergoing fragmentation and test whether they correlate with the B-factor from X-ray crystallography, a parameter that is predictive of the flexible regions of a protein.
MATERIAL AND METHODS
Chemicals and Proteins
Ammonium acetate, water, yeast alcohol dehydrogenase (ADH) form Saccharomyces cerevisiae, concanavalin A (ConA) from Canavalia ensiformis (Jack bean) were purchased from Sigma-Aldrich (St. Louis, MO). The FMO protein from the green sulfur bacterium Chlorobaculum (Chlorbium) tepidum was purified as previously described.36
Sample preparation for Native ESI
Lyophilized protein powder was dissolved in 100 mM ammonium acetate (pH = 6.5–7) to afford an assembly concentration at 5 µM. The sample was washed three times with equal volume 100 mM ammonium acetate solution in a Vivaspin 500 concentrator with 10,000 or 30,000 molecular-weight cut off (MWCO) (Vivaproducts Inc., Littleton, MA). Buffer exchange of purified FMO protein sample was conducted by Vivaspin 500 concentrators (30,000 MWCO) before the native ESI experiment.
MS of Protein complexes
The protein sample was delivered by syringe pump (Harvard PHD Ultra syringe pump, Instech Laboratories, Inc., Plymouth Meeting, PA) at flow rate 5–300 nL/min to a nano spray source, which was comprised of a custom-pulled nano spray tip (Sutter Instrument Co., Novato, CA) of silica capillary tubing (360 µm o.d., 150 µm i.d., Polymicro Technologies, Phoenix, AZ). Mass spectra were acquired with a Bruker Solarix 12 T FTICR mass spectrometer (Bruker Daltonics, Bremen, Germany). The capillary voltage was 0.9–1.3 kV. The drying-gas temperature was 100 °C; its flow was 2.5 L/min. The voltage for ISCID was varied from 0 to 100 V depending on the application. The ion-funnel RF amplitude was 300 Vpp, and the ion-funnel voltages were 200 V (funnel 1) and 18 V (funnel 2). RF frequencies used in all ion-transmission regions were the lowest available value: multipole 1 (2 MHz), quadrupole (1.4 MHz) and transfer line (1 MHz). The collision voltage for the collision cell was varied from 0 to 50 V, depending on the application. Ions were accumulated for 500 ms in the RF-hexapole ion trap before being transmitted to the infinity ICR trap. The time-of-flight was ~ 2.5 ms for the protein-assembly ions. The source region (PS1) pressure was 2.3 mbar; the quadrupole region (PS4) pressure was 4.4 × 10−6 mbar, and the trap-chamber pressure (PS6) was 1.3 × 10−9 mbar. The typical ECD pulse length was 0.06 s, ECD bias 0.6 V and ECD lens 10 V. The ECD hollow cathode heater current was 1.6 A. MS parameters were slightly modified in each individual sample to obtain an optimized signal. One to several hundred scans were averaged for each spectrum. External calibration was done by ESI of cesium perfluoroheptanoic acetate up to m/z 8500.
Data Analysis
Peak picking and spectra deconvolution were performed with Bruker Data Analysis software (Bruker Daltonics, Bremen, Germany). Bruker Biotools software was used for mapping the measured peak mass list with calculated fragment mass list from the protein sequences. To validate that the sequence information was sufficient to identify the constituent subunits, sequence tags were generated with the Biotools software based on the deconvoluted spectra and submitted to Mascot searching against NCBI database as previous reported.35 Alternative peak matching was conducted by Prosight PTM (v1.0, https://prosightptm.northwestern.edu)37.
RESULTS AND DISCUSSION
ECD of protein complexes
In these FTICR MS experiments, electrons from a cathode emitter interact with trapped protein-assembly ions to initiate electron-capture dissociation (ECD). We could select the ions based on their m/z before fragmentation either prior to the ICR trap, using a selection quadrupole or in the FTICR trap itself (Figure 1b). Ion selection allows the study of each species in a single charge state. Large protein-assembly ions in our experiment had m/z values close to 6000 (the upper selection limit on the quadrupole of the Bruker Solarix) because native spray produces lower charge states than normal ESI, where the proteins are denatured. Preselecting high m/z ions by the quadrupole was inefficient, giving poor signal intensity. To select ions in the FTICR trap, the waveforms used to excite an ion of interest also excite ions of nearby m/z. This excitation causes movement of high m/z ion packets from the trap’s central axis, reducing the overlap between protein-assembly ions and the emitted electrons needed for ECD.38
Because preselecting for ECD is not highly efficient, we chose not to preselect and instead to submit the entire charge distribution to ECD. This is not a problem here because the protein assembly was relatively pure, and native ESI generated a narrow charge-state distribution extending over ~ 4 charge states with one as most abundant. In our communication that described preliminary results, we also did no preselecting and obtained, using ECD, a set of c ions from the alcohol dehydrogenase (ADH) protein assemblies.35 A report from the Langridge-Smith group39 demonstrated a similar improvement in overall efficiency by applying ECD to several of the most abundant charge states of a protein-ligand complex.
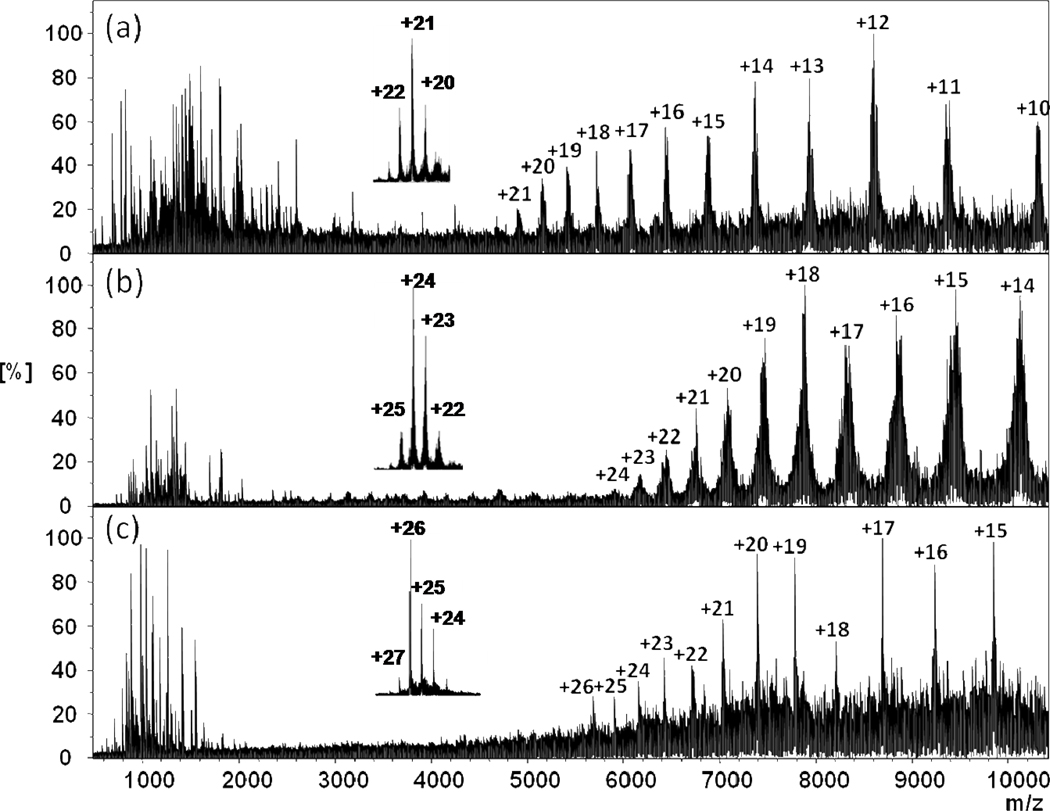
Having established a protocol, we conducted top-down ECD of protein assemblies of different sizes and oligomer states. In addition to the ADH assembly, other homogeneous assemblies, concanavalin A (ConA), and the photosynthetic Fenna-Matthews-Olson antenna protein complex (FMO), also fragment to give c or z ions and charge reduction (Figure 2). The results are predictive that ECD top-down fragmentation of protein assemblies will be useful for other assemblies. The approach affords subunit sequence information and MW information in single experiment.
Figure 2.
ECD mass spectra of intact protein assemblies: (a) concanavalin A (ConA) from Canavalia ensiformis (Jack bean); (b) FMO antenna protein from green sulfur bacterium Chlorobaculum tepidum; (c) yeast alcohol dehydrogenase (ADH) from Saccharomyces cerevisiae. The insets are native ESI spectra of each protein complex.
Subunit sequence and locating non-covalent metal binding sites
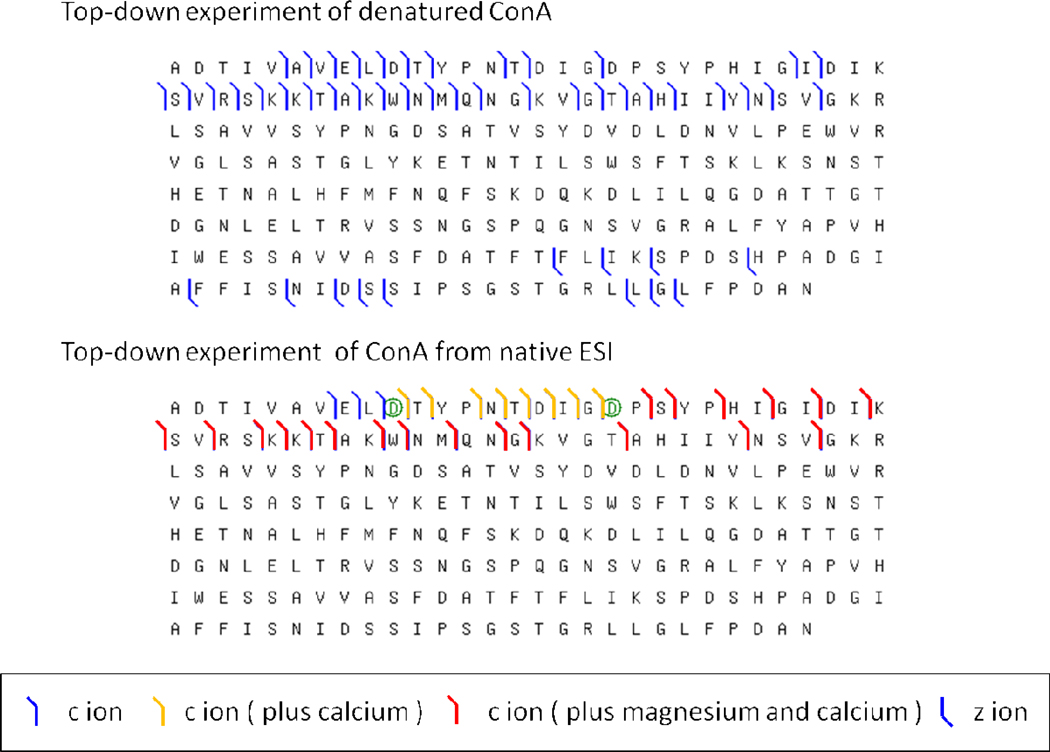
ECD of protein assemblies can produce ions representing both the full assembly and its fragment ions. Information extracted from this single experiment can also give subunit identification and stoichiometry. As for the previously reported ECD of the yeast ADH assembly, the ECD fragmentation extent is sufficient to identify the constituent subunit by sequence-database searching.35 The stoichiometry of the assembly can be determined on the basis of the molecular mass of the assembly and that of its subunits. For ConA, the intact mass of the assembly (103 kDa) is read out directly from the spectrum of native ESI. By applying ECD to the assembly, c fragment ions form, and their masses can be assigned within 20 ppm accuracy. Sequence information matched ConA (237 amino acid, 25,539 Da, UniProtKB database ID: CONA_CANVI). A total of 38 fragment ions, with and without metal adducts, covered the N terminus of ConA from the start to the 57th residue from the N terminus. The subunit identity and intact mass of the complex make it straightforward to conclude that ConA forms a tetramer.
ECD is capable of identifying labile modifications and non-covalent ligand binding sites.40 In the case of ConA, metal binding is preserved in the gas phase upon native ESI. Each lectin ConA protein has two metal-binding sites, one for Mn2+ at the S1 site and another for Ca2+ at the S2 site41; these sites must be occupied for ConA to interact with glycogen. Previous metal-binding studies demonstrated that several bivalent metal ions can bind to ConA as substitutes for Mn2+ and Ca2+ (e.g., Co2+, Ni2+, Cd2+, Mg2+ for the S1 site, Cd2+ for the S2 site).42, 43, 44 Fragment ions containing Ca2+ and Mg2+ are preserved in the ECD top-down study of tetrameric ConA. On the basis of ECD fragmentation (Figure 3a), residues Asp-19 and Asp-10 can be identified as part of major Mg2+ and Ca2+ binding sites; these assignments are in agreement with the S1 and S2 binding sites seen in the crystal structure.45 We note that an ECD top-down experiment with denatured ConA failed to reveal metal binding as the metal ion was lost upon either denaturation or ESI (Figure 3b). Precedents for this outcome come from other studies identifying phosphorylation and drug/metal-binding sites. For example, Loo and co-workers demonstrated that ECD in a top-down format can locate non-covalent drug binding sites.46, 47 Elucidation of the metal binding sites by top-down MS of proteins was also demonstrated by several groups.48, 49, 50
Figure 3.
Sequence coverage of ConA by top-down ECD for denatured ConA (24+ charge state, in 50% acetonitrile 50% water 1% formic acid) (top) and for ConA in its near native state (bottom).
In most protein-assembly studies by MS, the mass of the assembly is measured by native ESI whereas subunit identity and PTM information are provided by bottom-up LC-MS of the proteolytically digested assembly. The results reported here show that these two independent experiments can be integrated into one by using a top-down approach with fragmentation induced by ECD. More importantly, this approach should be applicable to the study of non-covalent ligand binding and labile PTMs (e.g., phosphorylation and glycosylation) of a protein assembly.
Structural information elucidated by ECD experiment
Gas-phase dissociation of protein assemblies is a rapidly growing area in MS.27 Protein assemblies are often dissociated by applying additional accelerating potential or heating during the transmission of the ions from ion source to detector.51, 52 The dissociation usually results in ejection of a few protein subunits from the assembly, providing a view of the assembly’s arrangement.25 Interestingly, ejected subunits usually carry more charges per mass than the remainder of the assembly. IM offers insight on this phenomenon; as a region unfolds, protons move from the core to sites on the periphery, causing it to charge disproportionately and then dissociate.27
Subunit unfolding can be investigated by a top-down approach using ECD. This “peel-an-onion” process occurs because as the internal energy of the assembly increases, the relatively flexible and unstructured arms of the constituent proteins become vulnerable to fragmentation. In our experiments, we increased the internal energy of the protein assembly by increasing the acceleration voltage to afford ISCID with nitrogen as the collision gas. We could follow the unfolding induced by increases in internal energy by analyzing the sites of ECD fragmentation. For example, we activated the tetrameric assembly of yeast ADH by increasing the acceleration voltage from 0 to 100 V (Figure S1). At each voltage setting, 100 ECD scans were averaged. Various c ions of higher m/z become more abundant with increasing acceleration voltage (Figure 4a). The results indicate that gas phase protein unfolding starts at the N-terminus and extends toward the core of the assembly.
Figure 4.
(a) Normalized abundances of the ECD fragment ions (c ions) from the ADH assembly at different acceleration potentials (ISCID). (b) Atomic displacement parameter (B-factor) plot for the C terminal region of ADH. The B-factor values are from the crystal structure of yeast ADH (PDB id: 2HCY). In the crystal structure, the protein has four different conformations represented by Chain A–D.
Correlation of fragmentation with the B-factor
We recognize in these results some clear opportunities for new structural insights from ECD. For example, the ECD pattern of fragments may serve as an indicator of flexibility in protein structure. In the x-ray crystal structure, the atomic displacement parameter (B-factor) reflects the flexibility and dynamics of a polypeptide chain; 53, 54 for example, a large B-factor indicates high mobility of individual atoms and side chains. When we plot the B-factor as a function of the location of the amino-acid residue for the N-terminal region of ADH, we see that the extent of fragmentation shows similar trends; we compared all B-factor values from different subunit conformations. The best correlation was observed on chain B conformation, which was from the crystal structure. The highest B-factor is on the end of the terminal region of the four conformations (Figure 4b). We interpret this as evidence that this region unfolds first, moving away from the protein core, adopting charge, and then undergoing subsequent fragmentation (Figure 5). There is evidence from previous IM studies on protein assemblies that subunit unfolding occurs before dissociation. A more detailed unfolding process can now be proposed by considering the ECD outcome and locating regions of structural flexibility. Given that protein flexibility is highly correlated with protein function, ECD in a top-down format may inform us about not only structure but also flexibility of proteins in assemblies. The correlation is not quantitative, but a rough correlation is sufficient because can be invaluable in designing proteins suitable for crystallization in x-ray crystallography.
Figure 5.
Tetrameric ADH crystal structure color-coded to show the B factor extent. Tetrameric ADH complex was assembled by crystal packing (PDB id: 2HCY). Dimer on the bottom of ADH complex is displayed with the B-factor scheme (the color and width represent the value of B factor) from crystal data (Chain B). In the yeast ADH crystal structure, there are four different conformations for subunit. We compared all B-factor values from different subunit conformations. The best correlation was observed on chain B conformation which was plot in the crystal structure
Structural elucidation by top-down ECD and CID
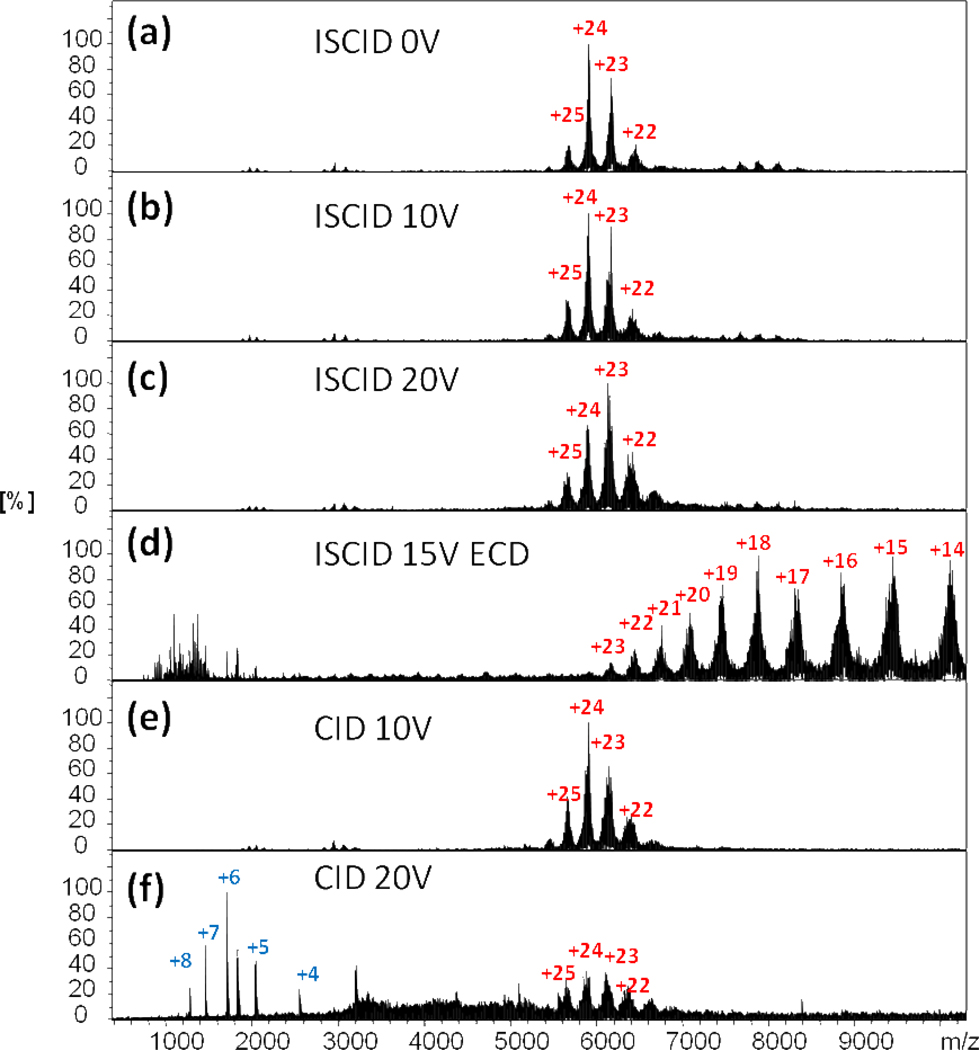
Although an ECD-based top-down approach to protein assemblies appears to identify the terminal regions of high flexibility, a flexible region in the middle of a protein sequence may not be sampled in the fragmentation. We decided to integrate CID in the platform to see if the combination of CID and ECD can provide more comprehensive structural information. In the study of the trimeric FMO photosynthetic antenna protein, only z ions from the C-terminal end of FMO are produced in the ECD top-down experiment (Figure 6), and these results are consistent with the C-terminal region of FMO being more flexible than the N-terminal region, which is heavily involved in forming the protein subunit interface.
Figure 6.
Native ESI and ECD/CID top-down spectra of the FMO antenna protein complex. (a–c) native ESI spectra of FMO with 0, 10, 20 V ISCID. No fragment ions were observed. (d) ECD spectrum of FMO protein with 15 V ISCID. (e) CID (10 V) spectrum of FMO; no fragment ions were observed. (f) CID (20 V) spectrum of FMO. Multiply charged fragment ions were observed at low m/z.
Interestingly, an unusual set of fragment ions of the FMO protein with charge states from 4+ to 8+ were observed in a CID experiment (Figure 6f). A database search indicates the fragment ion is not from the N- or C-terminal regions. Using the high mass resolving power and accurate mass measurement capabilities of FTICR MS, we identified this 10160.27 ± 0.01 Da species as originating from residues 201–295 (calculated as 10160.26 Da) in the middle of the FMO protein sequence (Figure S2).
The unique fragment patterns are consistent with the assembly’s structure. The FMO protein trimer contains 21 bacteriochlorophyll a (BChl a) pigments (seven BChl a pigments inside each subunit) by forming a tight trimeric entity held together primarily by salt bridges55. We now know there are three additional BChl a pigments on the surface of the FMO protein trimeric complex56. Each subunit contains a total of eight BChl a pigments (seven inside the subunit and one on the surface). For each subunit, a series of beta sheets form two parallel walls, like a “taco shell”, holding the seven BChl a pigments. The open end of the “taco shell” contains several alpha helixes and two BChl a pigments (BChl 1 and 2) and points to the center of the trimeric complex; all seven BChl a pigments are buried inside. Dissociation of the assembly will destroy those salt bridges and expose BChl a pigments, a process that requires high energy. Thus, no subunit ejection occurred upon CID. Alternatively, a loop within the region 201–295 unfolded first, fragmented upon collisional activation, and a large peptide from the middle of the protein was lost.
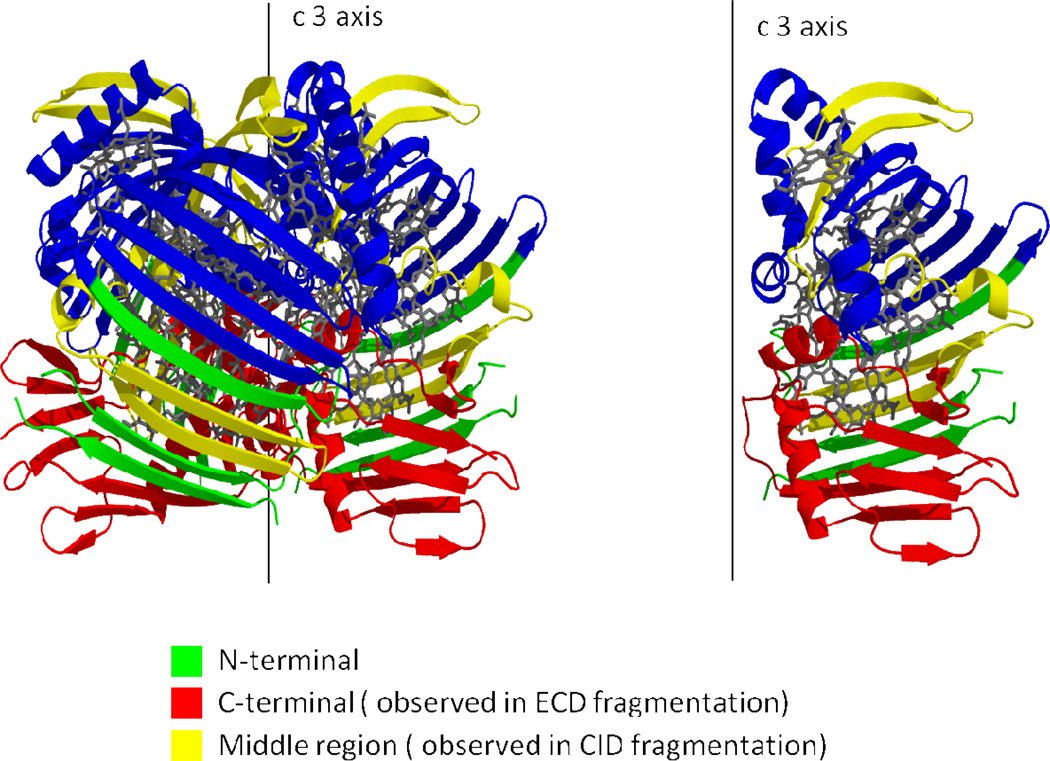
The crystal structure of trimeric FMO complex57 (Figure 7) shows that the C terminus of the protein is at the bottom of this complex, whereas the N-terminus is in a middle beta-sheet structure forming the side wall of the assembly. The region that undergoes CID is on the top of protein complex. Given our supposition that the locale for fragmentation is a flexible region of the protein structure, we tested again the B-factor as a correlate for the fragment pattern. Indeed, the region undergoing CID is one that has one of the highest B factors in the protein sequence. Moreover, the region 201–295 released upon CID overlaps with the region 275–366 that was sequenced by ECD. The combined outcomes of ECD and CID afford evidence that the unfolding starts at the middle region of protein. This initial unfolding also increases the flexibility of the polypeptide chain extending to the C-terminus; that chain then undergoes ECD.
Figure 7.
Crystal structure of the FMO antenna protein complex; the protein is displayed in cartoon mode; BChl a is depicted as a stick mode with gray color.
CONCLUSIONS
ECD in a top-down format has the potential to become a complementary tool for structural studies of protein assemblies and to give a unique view of the flexible regions of a protein assembly. The results from three protein complexes show that informative fragmentations occur (upon both CID and ECD) and that they are indicative of structure. The conclusion is supported by the results from three model assemblies: tetrameric yeast ADH, trimeric FMO antenna complex of green sulfur bacteria, and the tetrameric plant lectin concanavalin A. Specifically, we could obtain in one experiment, depending on the protein, (1) sequence information, (2) non-covalent metal-binding sites, (3) assembly stoichiometry, and (4) structural insights that pinpoint flexible regions. This approach is complementary not only to the traditional methods of x-ray crystal structure determination and NMR spectroscopy but also to the commonly applied combinations of native ESI, QToF MS, and ion mobility.
Although this FT-ICR based ECD top-down approach is still in its infancy, its promise will be realized with improvements in efficiency and selection capability and with its integration with IRMPD, other photochemical activation schemes, and possibly in-trap, high-energy collisional activation. Furthermore, different and important protein complexes of heterogeneous composition need to be investigated to demonstrate a broader application of this approach; some of these investigations are underway in our laboratory.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Center for Research Resource of the NIH (Grant P41RR000954), High-End Instrument Program of the NCRR (Grant 1S10 025101), and the Instrument Development for Biological Research Program of the NSF (Grant 0964199) to M.L.G. and U.S. Department of Energy (Grant DE-FG02-07ER15902) to R.E.B. This research is from the Photosynthetic Antenna Research Center (PARC), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences (Grant DE-SC 0001035). Additional support was provided by Merck. M.L.G. was a consultant for Merck.
REFERENCES
- 1.Dessen A, Xu W. Curr Opin Struct Biol. 2010;20:711–713. doi: 10.1016/j.sbi.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Fromme P. Hoboken, NJ: John Wiley & Sons, Inc.; 2008. [Google Scholar]
- 3.Petsko GA, Ringe D. In: Lawrence E, Robertson M, editors. London: New Science Press Ltd; 2004. [Google Scholar]
- 4.Ban N, Egelman EH. Curr Opin Struct Biol. 2010;20:207–209. doi: 10.1016/j.sbi.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Robinson CV, Sali A, Baumeister W. Nature. 2007;450:973–982. doi: 10.1038/nature06523. [DOI] [PubMed] [Google Scholar]
- 6.Krogan NJ, Cagney G, Yu H, Zhong G, Guo X, Ignatchenko A, Li J, Pu S, Datta N, Tikuisis AP, Punna T, Peregrin-Alvarez JM, Shales M, Zhang X, Davey M, Robinson MD, Paccanaro A, Bray JE, Sheung A, Beattie B, Richards DP, Canadien V, Lalev A, Mena F, Wong P, Starostine A, Canete MM, Vlasblom J, Wu S, Orsi C, Collins SR, Chandran S, Haw R, Rilstone JJ, Gandi K, Thompson NJ, Musso G, St Onge P, Ghanny S, Lam MH, Butland G, Altaf-Ul AM, Kanaya S, Shilatifard A, O'Shea E, Weissman JS, Ingles CJ, Hughes TR, Parkinson J, Gerstein M, Wodak SJ, Emili A, Greenblatt JF. Nature. 2006;440:637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- 7.Hasnain SS, Wakatsuki S. Curr Opin Struct Biol. 2010;20:584–586. doi: 10.1016/j.sbi.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Zhou M, Robinson CV. Trends Biochem Sci. 2010;35:522–529. doi: 10.1016/j.tibs.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Heck AJ. Nat Methods. 2008;5:927–933. doi: 10.1038/nmeth.1265. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzen K, Duijn EV. In: UNIT 17.12 Native Mass Spectrometry as a Tool in Structural Biology. Coligan JE, Dunn BM, Speicher DW, Wingfield PT, editors. vol. 62. John Wiley & Sons, Inc.; 2010. [Google Scholar]
- 11.Ganem B, li Y-T, Henion JD. J Am Chem Soc. 1991:7818–7819. [Google Scholar]
- 12.Benesch JL, Ruotolo BT, Simmons DA, Robinson CV. Chem Rev. 2007;107:3544–3567. doi: 10.1021/cr068289b. [DOI] [PubMed] [Google Scholar]
- 13.Heck AJ, Van Den Heuvel RH. Mass Spectrom Rev. 2004;23:368–389. doi: 10.1002/mas.10081. [DOI] [PubMed] [Google Scholar]
- 14.Loo JA. Mass Spectrom Rev. 1997;16:1–23. doi: 10.1002/(SICI)1098-2787(1997)16:1<1::AID-MAS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Leary JA, Schenauer MR, Stefanescu R, Andaya A, Ruotolo BT, Robinson CV, Thalassinos K, Scrivens JH, Sokabe M, Hershey JW. J Am Soc Mass Spectrom. 2009;20:1699–1706. doi: 10.1016/j.jasms.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Ruotolo BT, Giles K, Campuzano I, Sandercock AM, Bateman RH, Robinson CV. Science. 2005;310:1658–1661. doi: 10.1126/science.1120177. [DOI] [PubMed] [Google Scholar]
- 17.Uetrecht C, Versluis C, Watts NR, Wingfield PT, Steven AC, Heck AJ. Angew Chem Int Ed Engl. 2008;47:6247–6251. doi: 10.1002/anie.200802410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrera NP, Di Bartolo N, Booth PJ, Robinson CV. Science. 2008;321:243–246. doi: 10.1126/science.1159292. [DOI] [PubMed] [Google Scholar]
- 19.Barrera NP, Isaacson SC, Zhou M, Bavro VN, Welch A, Schaedler TA, Seeger MA, Miguel RN, Korkhov VM, van Veen HW, Venter H, Walmsley AR, Tate CG, Robinson CV. Nat Methods. 2009;6:585–587. doi: 10.1038/nmeth.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uetrecht C, Versluis C, Watts NR, Roos WH, Wuite GJ, Wingfield PT, Steven AC, Heck AJ. Proc Natl Acad Sci U S A. 2008;105:9216–9220. doi: 10.1073/pnas.0800406105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobott F, Hernandez H, McCammon MG, Tito MA, Robinson CV. Anal Chem. 2002;74:1402–1407. doi: 10.1021/ac0110552. [DOI] [PubMed] [Google Scholar]
- 22.Janis J, Pasanen S, Rouvinen J, Vainiotalo P. J Mass Spectrom. 2008;43:1376–1380. doi: 10.1002/jms.1413. [DOI] [PubMed] [Google Scholar]
- 23.Kitova EN, Kitov PI, Bundle DR, Klassen JS. Glycobiology. 2001;11:605–611. doi: 10.1093/glycob/11.7.605. [DOI] [PubMed] [Google Scholar]
- 24.van Duijn E. J Am Soc Mass Spectrom. 2010;21:971–978. doi: 10.1016/j.jasms.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Benesch JL. J Am Soc Mass Spectrom. 2009;20:341–348. doi: 10.1016/j.jasms.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Benesch JL, Aquilina JA, Ruotolo BT, Sobott F, Robinson CV. Chem Biol. 2006;13:597–605. doi: 10.1016/j.chembiol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Benesch JL, Robinson CV. Curr Opin Struct Biol. 2006;16:245–251. doi: 10.1016/j.sbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 28.El-Faramawy A, Guo Y, Verkerk U, Thomson BA, Siu M. 56th ASMS Conference on Mass Spectrometry and Allied Topics; Denver, Co: 2008. [Google Scholar]
- 29.Felitsyn N, Kitova EN, Klassen JS. Anal Chem. 2001;73:4647–4661. doi: 10.1021/ac0103975. [DOI] [PubMed] [Google Scholar]
- 30.Jones CM, Beardsley RL, Galhena AS, Dagan S, Cheng G, Wysocki VH. J Am Chem Soc. 2006;128:15044–15045. doi: 10.1021/ja064586m. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzen K, Versluis C, van Duijn E, van den Heuvel RHH, Heck AJR. Intl. J. Mass Spectrom. 2007;268:198–206. [Google Scholar]
- 32.Benesch JL, Ruotolo BT, Sobott F, Wildgoose J, Gilbert A, Bateman R, Robinson CV. Anal Chem. 2009;81:1270–1274. doi: 10.1021/ac801950u. [DOI] [PubMed] [Google Scholar]
- 33.Pagel K, Hyung SJ, Ruotolo BT, Robinson CV. Anal Chem. 2010;82:5363–5372. doi: 10.1021/ac101121r. [DOI] [PubMed] [Google Scholar]
- 34.Geels RB, van der Vies SM, Heck AJ, Heeren RM. Anal Chem. 2006;78:7191–7196. doi: 10.1021/ac060960p. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Cui W, Wen J, Blankenship RE, Gross ML. J Am Soc Mass Spectrom. 2010;21:1966–1968. doi: 10.1016/j.jasms.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen J, Zhang H, Gross ML, Blankenship RE. Proc Natl Acad Sci U S A. 2009;106:6134–6139. doi: 10.1073/pnas.0901691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeDuc RD, Taylor GK, Kim YB, Januszyk TE, Bynum LH, Sola JV, Garavelli JS, Kelleher NL. Nucleic Acids Res. 2004;32:W340–W345. doi: 10.1093/nar/gkh447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan S, Burlingame AL. J Am Soc Mass Spectrom. 2010;21:455–459. doi: 10.1016/j.jasms.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke DJ, Murray E, Faull PA, Hupp T, Barran P, Langridge-Smith P, Mackay CL. 58th ASMS Conference on Mass Spectrometry and Allied Topics; Utah: Salt Lake City; 2010. [Google Scholar]
- 40.Cooper HJ, Hakansson K, Marshall AG. Mass Spectrom Rev. 2005;24:201–222. doi: 10.1002/mas.20014. [DOI] [PubMed] [Google Scholar]
- 41.Hardman KD, Ainsworth CF. Biochemistry. 1972;11:4910–4919. doi: 10.1021/bi00776a006. [DOI] [PubMed] [Google Scholar]
- 42.Kalb AJ, Levitzki A. Biochem J. 1968;109:669–672. doi: 10.1042/bj1090669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders JN, Chenoweth SA, Schwarz FP. J Inorg Biochem. 1998;70:71–82. doi: 10.1016/s0162-0134(98)00016-6. [DOI] [PubMed] [Google Scholar]
- 44.Young NM. FEBS Lett. 1983;161:247–250. doi: 10.1016/0014-5793(83)81018-7. [DOI] [PubMed] [Google Scholar]
- 45.Edelman GM, Cunningham BA, Reeke GN, Jr, Becker JW, Waxdal MJ, Wang JL. Proc Natl Acad Sci U S A. 1972;69:2580–2584. doi: 10.1073/pnas.69.9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie Y, Zhang J, Yin S, Loo JA. J Am Chem Soc. 2006;128:14432–14433. doi: 10.1021/ja063197p. [DOI] [PubMed] [Google Scholar]
- 47.Yin S, Loo JA. J Am Soc Mass Spectrom. 2010;21:899–907. doi: 10.1016/j.jasms.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Erales J, Gontero B, Whitelegge J, Halgand F. Biochem. J. 2009;419:75–82. doi: 10.1042/BJ20082004. [DOI] [PubMed] [Google Scholar]
- 49.Hartinger CG, Tsybin YO, Fuchser J, Dyson PJ. Inorg. Chem. 2008;47:17–19. doi: 10.1021/ic702236m. [DOI] [PubMed] [Google Scholar]
- 50.Yin S, Loo JA. Int. J. Mass Spectrom. 2011;300:118–122. doi: 10.1016/j.ijms.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benesch JL, Sobott F, Robinson CV. Anal Chem. 2003;75:2208–2214. doi: 10.1021/ac034132x. [DOI] [PubMed] [Google Scholar]
- 52.Geels RB, Calmat S, Heck AJ, van der Vies SM, Heeren RM. Rapid Commun Mass Spectrom. 2008;22:3633–3641. doi: 10.1002/rcm.3782. [DOI] [PubMed] [Google Scholar]
- 53.Daggett V, Levitt M. Proc Natl Acad Sci U S A. 1992;89:5142–5146. doi: 10.1073/pnas.89.11.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parthasarathy S, Murthy MR. Protein Eng. 2000;13:9–13. doi: 10.1093/protein/13.1.9. [DOI] [PubMed] [Google Scholar]
- 55.Tronrud DE, Wen J, Gay L, Blankenship RE. Photosynth Res. 2009;100:79–87. doi: 10.1007/s11120-009-9430-6. [DOI] [PubMed] [Google Scholar]
- 56.Wen J, Zhang H, Gross ML, Blankenship RE. Biochemistry. 2011;50:3502–3511. doi: 10.1021/bi200239k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Camara-Artigas A, Blankenship RE, Allen JP. Photosynth Res. 2003;75:49–55. doi: 10.1023/A:1022406703110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.