Abstract
Loss of balance is often due to loss of vestibular hair cells. In mammals, regeneration of functional hair cells in the mature sensory epithelium is limited; therefore, loss of sensory cells can lead to debilitating balance problems. Delivery of the transcription factor atonal (atoh1) after aminoglycoside ototoxicity has previously been shown to induce the transdifferentiation of supporting cells into new hair cells and restore function. A problem with mouse aminoglycoside models is that the partial loss of hair cells seen in human disease is difficult to establish consistently. In order to more closely mirror human clinical balance dysfunction, we have used systemic application of 3,3’-iminodipropionitrile (IDPN), a vestibulotoxic nitrile compound known to cause vestibular hair cell loss, to induce a consistent partial loss of vestibular hair cells. To determine if balance function could be restored we delivered atoh1 using a new adenovirus vector based on Ad28. The Ad28 adenovector is based on a human serotype with a low seroprevalence that appears to target gene delivery to vestibular supporting cells. In order to further provide cell type selectivity of gene delivery, we expressed atoh1 using the supporting cell specific glial fibrillary acid protein (GFAP) promoter. Delivery of this vector to IDPN-damaged vestibular organs resulted in a significant recovery of vestibular hair cells and restoration of balance as measured by time on rotarod compared to untreated controls.
Keywords: vestibular hair cell regeneration, balance, Ad28, atoh1
Introduction
Balance disorders resulting from vestibular hair cell loss are debilitating and account for about 5 million physician visits a year in the United States. Loss of vestibular hair cells has a variety of causes including aminoglycoside ototoxicity or inner ear diseases like Meniere’s disease 1. In mammals, regeneration of functional hair cells in the mature sensory epithelium is limited, although partial recovery after vestibulotoxic insults has been reported 2-4. However, the extent of spontaneous recovery after damage is limited 2.
Currently, treatment of balance disorders consists of rehabilitation and sensory substitution. A variety of drugs have been used to decrease the debilitating symptoms of vertigo and nausea 5. However, some patients fail to benefit from the available treatment options that do not treat the causative hair cell loss. Gene therapy is a promising molecular approach to trigger regeneration of vestibular hair cells sufficiently to restore vestibular function. Temporal bone studies in humans have shown that even after profound damage, supporting cells and some residual hair cells are present 6. A known mechanism for replacement of hair cells is transdifferentiation of supporting cells into hair cells 7. Forcing the transdifferentiation of residual supporting cells in the vestibular system is thus a potential method of restoring lost hair cells.
Atoh1 is a basic helix-loop-helix transcription factor that plays a crucial role in hair cell development. Atoh1-null mice fail to generate hair cells 8,9. During development, atoh1 is also required for the expression of the neurotrophin BDNF by vestibular hair cells 10. BDNF is required for a proper innervation of the sensory epithelium. Forced expression of atoh1 alone is sufficient to cause supporting cells to transdifferentiate to new functional cochlear and vestibular hair cells that as shown by some studies are also able to attract afferent innervation 2,11-13. Atoh1 is therefore a promising gene for therapeutic approaches to restore vestibular function.
A prerequisite to study regeneration of vestibular hair cells is an animal model that closely mimics the pathological changes found in humans with symptomatic vestibular dysfunction. So far, local application of aminoglycosides has been widely used to study hair cell loss in animal models as systemic application of aminoglycosides in mice fails to induce sufficient hair cell loss 2,12,14. Local aminoglycoside delivery usually causes close to total hair cell loss and variability between individual animals makes it difficult to induce an intermediate extent of vestibular hair cell loss 14. A problem with models that result in complete loss of the integrity of the neuroepithelium is that they respond poorly to regeneration efforts 15. In humans, most clinically significant vestibular disease is associated with residual survival of both supporting cells and hair cells 6.
To more closely mirror the clinical condition of balance disease, we wanted to create a model in which hair cells were consistently reduced in number but in which there was not complete destruction of the neuroepithelium. We adapted a systemic ototoxicity model to create partial but physiologically relevant hair cell loss. 3,3’-iminodipropionitrile (IDPN) is a vestibulotoxic nitrile that causes dose-dependent vestibular hair cell loss 16-18. Exposure to low doses of IDPN results in hair cell extrusion, whereas increasing exposure results in apoptosis and, with further increases, necrosis 19. IDPN also decreases the presence of neurofilaments (NF) in terminals of vestibular calyx afferents that surround type I hair cells 20,21. The effect of IDPN on NFs is especially marked after subchronic exposure, whereas acute high-dose treatment induces major hair cell loss but only minor loss of NFs 22.
We have used systemic delivery of IDPN to model the partial bilateral vestibular hair cell loss that is often seen in a clinical population. To evaluate molecular approaches to treat balance disorders, we delivered a novel adenovirus vector that targets delivery of genes to supporting cells in the cochlea and vestibular system. The Ad28 vector expresses the atoh1 gene driven by a cell type selective promoter, glial fibrillary acidic protein (GFAP). After vector delivery to one inner ear in this bilateral vestibular injury model, inner ear histology and balance function were evaluated and compared to control and non-vector treated animals.
Results
Characterization of Ad28 gene delivery in the vestibular system ear
Distribution of vector
To evaluate the distribution of Ad28 delivered transgenes we examined delivery of GFP driven by the hCMV promoter. Ears in which Ad28eGFP was injected into the perilymph were stained for GFP to evaluate the temporal and spatial transfection pattern. GFP expression in utricle and saccule was predominately confined to supporting cells and was strong and stable for the duration of 14 days (Fig. 1). Hair cells expressed GFP only shortly after transfection in the saccule on day 3. This suggests that Ad28 vector delivery is limited to specific cells in the inner ear.
Figure 1. Adenovirus serotype 28 predominately transfects vestibular supporting cells.
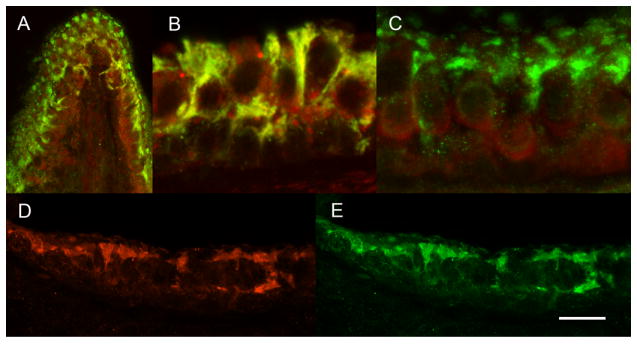
After delivery of 0.14 × 108 pu of Ad28GFP to the perilymph, GFP expression was evaluated at 14 days post delivery. Dual immunofluorecence for GFP (green) and myosin VII (red) is seen in the ampulla (A), saccule (B) and utricle (C). Hair cells did not express GFP. Green, GFP expressing supporting cells can be seen alternating with hair cells. Immunofluorescence for GFAP (red, D) and GFP (Green, E) suggests that Ad 28 transfects supporting cells.
Ad28 delivery does not alter balance in normal animals
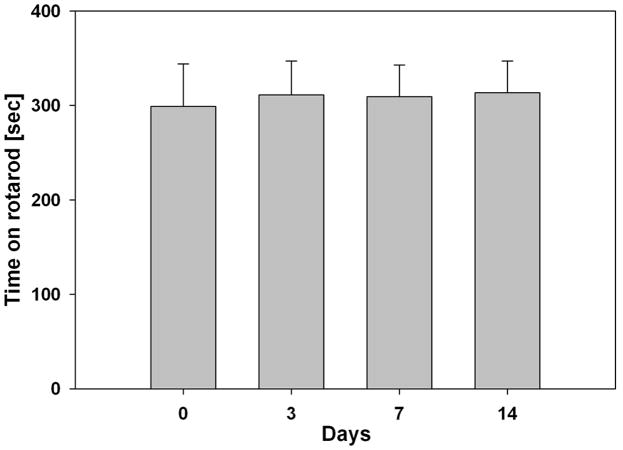
To test the administration approach and delivery of the Ad28 adenovector on the vestibular system, control mice received Ad28eGFP via the round window. Balance function was tested by evaluating the animals’ time on a rotarod. The preoperative baseline recording, i.e. the average time that animals managed to stay on the rotarod, was 299s ± 44.9 (n=4). The performance remained stable with 311s ± 35.6 on postoperative day 3, 309s ± 33.5 on day 7 and 314s ± 33.3 on day 14. There was no statistically significant change postop compared to preop (Fig. 2) (p>0.05).
Figure 2. Ad 28 delivery does not affect rotarod performance in control mice.
To evaluate the effect of Ad28 on the vestibular system, mice underwent delivery of Ad28GFP and were followed by measuring their ability to balance on a rotating rod (rotarod). Rotarod performance preop (day 0) was not changed after delivery of 0.14 × 108 pu Ad28eGFP (days 3, 7, 14).
Effect of IDPN treatment on balance
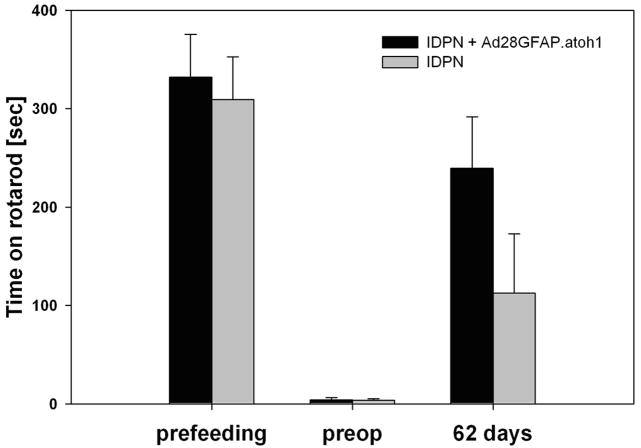
IDPN is known to cause loss of vestibular function 18. To quantify the effect of IDPN on balance, mice were fed IDPN followed by rotarod testing over a 2 month interval. The average of the baseline recording of rotarod performance was 309s ± 40.9 (n=6). After IDPN exposure the time on the rotorod decreased steadily and rapidly to 169s ± 88.4 (n=5) by day 3 and further to 26.8s ± 17.5 (n=6) by day 7 and to 3.6s ± 1.52 (n=5) by day 11 post exposure. Afterwards, there was a slight recovery of rotarod performance, yet no statistically significant difference to day 11 was seen for 17 more days (46.0s ± 2.1, day 28 post exposure, n=3). Then performance improved slowly to up to 113s ± 60.4 at the endpoint of the experiment (day 62 post exposure, n=5) (Fig. 3).
Figure 3. Atoh1 delivery results in restoration of balance after IDPN vestibulotoxicity.
Exposure to IDPN causes a rapid loss of balance function. At 10 days post IDPN feeding, mice showed a significant reduction of rotarod times. A second group of mice was treated with IDPN followed after 10 days with delivery of Ad.gfap.gfp to the inner ear. Before IDPN exposure and on day 10 after exposure (=preop) both IDPN only and the group that were to receive vector were statistically identical. Sixty-two days after vector delivery the atoh1-treated mice performed significantly better than the IDPN only group, showing a significant and sustained recovery of balance as measured by time on the rotarod (P=0.001).
Effect of supporting cell targeted atoh1 delivery on balance
A second group of mice was exposed to IDPN and likewise responded with a significantly reduced rotarod performance of 3.9s ± 2.5 (n=5) on day 11 (baseline prior to feeding 332s ± 43.6, n=7). At this time point, there was no statistically significant difference between this group and the IDPN only group (P=0.824). Mice were treated with Ad28GFAP.atoh1 (atoh1 driven by a supporting cell specific promoter) on day 11 after IDPN. The recovery rate, measured in additional seconds on the rotarod per day, was not statistically significant between the atoh1 treated animals during the first days after inoculation (6.77s/d ± 8.5 for the period from days 10-16) compared to the IDPN only group (3.63s/d ± 4.99 for the period from days 10-15). In the second week after vector delivery, atoh1-mice improved their rotarod performance significantly faster (11.46s/d ± 5.16 for the period from days 16-24, P=0.047) than the IDPN only controls (2.33s/d ± 0.82 for the period from days 15-28). The average rate of recovery after vector application on day 10, until termination of the experiment on day 62, was significantly better for atoh1-mice (4.17s/d ± 0.8) than IDPN only mice (2.01s/d ± 0.96, P = 0.001). At the end of the experiment, Ad28GFAP.atoh1 treated mice managed to stay on the rod for 237 sec ± 52.21 which is 73.5 % ± 14.2 of the baseline performance before IDPN exposure (Fig 3). In contrast, the IDPN only group demonstrated rotarod times of 113s ± 60.4, i.e. 34.1% ± 16.2, which is significantly shorter (P=0.005). This suggests that unilateral delivery of Ad28.GFAP.atoh1 results in a significant recovery of balance after IDPN vestibulotoxicity.
Atoh1 delivery restores sensory cells
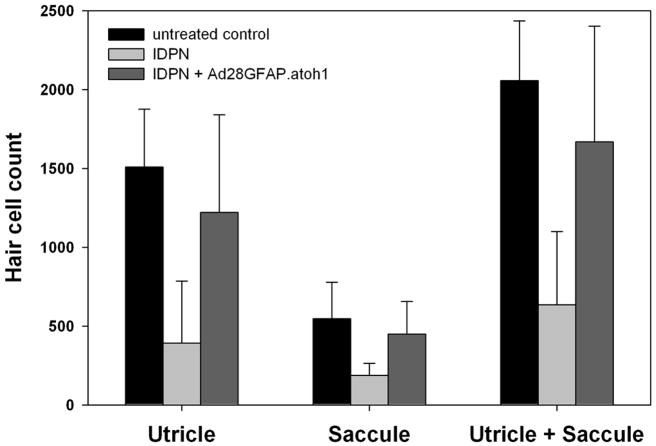
To verify the effects of atoh1 on the vestibular system, macular organs removed from treated and age matched control animals were immunostained for myosin VIIa, a selective marker for hair cells in the inner ear 23 to determine total hair cell counts in the macular organs. In untreated controls, the count of hair cells was 546.3 ± 282.8 in the saccule and 1509.8 ± 365.3 in the utricle yielding a total macular organ hair cell count of 2056 ± 378.5 (n=4). Sixty-two days after exposure to IDPN, the number of saccular hair cells decreased to 187 ± 75.8 (n=5); the number of utricular hair cells was reduced to 391.3 ± 393.1 (n=6) (Fig. 4). The total number of macular organ hair cells was 636.2 ± 462.3 (n=5) in the IDPN treated ears.
Figure 4. Atoh1 delivery results in recovery of macular organ hair cell counts.
To determine the effects of IDPN and IPDN followed by atoh1 delivery, macular organ hair cell counts were taken on day 62 after treatment. Exposure to IDPN significantly decreases the number of hair cells in the utricle as well as in the saccule (light grey bars) in comparison to untreated controls (black bars). Delivery of Ad28GFAP.atoh1 into the perilymph after treatment with IDPN increased hair cell numbers significantly (dark grey bars) up to control levels.
In mice that received Ad28GFAP.atoh1 after exposure to IDPN, the total hair cell count was significantly increased to 1668.3 ± 733.9 (n=4) compared to the IDPN only group. A statistically significant increase in hair cell numbers was found in the saccule (448.0 ± 207.3, n=4) as well as in the utricle (1220.5 ± 619.6, n=4). Statistical analysis revealed that there was no difference in hair cell numbers of Ad28GFAP.atoh1 treated mice compared to age matched healthy mice (P=0.384) (Fig. 4).
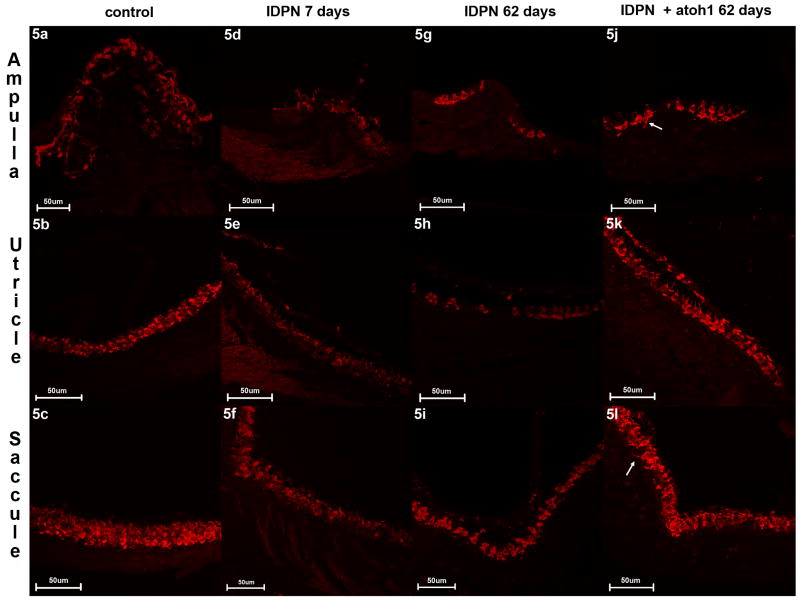
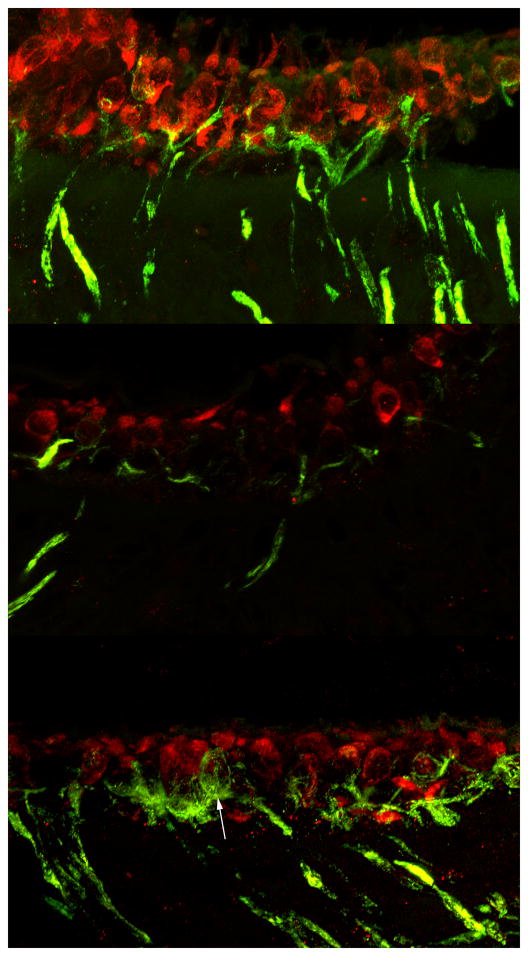
Histological analysis demonstrated a dense population of myosin VIIa-positive hair cells in ampulla (Fig. 5a), utricle (Fig. 5b) and saccule (Fig. 5c) of healthy controls. These hair cells had a strong and even expression of myosin VIIa throughout the cell body and the stereocilia. On day 7 after IDPN exposure most of the hair cell population appeared to disintegrate. Cell bodies were less well defined by myosin VIIa staining (Fig. 5d-f). The ampullary and utricular hair cells were more affected by these changes than the saccule which is similar to findings by Seoane and colleagues 19. Residual hair cells, as seen on day 62 after IDPN exposure, were nicely defined by myosin VIIa staining (Fig. 5g-i). In comparison to untreated controls the distribution of hair cells was patchy especially in the saccule and ampulla. In mice that received Ad28GFAP.atoh1 after IDPN exposure, hair cells showed well-defined myosin VIIa staining throughout the cell body and also in the stereocilia. Some cell bodies were comparatively slender and reached into the bottom layer of the sensory epithelium (Fig. 5j,l – arrow). Dual immunofluorescence for myosin VII and neurofilament (Fig 6) demonstrated the presence of innervation in both the IDPN only treated (Fig 6B) and the IDPN and Ad28GFAP.atoh1 treated inner ear (Fig 6C). As seen in figure 6C, innervation appears denser than in IDPN only treated animals (6B) and the regenerated epithelium has some calyces (6C arrow) suggesting the presence of type I vestibular hair cells.
Figure 5. Immunohistochemistry for myosin VIIa shows regenerated hair cells after IDPN-mediated hair cell loss.
Early after IDPN exposure hair cells are severely damaged (5d-f) but some of them seem to recover as the data from 62 days post exposure show (5g-i). However, these hair cells often lack stereocilia and they are unevenly distributed. After atoh1 treatment the sensory epithelia resemble these of control ears with well defined cell bodies and stereocilia (5j-l). Some hair cells have slender cell bodies that seem to originate from the bottom layer of the sensory epithelium (5j+l arrow).
Figure 6. Atoh1 deliveryafter IDPN exposure results in innervated neuroepithelium.
Saccular sections stained for PAN (green), a neuronal marker, and myosin-VIIa (red). In controls type-I hair cells are innervated by calyx type afferents. After IDPN exposure there continues to be an innervated population of hair cells with occasional calyces detectable (B). In contrast delivery of atoh1 after IDPN mediated damage results in the return of calyx innervated hair cells (arrow) suggesting the presence of type I vestibular hair cells (scale bar = 10μm).
Discussion
Optimization of molecular therapy for balance disorders requires development of relevant preclinical assays and a vector system designed to be specific for the targeted disease process. In this series of experiments, we have demonstrated that a consistent mouse model of partial severe vestibular hair cell loss can be developed and that use of a vector targeted to and limited in expression to supporting cells can be used to restore balance function in this model.
Adaption of the IDPN vestibulotoxicity model
In our study we adapted a model of IDPN vestibulotoxicity in mice that was established in male Hsd-ICR mice by Soler-Martin et al. 18. In a pilot study we dosed female C57B/L6 mice with different amounts of IDPN starting at 2.96 μg/g body weight, i.e. the dosage used by Soler-Martin, increasing the dosage until signs of vestibular dysfunction were visible. At 120 μg/g body weight we obtained reproducible vestibular dysfunction on the rotarod as well as histological changes (Figs 3,4,5). As the underlying mechanisms of IDPN toxicity are not yet known in detail, genetic differences between the two mice strains, as well as gender differences, might account for the discrepancy of the applied dosages.
The rotarod is an established method to test balance in rodents 24. We adapted this method to evaluate the effect of atoh1 delivery after IDPN treatment in mice. IDPN induces vestibular hair cell death resulting in vestibular dysfunction 17,18,25. As expected, the time that mice managed to stay on the rotating rotarod decreased significantly after exposure to IDPN (Fig. 3). Immunohistochemistry revealed a severely damaged hair cell population in saccule, utricle and ampulla on day 7, after IDPN exposure (Fig. 5). As previously described, the ampulla (Fig. 5d) and utricle (Fig. 5e) were more affected than the saccule (Fig. 5f) 19. Immunohistochemistry on day 62 after exposure showed residual vestibular hair cells that had more uniform myosin VIIa staining than on day 7 post IDPN (Fig. 5g-i).. These hair cells might have recovered from partial damage via intracellular self-repair or some spontaneous regeneration of the sensory epithelium might have occurred 2-4. Both processes can lead to abnormal hair cells and loss of stereocilia. Despite this ability of the sensory epithelium to recover from damage, its success is limited and incomplete. The damaged epithelium did appear to maintain innervation (Fig 6B) suggesting that the loss of balance seen in these animals was related to reduction of the hair cell number.
Overall, the number of surviving hair cells was significantly lower in IDPN treated mice than in healthy controls (Fig. 4), resulting in a severe functional deficit in the IDPN treated mice (Fig. 3). In summary, we developed a model that met our aim of consistent hair cell damage after systemic delivery thereby causing symptomatic and consistently measurable balance dysfunction. As this model resembles the pathology found in humans, it can be used for preliminary preclinical regeneration studies 6.
Atoh1 delivery results in restoration of hair cells and balance function
Atoh1 can cause supporting cells to transdifferentiate into functional hair cells 2,11-13. We found that adenovector delivered atoh1 was also able to restore balance function in an IDPN induced model of vestibular damage. Ten days after IDPN exposure and subsequent decline in rotarod performance, mice were treated with Ad28GFAP.atoh1 in one ear. We chose to treat only one ear so that the untreated ear could serve as a control. In addition, the functional recovery of one peripheral vestibular organ is often sufficient to significantly improve the functional outcome due to central compensation and adaption which can not occur in bilateral vestibular hypofunction.
Animals recovered quickly after surgery and their average time on the rotarod increased from the first week after vector delivery. Evaluation of the time course of recovery showed that rotarod times improved the fastest during the first 2 weeks after vector delivery. This finding corresponds with the observation that after toxic ablation of hair cells and subsequent delivery of atoh1, new myosin VIIa-positive vestibular hair cells are present from 10 days after atoh1 delivery 26. Taken together these observations suggest that vestibular function increases due to regeneration of vestibular hair cells. After that period, IDPN only and IDPN + atoh1 groups improved their rotarod times by 3.5s/d ± 1.5 (IDPN only group, time period days 35-62) and 2.3s/d ± 2.5 (IDPN + atoh1, time period days 31-62) which was statistically not significant (P=0.327). This improvement is likely to be due to learning. Cell counts of macular organs confirmed that hair cell regeneration had taken place, increasing hair cell numbers in saccule and utricle to numbers not statistically different from untreated controls indicating repopulation of the vestibular system (Fig. 4). Vestibular function never recovered to baseline over the observed time period probably due to the fact that only one side was treated. Overall, this suggests atoh1 induces regeneration of hair cells and restoration of function and that the regenerated hair cells persist and remain functional for prolonged time periods.
Selective atoh1 delivery using a novel D species human adenovector
Selectivity of molecular therapeutics can be improved through cellular retargeting or the use of tissue specific promoters. We increased the specificity for supporting cells by using a supporting cell specific promoter, GFAP. In previous studies, we and others have demonstrated that this promoter appeared selective for supporting cells and yielded efficient hair cell regeneration in vitro 27. Adenovirus serotype 28 is a rare serotype belonging to the human D group adenovectors with a low prevalence of neutralizing antibodies (NAbs) 28. Current studies suggest that the incidence of neutralizing antibodies to this vector is less than 10% 29. Ad28 had no impact on balance function in normal animals (Fig. 2). Immunohistochemistry showed that Ad28 predominately transfected vestibular supporting cells within the neuroepithelium which makes it a suitable vehicle to carry regenerative genes that target supporting cells (Fig. 1). Use of a vector that targets supporting cells adds an additional layer of specificity to the delivery of atoh1 that is driven by a supporting cell specific promoter. Currently, the receptor for Ad 28 on supporting cells is under investigation, however it is not CAR or CD46 29. Ad28 is a efficient and specific vector that posses the necessary features for a safe and successful use in further regeneration studies.
Conclusion
We have developed a mouse model of vestibular damage that was used to assess the efficacy of a molecular therapeutic approach to treating balance disorders. Utilization of a novel supporting cell specific vector allowed delivery of atoh1 to IDPN treated animals, resulting in restoration of balance function through regeneration of vestibular hair cells. (Ad28, atoh1, IDPN vestibulotoxicity).
Materials and Methods
Animals and Anesthesia
All studies used 2 months old female C57BL/6 mice and were approved by the University of Kansas guidelines for animal care and housing. Mice were anesthetized with an intraperitoneally administered mixture of Ketamine (100mg/kg), Xylazine (5mg/kg) and Acepromazine (2mg/kg).
Dosing and exposure to IDPN
Based on studies done by Llorens et al., female mice were fed IDPN 120μg/g body weight (>99%, Arcos Organics, Geel, Belgium) 18,19. Animals tolerated the feeding regime if the total dose was split into 8 smaller portions that were administered over two days. For feeding, we used a gavage needle (single use sterile animal feeding needle, size 20 × 1-1/2, Popper and Sons Inc., USA). Animals were rehydrated with saline (0.70cc sq) and allowed to recover on a heating pad for three hours before the next feeding took place.
Balance testing
To evaluate balance function mice were tested on a rotarod treadmill (ENV-575M, Med Associated Inc., Georgia, USA). The rod started at an initial velocity of 4 rpm and accelerated to 40 rpm at 300 sec. The time from the start of acceleration until the mouse fell completely off the rod was recorded. The test was stopped after a maximum of 360 sec. All mice were trained on the rod for two days prior to the baseline recording. Mice that were unable walk on the rotarod for greater than 300 seconds on both training days were rejected from the study.
Vector production and application
The Ad28 vectors for these studies are deleted in both adenovirus E1A and E1B (E1), are constructed and produced using 293-ORF6 cells as previously described 30 and contain the transgene expression cassette in the E1 region. The construction of Ad28 based adenovectors was conducted as described in Gall et al (31). For the construction of Ad28 vectors a full length Ad28 virus genome was cloned into the plasmid based system described. An Ad28 specific left end shuttle plasmid was built as described for Ad5 (31)l. The regions encoding the Ad28 E1A and E1B region were replaced with the transgene expression cassette in this shuttle plasmid. Full length E1 deleted Ad28 vector genomes were constructed by the standard procedures described (31) The Ad28 vectors for these studies deleted in both E1A and E1B (E1), are constructed and produced using 293-ORF6 cells. The 293-ORF6 cell line allows for growth of E1 deleted adenoviruses from multiple serotypes (32). The expression cassettes used in these studies include a human CMV ie promoter driven cassette and a human glial fibrillary acidic protein (GFAP) promoter driven cassette. The atonal expressing Ad28 adenovector used in these studies expresses the mouse atonal gene (Ad28GFAP.atoh1) driven by the human glial fibrillary acidic protein (GFAP) promoter with an SV40 poly-adenylation site and transcriptional stop 3’ of the open reading frame. Total particles are analyzed by a spectrophotometric assay that has been standardized and qualified to reliably and robustly quantitate the total particles (PU) within a lot of adenovirus. All dosing was done by total pu. After purification vector lots are aliquoted and stored at -80°C.
The vector was administered unilaterally, through the round window into the scala tympani as previously described 33. In short, a dorsal postauricular incision was made and the bulla was exposed and opened. After identifying the round window niche, 0.65 × 109 pu of Ad28GFAP.atoh1 or 0.14 × 108 pu of Ad28GFP was injected using a Hamilton micro syringe (Hamilton Company, USA). Vector dose was based on a maximum delivery of 1 microliter of vector. The opening was sealed with a piece of tissue and the wound was closed with stitches (Prolene 5-0, Ethicon Inc., USA).
To test the effect of Ad28 adenovector delivery on the healthy vestibular system we recorded a baseline rotarod time prior to vector application and repeated the measurement on days 3, 7 and 14 after delivery of 0.14 × 108 pu of Ad28GFP via the round window.
Effect of IDPN on balance
A baseline rotarod recording was performed prior to IDPN treatment and further recordings were done 3, 7, 11, 14, 21, 35, 42, 56 and 62 days after treatment.
After a baseline rotarod recording followed by IDPN treatment we retested the mice on days 3, 7 and 11 post IDPN exposure. Mice that showed no significant change in rotarod performance were excluded to control for ineffective feeding, the others received vector. After application of Ad28GFAP.atoh1 mice were tested every week on the rotarod for 62 days past feeding. For every test we took the average of two recordings. A recovery rate was calculated as increase in rotarod time during two recordings per days of that time period. The method of least-squares was used to determine the recovery rate for the whole period from vector application until the termination of the experiment.
At 62 days past IDPN treatment animals were processed for histology and macular organ hair cell counts. IDPN treated and Ad28GFAP.atoh1 administered animals (n=6) were compared to IDPN only treated animals (n=6) and untreated controls (n=4).
Tissue preparation and histological examination
Mice were anesthetized with Beuthanasia-D (10ml/kg, Schering-Plough Animal Health, USA) and euthanized via intra-cardiac perfusion with 4% paraformaldehyde. Both temporal bones were removed, postfixed in 4% paraformaldehyde overnight and decalcified in 10% EDTA for 48hrs before embedding in paraffin. The survival time for mice that received IDPN + Ad28GFAP.atoh1 and mice that received only IDPN was 62 days after IDPN treatment. To evaluate the damage by IDPN on day 10 past feeding, two mice were sacrificed at that time point. Mice that were inoculated with Ad28eGFP were sacrificed on days 3, 7 and 14 after vector delivery.
For immunohistochemistry 10μm sections were cut and deparaffinized. After antigen retrieval (Dako Target Retrieval Solution, Dako, Denmark) and incubation with Image-iT FX signal enhancer (Invitrogen, USA) sections were stained with polyclonal anti-myosin-VIIa (rabbit, Proteus Biosciences, USA, 1:50), monoclonal anti-neurofilament (PAN) (mouse, Invitrogen, USA, 1:100) or polyclonal anti-GFP (rabbit, Santa Cruz Biotechnology, USA, 1:100) as a primary antibody and fluorescent labeled secondary antibody (Alexa Fluor 555, goat anti-rabbit IgG, Invitrogen, USA, 1:50 and Alexa Fluor 488, goat anti-mouse IgF, Invitrogen, USA, 1:50). Sections were mounted with ProLong Gold antifade medium (Invitrogen, USA) and examined by confocal microscopy (Nikon Confocal Microscope C1, Nikon corp. Japan).
Macular organ hair cell counts
Total hair cell counts were obtained from saccule and utricle from control animals (n=4), animals fed with IDPN (n=5) and animals fed with IDPN followed by AdGFAP.atoh1 (n=4). The preparation of macular organs was performed as previously described 12. In short, mice were anesthetized with Beuthanasia-D (10mL/kg, Schering-Plough Animal Health, USA) and sacrificed via intracardiac perfusion with 4% paraformaldehyde. After decapitation, the otic capsule was exposed and the macular organs identified by finding the otolithic membranes. The saccule and utricle were removed and fixed in 4% paraformaldehyde for 15 min. Otoliths were removed by incubation in 10% EDTA for 15 min. Following blocking in 10% FBS, macular organs were incubated with polyclonal anti-myosin-VIIa (rabbit, Proteus Biosciences, USA, 1:50) for 24h followed by fluorescent-labeled secondary antibody (Alexa Fluor 555, goat anti-rabbit IgG, Invitrogen, USA, 1:50) overnight. The organs were then mounted onto Superfrost/Plus Microscope Slides (Fisher Scientific Int., Pittsburgh, PA) using ProLong Gold antifade medium (Invitrogen, USA).
The macular organs were imaged with confocal microscopy (Nikon Confocal Microscope C1, Nikon corp. Japan) at a magnification of 100×. Images were photomerged and analysed with Adobe Photoshop CS3 Extended (Adobe Systems Incorporated, USA).
Statistics
Statistical analysis was done with ANOVA and to determine the statistical significance of any change (p < 0.05). Error bars represent standard deviation.
Acknowledgments
Supported by NIDCD R01DC008424 and NICHD HD02528
Footnotes
Conflict of Interest: There are no conflicts of interest to report.
References
- 1.Tsuji K, Velazquez-Villasenor L, Rauch SD, Glynn RJ, Wall C, 3rd, Merchant SN. Temporal bone studies of the human peripheral vestibular system. Meniere’s disease. The Annals of otology, rhinology & laryngology. 2000;181:26–31. doi: 10.1177/00034894001090s505. [DOI] [PubMed] [Google Scholar]
- 2.Kawamoto K, Izumikawa M, Beyer LA, Atkin GM, Raphael Y. Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity. Hearing research. 2009;247:17–26. doi: 10.1016/j.heares.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Science. Vol. 259. New York, NY: 1993. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans; pp. 1619–1622. [DOI] [PubMed] [Google Scholar]
- 4.Zheng JL, Keller G, Gao WQ. Immunocytochemical and morphological evidence for intracellular self-repair as an important contributor to mammalian hair cell recovery. J Neurosci. 1999;19:2161–2170. doi: 10.1523/JNEUROSCI.19-06-02161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zajonc TP, Roland PS. Vertigo and motion sickness. Part II: Pharmacologic treatment. Ear, nose, & throat journal. 2006;85:25–35. [PubMed] [Google Scholar]
- 6.Tsuji K, Velazquez-Villasenor L, Rauch SD, Glynn RJ, Wall C, 3rd, Merchant SN. Temporal bone studies of the human peripheral vestibular system. Aminoglycoside ototoxicity. The Annals of otology, rhinology & laryngology. 2000;181:20–25. doi: 10.1177/00034894001090s504. [DOI] [PubMed] [Google Scholar]
- 7.Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neuroscience letters. 1996;205:17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- 8.Akazawa C, Ishibashi M, Shimizu C, Nakanishi S, Kageyama R. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. The Journal of biological chemistry. 1995;270:8730–8738. doi: 10.1074/jbc.270.15.8730. [DOI] [PubMed] [Google Scholar]
- 9.Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, et al. Science. Vol. 284. New York, NY: 1999. Math1: an essential gene for the generation of inner ear hair cells; pp. 1837–1841. [DOI] [PubMed] [Google Scholar]
- 10.Fritzsch B, Matei VA, Nichols DH, Bermingham N, Jones K, Beisel KW, et al. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev Dyn. 2005;233:570–583. doi: 10.1002/dvdy.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izumikawa M, Minoda R, Kawamoto K, Abrashkin K, Swiderski DL, Dolan DF, Brough D, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nature medicine. 2005:11. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 12.Staecker H, Praetorius M, Baker K, Brough DE. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol. 2007;28:223–231. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- 13.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 14.Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol. 2008;9:65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hearing research. 2008;240:52–56. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delay J, Pichot P, Thuillier J, Marquiset JP. Effect of aminodipropionitrile on motor behavior of the white mouse. Comptes rendus des seances de la Societe de biologie et de ses filiales. 1952;146:533–534. [PubMed] [Google Scholar]
- 17.Llorens J, Dememes D, Sans A. The behavioral syndrome caused by 3,3’-iminodipropionitrile and related nitriles in the rat is associated with degeneration of the vestibular sensory hair cells. Toxicology and applied pharmacology. 1993;123:199–210. doi: 10.1006/taap.1993.1238. [DOI] [PubMed] [Google Scholar]
- 18.Soler-Martin C, Diez-Padrisa N, Boadas-Vaello P, Llorens J. Behavioral disturbances and hair cell loss in the inner ear following nitrile exposure in mice, guinea pigs, and frogs. Toxicol Sci. 2007;96:123–132. doi: 10.1093/toxsci/kfl186. [DOI] [PubMed] [Google Scholar]
- 19.Seoane A, Dememes D, Llorens J. Relationship between insult intensity and mode of hair cell loss in the vestibular system of rats exposed to 3,3’-iminodipropionitrile. The Journal of comparative neurology. 2001;439:385–399. doi: 10.1002/cne.1357. [DOI] [PubMed] [Google Scholar]
- 20.Anniko M, Thornell LE, Gustavsson H, Virtanen I. Intermediate filaments in the newborn inner ear of the mouse. ORL; journal for oto-rhino-laryngology and its related specialties. 1986;48:98–106. doi: 10.1159/000275854. [DOI] [PubMed] [Google Scholar]
- 21.Dechesne CJ, Scarfone E, Atger P, Desmadryl G. Neurofilament proteins form an annular superstructure in guinea-pig type I vestibular hair cells. Journal of neurocytology. 1994;23:631–640. doi: 10.1007/BF01191557. [DOI] [PubMed] [Google Scholar]
- 22.Seoane A, Dememes D, Llorens J. Distal effects in a model of proximal axonopathy: 3,3’-iminodipropionitrile causes specific loss of neurofilaments in rat vestibular afferent endings. Acta neuropathologica. 2003;106:458–470. doi: 10.1007/s00401-003-0744-8. [DOI] [PubMed] [Google Scholar]
- 23.Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, Mooseker MS. Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9815–9819. doi: 10.1073/pnas.92.21.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter RJ, Morton J, Dunnett SB. Motor coordination and balance in rodents. Current protocols in neuroscience / editorial board, Jacqueline N Crawley [et al. 2001;Chapter 8(Unit 8):12. doi: 10.1002/0471142301.ns0812s15. [DOI] [PubMed] [Google Scholar]
- 25.Llorens J, Dememes D. Hair cell degeneration resulting from 3,3’-iminodipropionitrile toxicity in the rat vestibular epithelia. Hearing research. 1994;76:78–86. doi: 10.1016/0378-5955(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 26.Baker K, Brough DE, Staecker H. Repair of the vestibular system via adenovector delivery of Atoh1: a potential treatment for balance disorders. Advances in oto-rhino-laryngology. 2009;66:52–63. doi: 10.1159/000218207. [DOI] [PubMed] [Google Scholar]
- 27.Praetorius M, Hsu C, Baker K, Brough DE, Plinkert P, Staecker H. Adenovector-mediated hair cell regeneration is affected by promoter type. Acta oto-laryngologica. 2009:1–8. doi: 10.3109/00016480903019251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP, Kostense S, Penders G, Helmus N, Koudstaal W, Cecchini M, Wetterwald A, Sprangers M, Lemckert A, Ophorst O, Koel B, van Meerendonk M, Quax P, Panitti L, Grimbergen J, Bout A, Goudsmit J, Havenga M. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. Journal of virology. 2003;77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahl CA, Bonnell J, Hiriyanna S, Fultz M, Nyberg-Hoffman C, Chen P, et al. Potent immune responses induced by vaccine regimens with 1 a novel adenovirus vector based on rare human serotype 28. Journal of virology. 2009 doi: 10.1016/j.vaccine.2010.06.050. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brough DE, Lizonova A, Hsu C, Kulesa VA, Kovesdi I. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. Journal of virology. 1996;70:6497–6501. doi: 10.1128/jvi.70.9.6497-6501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gall JG, Lizonova A, Ettyreddy D, McVey D, Zuber M, Kovesdi I, Aughtman B, King CR, Brough DE. Rescue and production of vaccine and therapeutic adenovirus vectors expressing inhibitory transgenes. Mol Biotechnol. 2007;35:263–273. doi: 10.1007/BF02686012. [DOI] [PubMed] [Google Scholar]
- 32.Abrahamsen K, Kong HL, Mastrangeli A, Brough DE, Lizonova A, Crystal RG, Falk-Pedersen E. Characterization of a replication-defective nonsubgroup C adenovirus vector. Journal of Virology. 1997;71:8946–8951. doi: 10.1128/jvi.71.11.8946-8951.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Praetorius M, Baker K, Brough DE, Plinkert P, Staecker H. Pharmacodynamics of adenovector distribution within the inner ear tissues of the mouse. Hearing research. 2007;227:53–58. doi: 10.1016/j.heares.2006.07.002. [DOI] [PubMed] [Google Scholar]