Abstract
Objective
To perform an open label, pilot study of an orally administered liquid formulation of immediate release pentoxifylline (PTX) to patients with Duchenne muscular dystrophy (DMD). Treatment efficacy, safety and tolerability were assessed.
Methods
The tolerability and safety of PTX, measures of muscle strength and function were evaluated during 12 months of treatment with PTX.
Results
Seventeen boys with DMD, between 4 and 8 years, were enrolled at one of 5 Cooperative International Neuromuscular Research Group (CINRG) centers. Only 9 were able to complete the 12 month PTX treatment phase; the primary reasons for discontinuation were adverse events. Intolerable gastrointestinal side effects were experienced by 65% of participants. Two participants experienced severe leukopenia that resolved with medication withdrawal.
Conclusions
Open-label treatment with liquid formulation of immediate release PTX resulted in a high level of adverse event in boys with DMD. Poor tolerability of this PTX formulation precluded adequate assessment of efficacy.
Keywords: Duchenne, Muscular Dystrophy, Leukopenia, Pentoxifylline, Clinical Trial
Introduction
Duchenne muscular dystrophy (DMD) is a progressive myopathy that results in weakness and cardiorespiratory failure. Untreated, DMD patients usually die by the third decade of life. Corticosteroids and supportive care have improved this natural history but do not prevent the relentless progression of disability leading to early death.1
The lack of sub-sarcolemmal dystrophin in DMD muscle causes activation of downstream pathways that culminate in muscle cell death.1 Pentoxifylline (PTX) was a promising pharmaceutical candidate to ameliorate downstream pathological effects in DMD muscle based on its action in other disease models.2,3 PTX suppresses inflammation by inhibiting synthesis of tumor necrosis factor-α and other pro- and anti-inflammatory cytokines. PTX prevents endotoxin-induced neutrophil degranulation and inhibits the production of reactive oxygen species. Finally, PTX is a nonspecific inhibitor of phosphodiesterase. PTX has been marketed for adults since 1972 and is currently indicated for patients with occlusive arterial disease of the limbs. The safety and efficacy have not been established in the pediatric population.4
PTX demonstrated benefit in a preclinical study in the mdx mouse model of DMD. In an exercise paradigm, PTX-treated mice showed 51% less deterioration of muscle strength compared to sham-treated mdx mice.5
We conducted an open label, pilot study of treatment with orally administered, liquid formulation, immediate release PTX for 12 months in young DMD boys. Poor tolerability of this PTX formulation precluded adequate assessment of the effect of the drug on muscle strength.
Methods
Eligibility and enrollment
Five CINRG centers received Institutional Review Board approval. Twenty DMD boys between ages 4 and 8 years, who had not used glucocorticosteroids for at least one year, were consented to participate in the study. Of the enrolled 17 participants (Figure 1), 15 were Caucasian and 1 each were of African and Hispanic descent. The mean and median age of participants were 6.0 and 5.8 years, respectively (range 4.3 to 8.5 years). Patients with significant concomitant illness and presence of coagulopathy or other hematological disorders were excluded.
Figure 1.
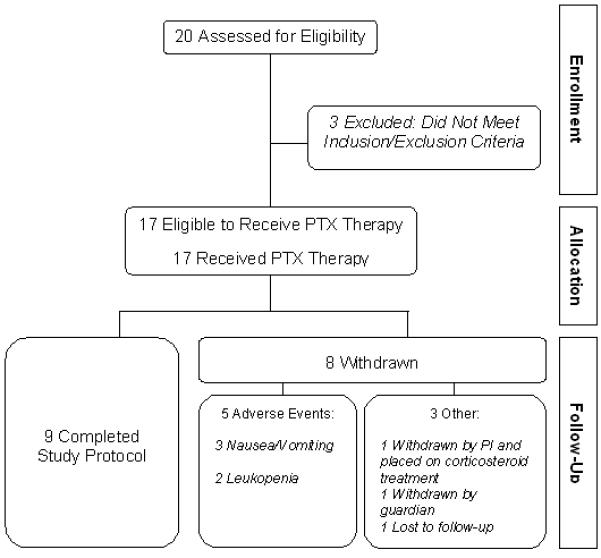
Participant flow through the trial.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Institutional Review Board at each institution. Written informed consent was obtained for all participants. The trial was registered at the NIH website (ClinicalTrials.gov: NCT00102453).
Treatment and Evaluation
A 3-month lead-in period established pre-treatment parameters of longitudinal muscle strength and function for each participant. During 12 months of open label treatment, PTX was administered as a flavored alcohol-free syrup (IND 65,106) to facilitate dosing based on participant weight (20 mg/kg/day in a 20mg/mL solution). Interval clinical evaluations included medication use review and adverse event (AE) reporting utilizing the Common Terminology Criteria for Adverse Events (CTCAE) grading scale,6 quantitative muscle testing (QMT), manual muscle testing (MMT) and safety parameters.
Statistical Analysis
The original analyses plan included a report of total QMT score as the primary endpoint and individual QMT scores (arm, leg and grip), MMT score and functional tests (10 meter timed walk, time to climb 4 steps and time to rise from the floor) as secondary endpoints. A paired two-sided t-test was planned to compare the mean difference, as a sample size of 17 allowed for a difference of 1 SD with 80% power, while controlling for a two-sided Type I error of 0.05. However, due to the high drop-out rate, this analysis method was not utilized. We instead compared baseline characteristics between patients who did and did not complete the study using two-sample, two-sided t-tests.
Results
Only 9 of the 17 participants who began PTX treatment were able to complete the study. Five participants discontinued PTX because of AEs: 3 had irresolvable nausea and vomiting in temporal association with PTX intake, and 2 had leukopenia. Three other participants discontinued PTX: 1 was withdrawn from the study by the site investigator and placed on steroid treatment due to rapid deterioration in strength, 1 was withdrawn electively by his family and 1 was lost to follow-up at treatment month 8.
Nausea and vomiting were reported in the majority of participants (65%). The severity of this AE ranged from a single episode of vomiting immediately after PTX dosing to extended periods of paroxysmal vomiting. Change in syrup flavor did not improve tolerance of liquid formulation PTX.
The development of severe leukopenia in 2 participants was unexpected, serious and ultimately led to withdrawal from the study. One participant experienced flu-like symptoms and fever for a 24-hour period within 4 weeks of starting PTX. A decreased white blood count (WBC) of 2.0 K/uL (normal: 4.5-13.5) and a decreased absolute neutrophil count (ANC) of 0.19 K/uL (normal: 2.3 -8.10) resulted in permanent discontinuation of PTX. Leukopenia resolved by 9 days later (WBC 7.5 K/uL, ANC 4.22 K/uL).
The second participant who developed leukopenia experienced a decrease in WBC to 3.5 K/uL (normal: 5.0-15.5 K/uL) 4 months after initiation of treatment. PTX dose was first decreased to 15mg/kg and then was stopped 6 days later. After a drug holiday of approximately one month when his WBC had returned to the normal range, he was re-challenged with 15mg/kg PTX. Two weeks later, his WBC decreased to 2.4 K/uL and ANC to 0.4 K/uL. Therefore, PTX was permanently stopped; WBC and ANC returned to normal after approximately 40 days.
To investigate the unexpected study drug toxicity, we compared participant characteristics at study entry. We found no significant difference in age at study entry, baseline QMT and MMT strength scores, and baseline timed function tests between patients who completed the study and those who discontinued (data not shown). Figure 2 shows the QMT scores for each participant plotted as a function of time.
Figure 2. Longitudinal assessment of total QMT score for enrolled participants.
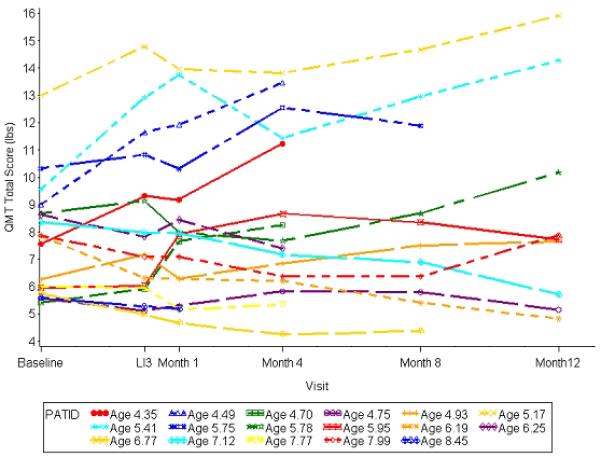
Trends in quantitative muscle testing (QMT) total score are shown for each individual participant over the course of the study comprising a 3-month lead-in phase (LI3) followed by a 12-month treatment phase.
Discussion
The original intent of this study was to assess efficacy of PTX to diminish loss of strength in DMD and to indicate the feasibility of a larger, randomized, controlled efficacy trial. While the immediate release, oral, liquid formulation of PTX provided a method for accurate dosing based on participant weight, it was not well tolerated.
The occurrence of leukopenia in 2 pediatric DMD participants (12% of study participants) contrasted with the reported incidence in adults of less than 1%.4 There are no published reports of PTX-related leukopenia in children. In our participants, leukopenia resolved upon PTX withdrawal. One participant was re-challenged and leukopenia rapidly recurred.
The 65% incidence of vomiting in pediatric DMD participants treated with PTX was also markedly higher than expected compared to a 4.5% reported incidence in adults.4 The striking difference in the incidence of vomiting associated with immediate release PTX administration between adults in reported studies and children in this study underscores the importance of performing pediatric safety trials. Differences in adverse events due to PTX between the participants in this study and those in previously published studies include the age of participants (adults vs. children), disease (non-DMD vs. DMD) and formulation of the immediate release PTX (capsule vs. liquid). These differences add to the complexity of identifying the relative contribution of each of these factors to toxicity.
Similar to multiple other studies, the unexpectedly high level of toxicity experienced by participants in this pilot study was not anticipated from the preclinical studies in the mouse model. The most prevalent toxicity in the human participants was vomiting, which would not be detected in a non-vomiting species such as mice,7 highlighting a limitation of the murine model for preclinical studies.
Approximately half of the participants finished the full 12-month treatment course. There was no significant difference in baseline characteristics between those participants who finished the 12 months of PTX treatment and those who did not finish. To the extent that participants who did not finish the trial were followed, we did not observe substantive changes in strength and motor function, other than the one participant who discontinued the study because of rapid deterioration. Because of the high study discontinuation rate among participants, a possible beneficial effect of a well-tolerated formulation of PTX on DMD cannot be excluded.
It is interesting to consider whether dystrophin deficiency may have contributed to the high frequency of gastrointestinal side effects found in this study. Children with dystrophin-deficient muscular dystrophy have been shown to have a derangement of gastric motility.8 In ex vivo preparations of the gut comparing mdx to normal mice, nitric oxide production was less in mdx, and nNOS expression was reduced in mdx stomach smooth muscle cells.9 The amplitude of spontaneous muscular contractions of the mdx mouse ileum ex vivo was increased, and it decreased to normal by application of relaxin, a hormone that upregulates NO biosynthesis.10,11 It is possible that PTX could compound the gut hypercontractility associated with dystrophin deficiency, and this may explain why the intensity and frequency of gastrointestinal side effects due to PTX were not anticipated based on prior non-DMD human studies done in adults and children.
In summary, the liquid formulation of immediate release PTX was poorly tolerated in boys with DMD largely because of gastrointestinal side effects and leukopenia. It remains possible that a different formulation of PTX would be better tolerated and could be more adequately tested for its ability to slow DMD disease progression.
Acknowledgements
We thank the patients and their families for their participation in the study. We also thank the collaborating CINRG centers (Principal Investigator, Co-Investigator(s), Coordinator(s), and Clinical Evaluator(s)) who were responsible for conducting all protocol related activities: Washington University in St. Louis, MO (A. Connolly, A. Pestronk, J. Florence, C. Wulf, R. Renna, B. Malkus), Mayo Clinic in Rochester, MN (N. Kuntz, W. Korn-Peterson, B. Kotajarvi), Texas Scottish Rite Hospital for Children in Dallas, TX (S.T. Iannaccone, B. Tietell, C. Rushing, L. Nelson, H. Owens), Children’s National Medical Center in Washington, DC (D.M. Escolar, R. Leshner, C. Tesi-Rocha, A. Zimmerman, K. Parker, M. Birkmeier), and Children’s Hospital of Pittsburgh of UPMC in Pittsburgh, PA (P.R. Clemens, H. Wessel, L.P. Hache, K. Karnavas, K. Paulukonis). We thank Robert Leshner, Jean Mah and Avital Cnaan for critical review of the manuscript. We would also like to thank the CINRG Data and Safety Monitoring Board Committee.
Study Funding Supported by a grant from the National Center for Research Resources (NCRR), and the National Institutes of Health (NIH): Grant #M01RR020359 (M.B.); #M01RR00084 (D.P.); #ULIRR024992 (K.F.P.).
The authors take full responsibility for the contents of this paper, which do not represent the views of the Department of Veterans Affairs or the United States Government.
Abbreviations
- AE
Adverse Event
- ANC
Absolute neutrophil count
- CINRG
Cooperative International Neuromuscular Research Group
- CTCAE
Common Terminology Criteria for Adverse Events grading scale
- DMD
Duchenne muscular dystrophy
- MMT
Manual muscle testing
- PTX
Pentoxifylline
- QMT
Quantitative muscle testing
- WBC
White blood count
Footnotes
Published Disclosures This study was funded by the NIH.
Ms. Zimmerman has received staff grant funding from Department of Defense, NIH, and Department of Education.
Dr. Tesi-Rocha reports no disclosures.
Dr. Clemens receives grant funding from Genzyme corporation, the NIH, Veterans Administration and Department of Defense.
Dr. Connolly has received grant funding from the Muscular Dystrophy Association, National Institutes of Health, and PTC Therapeutics.
Dr. Iannacone receives consulting honoraria from PTC Therapeutics and the Muscular Dystrophy Association.
Dr. Kuntz has received funding from the Mazza Foundation Neuromuscular Program.
Ms. Arrieta receives staff grant funding from Department of Defense, NIH, and Department of Education.
Ms. Hache receives staff grant funding from Department of Defense, NIH, and Department of Education.
Mr. Henricson receives consulting honoraria from PTC Therapeutics.
Ms. Hu receives staff grant funding from Department of Defense, NIH, and Department of Education and was a consultant for Procenza.
Ms. Mayhew served as a consultant for Genzyme and currently works as a consultant to Enobia Pharma.
Dr. Escolar receives consulting honoraria from Acceleron Pharma. During the performance of the study Dr. Escolar received grant funding from the NIH.
References
- 1.Engel AG, Yamamoto M, Fischbeck KH. In: Dystrophinopathies. Engel AG, Franzini-Armstrong C, editors. McGraw-Hill, Inc.; Myology. New York: 1994. pp. 1133–1187. [Google Scholar]
- 2.Coimbra R, Melbostad H, Hoyt DB. Effects of phosphodiesterase inhibition on the inflammatory response after shock: role of pentoxifylline. J Trauma. 2004;56(2):442–449. doi: 10.1097/01.TA.0000096642.54111.E8. [DOI] [PubMed] [Google Scholar]
- 3.Shaw SM, Shah MKH, Williams SG, Fildes JE. Immunological mechanisms of pentoxifylline in chronic heart failure. European Journal of Heart Failure. 2009;11:113–118. doi: 10.1093/eurjhf/hfn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanofi-Aventis U.S.LLC TRENTAL - pentoxifylline Package Insert. 2006.
- 5.Granchelli JA, Pollina C, Hudecki MS. Pre-clinical screening of drugs using the mdx mouse. Neuromuscul Disord. 2000;10(4-5):235–239. doi: 10.1016/s0960-8966(99)00126-1. [DOI] [PubMed] [Google Scholar]
- 6. [Publish Date: August 9, 2006];Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events. (Version 3.0). 2003 March 31; DCTD, NCI, NIH, DHHS. http://ctep.cancer.gov.
- 7.Hatcher RA. The mechanism of vomiting. Physiol. Rev. 1924;4:479–504. [Google Scholar]
- 8.Borrelli O, Salvia G, Mancini V, Santoro L, Tagliente F, Romeo EF, Cucchiara S. Evolution of gastric electrical features and gastric emptying in children with Duchenne and Becker muscular dystrophy. American Journal of Gstroenterology. 2005;100:695–702. doi: 10.1111/j.1572-0241.2005.41303.x. [DOI] [PubMed] [Google Scholar]
- 9.Mule F, Amato A, Vannucchi MG, Gaussone-Pellegrini MS, Serio R. Altered tachykinergic influence on gastric mechanical activity in mdx mice. Neurogastroenterology & Motility. 2006;18:844–852. doi: 10.1111/j.1365-2982.2006.00820.x. [DOI] [PubMed] [Google Scholar]
- 10.Baccari MC, Nistri S, Vannucchi MG, Calamai F, Bani D. Reversal by relaxin of altered ileal spontaneous contractions in dystrophic (mdx) mice through a nitric oxide-medicated mechanism. American Journal of Physiology – Regulatory Integrative & Comparative Physiology. 2007;293:R662–668. doi: 10.1152/ajpregu.00214.2007. [DOI] [PubMed] [Google Scholar]
- 11.Baccari MC, Calamai F, Chiappini L, Vannucchi MG, Bani D. Relaxin restores altered ileal spontaneous contractions in dystrophic (mdx) mice. Annals of the New York Academy of Sciences. 2005;1041:308–310. doi: 10.1196/annals.1282.047. [DOI] [PubMed] [Google Scholar]


