INTRODUCTION
The metabolic syndrome is a constellation of risk factors of metabolic origin that are accompanied by increased risk for cardiovascular disease and type 2 diabetes mellitus. Insulin resistance, which results in this risk-factor clustering, may contribute to many of the untoward outcomes attributed to the metabolic syndrome (1). The clinical risk factors are atherogenic dyslipidemia (low high density lipoprotein and high triglycerides levels), elevated blood pressure, elevated plasma glucose, a prothrombotic state, and a pro-inflammatory state (Figure 1). Metabolic syndrome is also characterized by hyperinsulinemia, low glucose tolerance and truncal obesity. Lipid profile abnormalities, such as elevated serum triglyceride levels and reduced high density lipoproteins, have been found in the majority of studies of intermittent claudication (2) and there is a strong inverse relationship between high-density lipoprotein levels and claudication severity (3). It has been estimated that 50% of diabetic patients have evidence of peripheral artery disease (PAD) (4). However, a study of 100 consecutive ‘non-diabetic’ patients attending a vascular clinic showed abnormal glucose tolerance tests in 40% (5). This was despite all 40 patients having normal random or fasting blood glucose levels. Two definitions of metabolic syndrome predominate in the literature, the National Cholesterol Education Program (NCEP) and the World Health Organization (WHO) (Table 1). Metabolic syndrome is defined as the presence of 3 or more of the following: (1) waist circumference ≥88 cm in women and ≥102 cm in men; (2) fasting triglycerides ≥150 mg/dL or drug treatment for elevated triglycerides; (3) HDL-cholesterol <50 mg/dL in women and <40 mg/dL in men or drug treatment for reduced HDL-cholesterol; (4) BP ≥130/85 mm Hg or use of BP-lowering medication; and (5) fasting glucose ≥100 mg/dL or use of glucose-lowering medication (6). If waist circumference is not available, a body mass index (BMI) >30 kg/m2 can be used as a determinant for abdominal obesity (7). The prevalence of metabolic syndrome in the adult population in developing countries is 22–39% and varies depending on the definition used and on ethnicity (8–10). Using either WHO or NCEP definitions (Table 1) metabolic syndrome is associated with future coronary heart disease events and type 2 diabetes. Both definitions will predict cardiovascular mortality, while the NCEP definition can predict all cause mortality. While the age-dependent prevalence of the metabolic syndrome is between 20% and 40% in healthy subjects (11), metabolic syndrome is present in approximately 45% of patients with symptomatic peripheral vascular disease and is higher in those with lower extremity atherosclerotic occlusive disease (prevalences of 52% to 58%) (12, 13).
Figure 1.
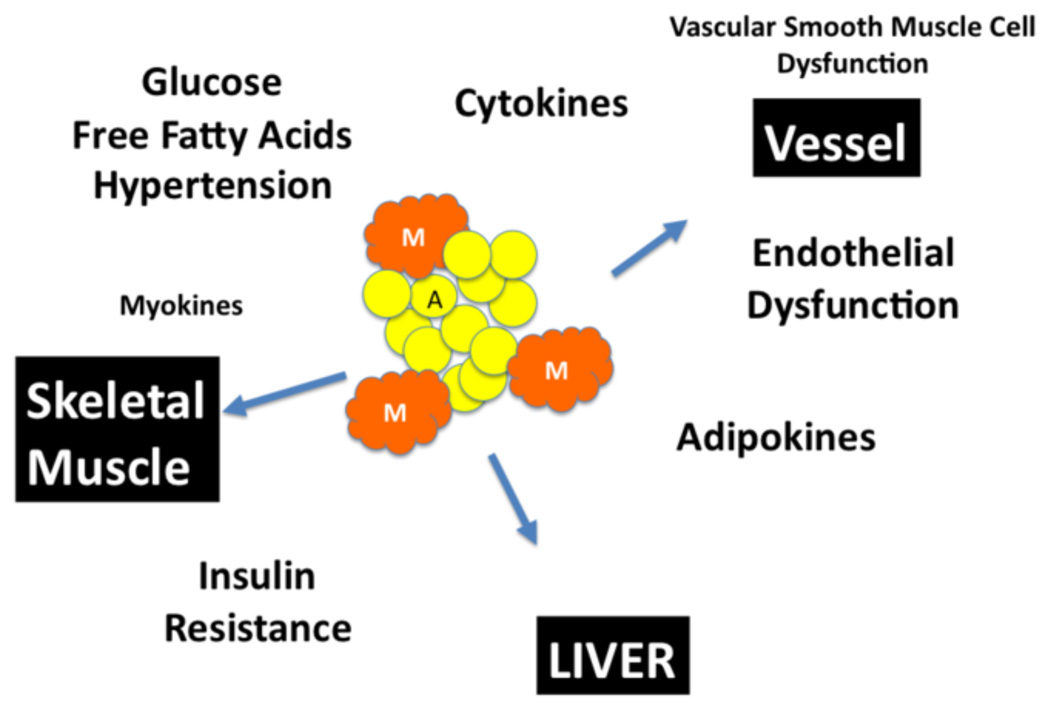
Metabolic syndrome is characterized by atherogenic dyslipidemia (low high density lipoprotein and high triglycerides levels), elevated blood pressure, elevated plasma glucose, a prothrombotic state, and a pro-inflammatory state. This is a result of an increase in adipocyte mass (A) and infiltration of macrophage infiltration (M) in the fat tissue and in organs. The presence of elevated blood pressure, elevated blood glucose and serum free fatty acids, hyperinsulinemia and insulin resistance leads dysfunction in skeletal muscle, liver and microcirculations. The secretion of myokines, adipokines and cytokines acts synergistically to enhance the metabolic state and drive the end-organ dysfunction.
Table 1.
Definitions of Metabolic Syndrome
| NCEP ATP III | WHO | |
|---|---|---|
| Absolutely required | None | Insulin resistance* (IGT, IFG, T2D or other evidence of IR) |
| Criteria | Any three of the five criteria below | Insulin resistance or diabetes, plus two of the five criteria below |
| Obesity | Waist circumference: >40 inches (M), >35 inches (F) | Waist/hip ratio: >0.90 (M), >0.85 (F); or BMI >30 kg/m2 |
| Hyperglycemia | Fasting glucose 100 mg/dl or On Therapy | Insulin resistance already required |
| Dyslipidemia | Triglycerides ≥150 mg/dl or On Therapy | Triglycerides ≥150 mg/dl or HDL-C: <35 mg/dl (M), <39 mg/dl (F) |
| Dyslipidemia (second, separate criteria) | HDL cholesterol: <40 mg/dl (M), <50 mg/dl (F) or On Therapy | |
| Hypertension | >130 mmHg systolic or >85 mmHg diastolic or On Therapy | ≥140/90 mmHg |
IGT, impaired glucose tolerance; IFG, impaired fasting glucose;
T2D, type 2 diabetes; IR, insulin resistance.
OBESITY, ADIPOCYTES, LIPIDS AND ADIPOKINES
Obesity/Adipose Tissue
In humans, two types of adipose tissue have been defined which are considered to have essentially antagonistic functions: white adipose tissue (WAT) stores excess energy as triglycerides and brown adipose tissue (BAT) is responsible for the dissipation of energy through the production of heat. BAT is abundant in small mammals and in newborns and helps them to survive cold temperatures (14). Recent investigations, however, have shown that adults also have metabolically active BAT and that BAT may play an important role in energy homeostasis. WAT stores energy in the form of triglycerides and accumulated WAT is the adipose tissue responsible for metabolic syndrome.
Adipose tissue is a heterogeneous mix of adipocytes, stromal pre-adipocytes, immune cells, and endothelium (15). Adipose tissue develops in several distinct anatomical depots within the body, and the differential expansion of these depots is of great importance. Expansion of visceral or abdominal white adipose tissue has been most strongly correlated to insulin resistance and cardiovascular disease in humans and animals (16). Conversely, expansion of subcutaneous WAT does not appear to have the same negative systemic consequences on metabolism (17). Adipose tissue possesses a relatively dense network of blood capillaries, ensuring adequate exposure to nutrients and oxygen. WAT varies in its vascularity, both between depots and within the tissue itself (18). The balance between angiogenesis and hypoxia has a significant impact on the modulation of “good” versus “bad” tissue expansion, thereby implicating the local microvasculature as a key modulator of the systemic impact adipose depots (17, 19, 20).
The adipose tissue of obese humans contains increased numbers of macrophages, and once activated, these macrophages secrete a range of cytokines such as TNF-α, IL-6, and IL-1 (21). The adipose tissue resident macrophages are responsible for the expression of most of the tissue’s TNF-α and IL-6. The expression of macrophage markers in human adipose tissue is high in subjects with obesity and insulin resistance, and can be correlated with the expression of TNF-α and IL-6 (22, 23). Adipocytes express low levels of monocyte chemoattractant protein (MCP)-1, and increased expression is found in obese subjects (23). Recent studies suggest that macrophages infiltrate adipose tissue as part of a scavenger function in response to adipocyte necrosis. Immunohistological studies of human adipose tissue have demonstrated that most of the macrophages in adipose tissue are surrounding dead adipocytes and form a syncytium, often referred to as a “crown-like structure” (24), and the macrophage burden surrounding necrotic adipocytes increases significantly (25). The adipocyte secretes several factors that modulate the production of new blood vessels (e.g., angiopoietin-1, angiopoietin-2, vascular endothelial growth factor (VEGF), transforming growth factor β (TGF-β), hepatic growth factor (HGF), stromal derived factor 1 (SDF-1), TNF-α, resistin, leptin, tissue factor, placental growth factor (PGF), and insulin-like growth factors (IGF)) (26). There is evidence suggesting that the vascular network forms before the mature lipid-carrying adipocytes reside in the area, thus providing access of secreted cytokines and adipokines to the circulation (27). Despite this great angiogenic potential, the rapidly expanding fat pad still experiences hypoxia and necrosis (28, 29). The induction of adipocyte hypoxia in vitro results in the expression of a number of inflammatory cytokines (30–32). The adipose tissue expandability hypothesis states that a failure in the capacity for adipose tissue expansion, rather than obesity per se is the key factor linking positive energy balance and type 2 diabetes. Each individual possesses a maximum capacity for adipose expansion, which is determined by both genetic and environmental factors. Once the limit of adipose tissue expansion is reached, adipose tissue ceases to store energy efficiently and lipids begin to accumulate in other tissues. Ectopic lipid accumulation in non-adipocyte cells induces insulin resistance, apoptosis and inflammation (33). Adipocytes are potent sources of such adipokines, which mediate many parts of the biology encountered. There are two types of adipocytokines: adipose-tissue-specific bioactive substances (true adipokines) and adipokines that are abundantly secreted from adipose tissue, but which are not specific for adipose tissue.
Lipoprotein Metabolism
Obesity, an increased mass of activated adipocytes and their linked changes in lipoproteins, is a major component in the biology of metabolic syndrome. Chronic inflammation associated with visceral obesity induces altered lipoprotein metabolism and insulin resistance in the liver (34). Abnormalities in the transport of lipoprotein diminish the catabolism of the very low density lipoprotein (VLDL) and increase the catabolism of the high density lipoprotein (HDL), which creates insulin resistance. This process is associated with a lower concentration of the adipokine, adiponectin (vide infra) that in turn regulates the catabolism of VLDL and HDL, consequently increasing the flow of fatty acids from the adipose tissue to the liver and muscles (35). Accumulation of lipid metabolites within non-adipose tissues can induce chronic inflammation by promoting macrophage infiltration and activation and tissue damage. Oxidized and glycated lipoproteins, free fatty acids (FFA), free cholesterol, triacylglycerols, diacylglycerols and ceramides induce cellular dysfunction through their pro-inflammatory and pro-apoptotic properties (36). Emerging evidence also suggests that macrophage activation by lipid metabolites and further modulation by lipid signaling, represents a common pathogenic mechanism underlying lipotoxicity in atherosclerosis, obesity-associated insulin resistance, liver steatosis and chronic kidney disease. Lipid derivatives, through modulation of macrophage function, promote plaque instability in the arterial wall, impair insulin responsiveness and contribute to inflammatory liver, muscle and kidney disease (36). In metabolic syndrome, the regulation of fat storage and energy supply by adipose tissue is impaired leading to elevated plasma FFA levels, excessive metabolism of FFAs, and high levels of FFA metabolites in non-adipose tissue (37). FFAs and their metabolites act as metabolic mediators of insulin resistance. It has been proposed that insulin resistance occurs in adipose tissue before muscle insulin resistance (38). In adipose tissue, insulin resistance of obesity is characterized by an inadequate insulin action in the fed state that resembles conditions in the normal fasting state. This results in the release of FFAs into the circulation to deliver energy to skeletal muscle. Thus, postprandial FFAs are directly transported to the skeletal muscle and liver, where energy is not needed. FFAs and their metabolites also act as signaling molecules interacting with insulin signaling (39) and have direct effects on glucose transport (37, 40, 41). Reducing ectopic fat in the skeletal muscle of diabetic patients might therefore be a promising target for diabetes therapy and might partly explain the action of the diabetic drug, thiazolidinedione (42).
Adipokines
Adipocytes secrete a variety of hormones, cytokines, growth factors and other bioactive substances, conceptualized as adipocytokines, including plasminogen activator inhibitor 1 (PAI-1), tumor necrosis factor (TNF-α), leptin and adiponectin (Table 2) (43). Dysregulated production of these adipocytokines is part of the pathogenesis of metabolic syndrome (Figure 2). Increased productions of PAI-1 and TNF-α from accumulated fat contribute to the formation of thrombosis and insulin resistance in obesity, respectively. A lack of leptin causes metabolic syndrome. Adiponectin exerts insulin-sensitizing and anti-atherogenic effects, hence decrease of plasma adiponectin is causative for insulin resistance and atherosclerosis in obesity (44).
Table 2.
Signaling molecules of Metabolic Syndrome
| CYTOKINES | ADIPOKINES | COAGULATION | HYPERTENSION | ADIPOCYTE RELATED |
|---|---|---|---|---|
| TNF-α | Leptin | PAI-1 | Angiotensinogen | Acylation Stimulating Protein |
| IL-6 | Adiponectin | Tissue Factor | Prostaglandins | Monobutyrin |
| IL-8 | Resistin | TIMP-1 | Oxygen Free Radicals | Adipsin |
| IL-10 | Visfatin | Adiophilin | ||
| L-1Rα | Apelin | ApoE | ||
| L-1RA | Vaspin | Pentraxin-3 | ||
| TGF-β | Chemerin | SAA | ||
| IGF1 | Hepcidin | Agouti | ||
| HBEGF | RBP-4 | α1 acid glycoprotein | ||
| MCP-1 | Omentin |
Figure 2.
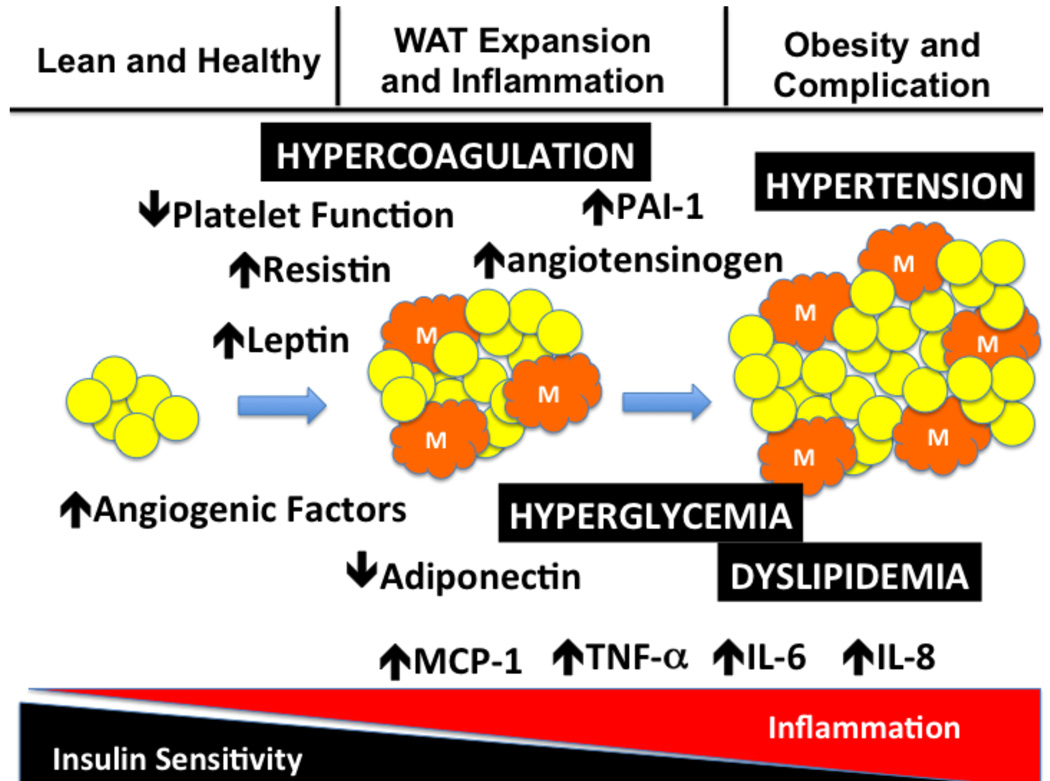
The development of metabolic syndrome is a journey from lean and healthy to white adipose tissue (WAT) expansion and inflammation which progresses to obesity and its associated complications. Expansion of the WAT is associated with an angiogenic response and an increase in the infiltration of macrophages into the WAT. The visceral and truncal WAT alter their production of adipokines and cytokines which leads to enhanced inflammation and a loss of insulin sensitivity (development of insulin resistance). The result of the changes in adipokines and cytokines leads to hypertension, hyperglycemia, dyslipidemia and a hypercoaguable state
Leptin
Leptin is a 167-amino acid hormone secreted largely by adipose tissue that controls food intake and energy expenditure (45). Circulating levels of leptin parallel fat cell stores, increasing with overfeeding and decreasing with starvation. While the absence of leptin or a mutation in leptin receptor genes induces a massive hyperphagia and obesity in humans (46, 47), the prevalence of these mutations in obese humans is rare. The effects of leptin are mediated by receptors, mainly located in the central nervous system, and in other tissues, including adipocytes and endothelial cells. Leptin receptor belongs to the class I family of cytokine receptors, and it engages both the signal transducer and activator of transcription-3 (STAT3) pathway and the insulin receptor substrate phosphoinositide-3 kinase pathway (48). It has been shown that STAT3 is essential for mediating food intake, liver glucose production, and gonadotropin secretion (48); however, the control of adipose tissue metabolism by leptin is STAT3 independent (49). Infusion of leptin into the hypothalamus led to the suppression of lipogenesis in adipose tissue through activation of the phosphoinositide-3 kinase pathway, the sympathetic nervous system, and the engagement of the adipose tissue endocannabinoid system (49). Leptin modulates the T-cell immune response, stimulates proliferation of T-helper cells, and increases production of pro-inflammatory cytokines by regulating different immune cells (50, 51).
Adiponectin
Adiponectin is a 30-kDa protein secreted from adipocytes (52). Circulating adiponectin is found in several different isoforms: trimer, low-molecular weight (hexamers), and high-molecular weight (HMW) (18mers) forms (53, 54), each with distinct biological functions (55). The HMW isoform is linked to the insulin-sensitizing effects of adiponectin, while the central effects of adiponectin are linked to the hexamer and trimer isoforms (56). Adiponectin is present in cerebrospinal fluid largely in the trimer and hexamer forms (57). Adiponectin increases food intake by enhancing hypothalamic AMPK activity in fasting conditions (58). Circulating levels of adiponectin are decreased in obesity-induced insulin resistance (59, 60). The HMW oligomer of adiponectin is inversely associated with the risk for diabetes independent of total adiponectin levels (61), and is responsible for the association of adiponectin with traits of metabolic syndrome (62, 63). Adiponectin binds two transmembrane receptors (AdipoR1 and AdipoR2) that are ubiquitously expressed. AdipoR1 is predominantly expressed in skeletal muscle with a preference for binding to globular adiponectin, whereas AdipoR2 is most abundant in the liver with a preference for binding to full-length adiponectin (64). Adiponectin improves insulin sensitivity by increasing energy expenditure and fatty acids and by the expansion of subcutaneous adipose tissue with decreased levels of macrophage infiltration (65), similar to the actions of PPARγ agonists. Thiazolidinediones are known to increase circulating levels of adiponectin, mostly the HMW form, by 2- to 3-fold (66–68), and improve insulin resistance by diversion of fat from ectopic sites to subcutaneous adipose tissue (69). Interestingly, the insulin-sensitizing effects of Thiazolidinediones are significantly diminished in the absence of adiponectin (70), suggesting an important role of adiponectin in reduction of the lipotoxicity and inflammation associated with obesity. Adiponectin has also had vasculoprotective effects through an increase in endothelial nitric oxide (NO) production and modulation of expression of adhesion molecules and scavenger receptors (71, 72).
Resistin
Resistin is a 12-kDa peptide that is part of a gene family of “Resistin-like molecules,” produced by resistin (73). Resistin is expressed by adipocytes in mice but is expressed by the macrophages of humans (74). Resistin is a 12 kDa protein that circulates as either a trimer (monomeric form of the peptide hormone) or hexamer (dimeric form of resistin). The monomeric form was shown to impair hepatic insulin action more potently than the dimerized form (75). In contrast, the dimerized form of resistin was shown to be more effective in antagonizing insulin-stimulated glucose uptake in adult murine cardiomyocytes (75). A number of studies have examined plasma resistin levels or adipose resistin expression, and have found variable associations with insulin resistance (76–79). High plasma levels of resistin correlate with proatherogenic inflammatory markers, metabolic syndrome, increased cardiovascular risk, unstable angina, and poor prognosis in coronary artery disease. Resistin increases pro-inflammatory markers and induces the release of endothelin 1 and the production of vascular cell adhesion molecule 1 and MCP-1. A recent large study involving the Framingham offspring cohort found a significant relationship between insulin resistance and resistin; however, this relationship was considerably weaker than the relationship with adiponectin, and was lost after adjustment for BMI (80). Resistin decreases after Thiazolidinediones treatment of humans, although resistin was also decreased by metformin treatment (66, 81).
Retinol binding protein 4
Retinol binding protein 4 (RBP4) is a highly-expressed circulating adipokine that can lead to insulin resistance. Many studies have demonstrated a positive association between RBP4 and insulin resistance or obesity, whereas others have not found such a relationship. There are suggestions of an age-related difference with younger people demonstrating the relation while older persons do not (82). RBP4 is associated with adipose tissue macrophage markers, suggesting a link between RBP4 and inflammation within adipose tissue (83). RBP4 circulates bound to transthyretin, which decreases RBP4 renal clearance, and transthyretin plasma levels are increased 4-fold in murine models of metabolic syndrome (84). There is an association of RBP4 and Glut4, which is presumed to play a role in fuel sensing in the adipocyte.
Visfatin
Visfatin is expressed in many cells and tissues, and was previously identified as a protein involved in B-cell maturation (pre-B colony enhancing factor) (85, 86). Visfatin has insulin-like functions, and is predominantly found in visceral adipose tissue (87). In human studies, a positive correlation between visceral adipose tissue visfatin gene expression and BMI was noted, along with a negative correlation between BMI and subcutaneous fat visfatin (88, 89), suggesting that visfatin regulation in these different depots is different, and adipose depot ratios are highly dependent on the obesity of the subjects. No difference in visfatin expression between fat depots of humans was noted (88, 89), and visfatin was expressed predominantly by non-macrophage cells in the adipose tissue stroma (89). Plasma visfatin can be positively associated with BMI (88). Visfatin can up-regulate IL-6 and TNF-α in vivo and in vitro (75). Visfatin can increase matrix metalloproteinase-9 (MMP-9) activity in monocytes, and TNF-α and IL-8 in peripheral blood mononuclear cells (90).
Tissue Inhibitor of Metalloprotease-1 (TIMP-1)
TIMP-1 is another new candidate adipokine. TIMP-1 is the constitutive inhibitor for the gelatinase MMP-9. The expression and secretion of TIMP-1 are upregulated by pro-inflammatory cytokines in obese patients and in vitro so it is possible that TIMP-1 has a role in maintaining adipose tissue mass in obesity (91, 92).
Heparin-binding epidermal-growth-factor-like growth factor
Heparin-binding growth factors are a family of mitogenic proteins that have varying affinities for heparin and heparin-like molecules. They include platelet-derived growth factor (PDGF), acidic fibroblast growth factor (aFGF), basic FGF (bFGF), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and heparin-binding epidermal growth factor (HBEGF). HBEGF is expressed in adipocytes and its plasma levels increase with the extent of obesity. HBEGF is a 22-kDa growth factor that is mitogenic for fibroblasts and smooth muscle cells. It acts by binding to the EGF receptor and, in fact, has a greater affinity for EGF receptors on smooth muscle cells and is a more potent mitogen for smooth muscle cells than EGF itself.
Angiotensinogen
The angiotensinogen gene is detected in adipose tissue, although mRNA expression is not correlated with adiposity. Angiotensinogen is the precursor of angiotensin-I which, after conversion to angiotensin-II, plays a major role in blood pressure regulation. Angiotensinogen mRNA expression is increased in visceral fat (93, 94), which partially explains the relationship between systemic hypertension and obesity in the metabolic syndrome. Angiotensinogen knockout mice with selective overexpression of angiotensinogen in adipose tissue develop obesity with a high-fat diet and especially hypertension, which is compatible with involvement of adipose-tissue angiotensinogen secretion in the genesis of this phenotype (95).
Omentin
Omentin is a secretory protein that has been recently identified as a new adipokine encoded by two genes (1 and 2) that is highly and selectively expressed in visceral adipose tissue. Omentin may regulate insulin action. Omentin 1 plasma levels and adipose tissue gene expression are decreased with obesity, and they correlate positively with plasma adiponectin and high-density lipoprotein and negatively with waist circumference, BMI, and insulin resistance (96). Omentin transcripts are strongly expressed in visceral adipose tissue, but poorly in subcutaneous fat. Omentin is present in the stromal vascular cells of omental adipose tissue, but not in mature fat cells. Human omentin is a peptide of 313 amino acids, and contains a secretory signal sequence and a fibrinogen-related domain (97). It is secreted in the culture medium of omental, but not subcutaneous, fat explants. Interestingly, it increases insulin-stimulated glucose uptake in both omental and subcutaneous adipocytes, and promotes Akt phosphorylation (98).
Chemerin
Chemerin is a novel and promising adipokine, whose plasma levels in humans have been found to be significantly associated with BMI, circulating triglycerides, and blood pressure (99). It has been demonstrated that chemerin or chemerin receptor knockdown impairs differentiation of 3T3-L1 cells into adipocytes, reduces the expression of adipocyte genes involved in glucose and lipid homeostasis, including adiponectin and leptin, and alters metabolic functions in mature adipocytes (100).
Apelin
Apelin is an adipokine produced and secreted by adipocytes, whose plasma levels are significantly elevated by obesity and insulin in humans, which was found to be the endogenous ligand for the G protein-coupled APJ receptor. The gene that encodes the apelin receptor shares the greatest sequence identity with the angiotensin AT1 receptor (101). Apelin production is up-regulated by hypoxia. Apelin has been shown to exert potent positive inotropic effects on both normal and failing myocardium. The cardiac apelin system is down-regulated by angiotensin II, and its restoration is reached by treatment with angiotensin type 1 receptor blocker.
Vaspin
Vaspin is an adipokine recently identified as a member of the serine protease-inhibitor family (102). It is strongly expressed in visceral adipose tissue and is stimulated in mouse and human obesity (103). Its tissue expression and plasma levels are normalized in the presence of insulin or an insulin-sensitizing drug (pioglitazone) (104).
Serum amyloid A
Serum amyloid A (SAA) is an inflammatory acute-phase protein associated with systemic inflammation and atherosclerosis, and is used as a predictive marker in coronary accidents or cardiovascular events. Circulating levels of SAA are significantly correlated with insulin resistance in obesity and type 2 diabetes (105). SAA is expressed in adipose tissue, and this expression is largely increased in obesity and diabetes (106, 107). Also, SAA could participate in lipoprotein metabolic alterations, promoting the linking of HDL-cholesterol to macrophages, thus reducing their cardiovascular-protective effect.
C-reactive protein
C-reactive protein (CRP) mRNA is detected in adipocytes. Adiponectin knockout mice show higher plasma CRP levels than wild-type mice. There is a strong negative correlation between adiponectin mRNA and CRP mRNA expression in human adipose tissue suggesting that a decrease in adiponectin leads to a rise in CRP. CRP can inhibit insulin-evoked NO production in endothelial cells through specific inactivation of the PI3K/Akt/eNOS pathway (108, 109). Similar to TNF-α, CRP simultaneously increases endothelial ET-1 production (109).
Thrombospondin-1 and CD36
One of the newer adipokines is Thrombospondin-1 (TSP1), a multi-domain, multi-functional glycoprotein synthesized by many cell types, which modulates cell adhesion and proliferation. Thrombospondin-1 is a major activator of TGF-β1 in vivo (110). As a result, TSP1 is involved in angiogenesis, inflammation and wound healing. CD36 is the receptor for thrombospondin-1 and is present on platelets, mononuclear phagocytes, adipocytes, hepatocytes, myocytes, and some epithelia (111). On phagocytes, it functions as a scavenger receptor, recognizing specific oxidized phospholipids and lipoproteins. CD36 also binds long-chain fatty acids and facilitates their transport into cells, thus participating in muscle lipid utilization, adipose energy storage, and gut fat absorption and possibly contributing to the pathogenesis of metabolic disorders, such as diabetes and obesity. The chemotactic properties of TSP-1 (112) provide a link between TSP1 and macrophage-mediated adipocyte inflammation. In addition, adipocyte-macrophage co-culture experiments demonstrated TSP1 gene and protein up-regulation by both cell types, suggesting a feed-forward inflammatory mechanism in adipose tissue (113). TSP1 may be an important component of inflammation and coagulation in the metabolic complications of obesity.
INSULIN RESISTANCE AND HYPERGLYCEMIA
Insulin resistance
Insulin resistance is the second key biological component of the metabolic syndrome. Chronic inflammation associated with visceral obesity induces insulin resistance in the liver (34). This chronic inflammation is characterized by the production of abnormal adipokines and cytokines such as TNF-α, FFA, IL-1, IL-6, leptin and resistin. These factors inhibit insulin signaling, which in turn causes impaired suppression of glucose production by insulin in hepatocytes, leading to hyperglycemia. An important and early complication of hepatic insulin resistance is the induction of hepatic VLDL production, via changes in the rate of apoB synthesis and degradation and de novo lipogenesis, or increased free fatty acid flux from adipose tissue into the liver. Insulin resistance also stimulates the production of CRP and PAI-1, both markers of an inflammatory state. All metabolic abnormalities related to hepatic insulin resistance have been shown to directly or indirectly promote atherosclerosis. Insulin has several direct vascular actions that contribute to either vascular protection or injury, depending on the cell type. Vascular protective effects of insulin include stimulation of endothelial cell production of the vasodilator nitric oxide. This, in turn, inhibits formation of lesions dependent on migration and proliferation of vascular smooth muscle cells, attenuates binding of inflammatory cells to the vascular wall, and inhibits thrombosis by reducing platelet adhesion and aggregation. However, insulin also promotes a host of deleterious vascular effects by stimulating the actions of various growth factors including angiotensin II and PAI-1. Glucocorticoid metabolism is also abnormal in insulin-resistant states. Adipocyte-derived hormones, including adiponectin and leptin, regulate systemic insulin sensitivity in accordance to existing triglyceride reserves. Leptin levels reflect existing fat mass and the adipokine negatively regulates insulin action in adipose tissue. Adiponectin, on the other hand, preserves insulin sensitivity via transient increments of AMPK activity and its circulating levels seem to reflect the adipogenic capacity of adipose tissue. Due to the fact that adiponectin and insulin are synergistic, inadequate adiponectin production contributes to systemic insulin resistance. In insulin-resistant states associated with impaired PI3K-dependent insulin signaling pathways, insulin-mediated ET-1 secretion is augmented and blockade of ET-1 receptors significantly improves insulin sensitivity and peripheral glucose uptake in the context of insulin resistance (114, 115).
Elevated blood glucose levels induce a series of alterations within the vasculature including endothelial dysfunction, cellular proliferation, changes in extracellular matrix conformation and impairment of LDL receptor-mediated uptake decreasing the in vivo clearance of LDL concentrations (116–120). The presence of high glucose concentrations also increases lipoprotein oxidation (121). Elevated glucose leads to the activation of the sorbital pathway, cellular oxidative stress and formation of advanced glycation endproducts (AGE). AGE can be processed by macrophages through a recently characterized series of high-affinity receptors: scavenger receptors types I and II, the receptor for advanced glycation endproducts (RAGE), oligosaccharyl transferase-48 (OST-48, AGE-R1), 80K-H phosphoprotein (AGE-R2) and galectin-3 (AGE-R3). Coupling of AGE proteins to their AGE receptor results in TNF-α and IL-1 synthesis and secretion. Evidence provided by both clinical and pre-clinical studies regarding a central involvement of the receptor for advanced glycation endproducts (RAGE) in vascular disease continues to mount. RAGE is upregulated as a consequence of diverse inflammatory stimuli including hyperglycemia, oxidized low density lipoprotein (oxLDL) and reduced shear stress. RAGE may maintain and amplify inflammatory responses in the vasculature if ligand for the receptor is present. RAGE binding by circulating AGEs or S100 protein released by activated leukocytes results in the generation of reactive oxygen species (ROS) and further activation of NF-αB. This leads to upregulation of adhesion molecules for circulating monocytes as well as further upregulation of RAGE itself. In addition, these ROS may scavenge and reduce bioavailability of the labile vasodilator NO, reducing its anti-inflammatory effects and possibly compromising control of vascular tone directly.
ENDOTHELIAL DYSFUNCTION AND INFLAMMATION
Adipose tissue resident macrophages and adipocytes in the adipose tissue and the consequences of hyperglycemia, altered lipoproteins and hyperinsulinema in the vasculature and within organ microcirculations induce dysfunctional endothelia and a pro-inflammatory state in metabolic syndrome (122).
Endothelial dysfunction
Endothelial dysfunction is an important component of the metabolic syndrome. Deficiency of endothelial-derived NO is believed to be the primary defect that links insulin resistance and endothelial dysfunction. NO deficiency results from decreased synthesis and/or release, in combination with exaggerated consumption in tissues by high levels of reactive oxygen and nitrogen species due to cellular disturbances in glucose and lipid metabolism. Insulin may stimulate endothelial nitric oxide production or may act directly on vascular smooth muscle via stimulation of the Na+-H+ exchanger and Na+/K+-ATPase, leading to hyperpolarization of the cell membrane and consequent closure of voltage-gated Ca2+ channels. Endothelial dysfunction contributes to impaired insulin action by altering the transcapillary passage of insulin to target tissues. Reduced expansion of the capillary network, with attenuation of microcirculatory blood flow to metabolically active tissues, contributes to the impairment of insulin-stimulated glucose and lipid metabolism. Insulin-induced vasodilation, which is mediated by the release of NO, is impaired in obese individuals who display insulin resistance, possibly due to suboptimal levels of (6R)-5,6,7,8-tetrahydrobiopterin (BH4), the natural and essential cofactor of NO synthases (NOS), and accelerated inactivation of NO by O2− within the vascular wall was observed. A 'third factor' may cause both insulin resistance and endothelial dysfunction in cardiovascular disease. Candidates include skeletal muscle fibre type and capillary density, distribution of adiposity and endogenous corticosteroid production. A complex interaction between endothelial dysfunction, abnormal skeletal muscle blood flow and reduced insulin-mediated glucose uptake may be central to the link between insulin resistance, blood pressure, impaired glucose tolerance and the risk of cardiovascular disease.
TNF-α
Of the pro-inflammatory cytokines, TNF-α is the best described in disturbed insulin signaling. Mice lacking TNF-α or TNF-α receptors are resistant to the development of obesity induced insulin resistance (123, 124). In adipose tissue, TNF-α is mostly secreted by macrophages in the stromal vascular fraction. Circulating TNF-α and adipose tissue TNF-α gene expression are increased in insulin resistance (125), and acute infusion of TNF-α inhibited insulin-induced glucose uptake in healthy subjects (126). Neutralization of TNF-α in rodents has improved insulin resistance (127), whereas attempts to neutralize TNF-α in humans to improve insulin resistance have generally not been successful (128)), although more recent studies have shown slight improvement in insulin resistance with TNF-α inhibition (129–131). Limited effects of TNF-α blockade on insulin resistance could be explained by the paracrine actions of TNF-α. Further investigations on the mechanisms involved in TNF-α overexpression associated with obesity and molecular signals underlying TNF-α-induced metabolic dysregulation are warranted. TNF-α increases ET-1 secretion and inhibits insulin's stimulating effect on endothelium-dependent vasodilation in humans (132, 133).
IL-6
IL-6 is also overexpressed in adipose tissue of the obese (125). The role of IL-6 in metabolic changes associated with obesity is unclear. There are some reports of IL-6 causing impaired insulin signaling in the liver and adipocytes by inducing ubiquitin-mediated degradation of insulin receptor substrate through suppressor of cytokine signaling (SOCS) 1 and 3 (134, 135). However, effects of IL-6 on insulin sensitivity in skeletal muscle are controversial (135). Exercise that is associated with increased insulin action in skeletal muscle increases circulating IL-6 levels dramatically (136), suggesting possible anti-inflammatory roles for IL-6 in skeletal muscle. The data on the increased onset of obesity and diabetes in mice lacking IL-6 are conflicting (137, 138). IL-6 also inhibits insulin-stimulated increases in eNOS activity and NO production in the endothelium (139). IL-1 together with IL-6 concentrations reportedly predicts the risk for Type 2 diabetes in humans better than either cytokine alone (140).
IL-10
Decreased production of IL-10, an anti-inflammatory cytokine, has been associated with the development of Type 2 Diabetes, and IL-10 plasma levels can be positively correlated with insulin sensitivity (141, 142). Lower IL-10 levels have been associated with the metabolic syndrome in obese, insulin-resistant postmenopausal women compared with women who are obese but do not satisfy the criteria for metabolic syndrome (143). IL-10 decreases IL-6-induced insulin resistance in muscle and liver in mice co-treated with IL-6 and IL-10 (144). A recent study showed that IL-10 is expressed in macrophages derived from adipose tissue and that the IL-10 receptor is expressed in adipocytes and not immune or endothelial cells in fat (145). Several studies indicate that IL-10 is an anti-inflammatory factor produced by immune cells in adipose tissue that acts on adipocytes to improve insulin signaling, potentially decreasing further macrophage recruitment.
MCP
Adipocytes secrete various chemoattractants that draw monocytes from circulation into adipose tissue. MCP-1, also known as chemokine (C-C motif) ligand 2 (CCL-2), is one the chemoattractants that plays an important role in the recruitment of macrophages to the adipose tissues. Moreover, obesity is associated with increased plasma levels of MCP-1 and overexpression in adipose tissue (146, 147). Mice lacking MCP-1 receptor (CCR-2) have decreased adipose tissue macrophage infiltration and improved metabolic function (148, 149). Other candidates might likely contribute to the recruitment of macrophages into the adipose tissue, such as macrophage inflammatory protein-1 (150) and osteopontin (151, 152). Osteopontin is an extracellular matrix protein that promotes monocyte chemotaxis, and the lack of osteopontin in mice caused improved insulin sensitivity and decreased macrophage infiltration into adipose tissue (152).
HYPERTENSION
Essential hypertension is a complex, multi-factorial, quantitative trait under polygenic control. Increased peripheral resistance due primarily to changes in vascular structure and function appear to be the fundamental hemodynamic abnormality in hypertension. These changes include arterial wall thickening and abnormal vascular tone, and are due to alterations in the biology of the cellular and non-cellular components of the arterial wall. Multiple interacting humoral and mechanical factors as well as oxidative stress stimulate complex signaling pathways, which modulate vascular smooth muscle cell contraction and growth (153). Hypertension is one of the commonest components of metabolic syndrome and is linked to both essential hypertension and obesity-related hypertension (154). Excess weight gain is likely the major cause of essential hypertension, and abnormal kidney function appears to be a cause as well as a consequence of obesity hypertension. Excess renal sodium re-absorption and a hypertensive shift of pressure natriuresis play a major role in mediating increased blood pressure associated with weight gain. Activation of the renin-angiotensin and sympathetic nervous systems and physical compression of the kidneys appear to contribute to obesity-induced increases in sodium re-absorption and hypertension.
COAGULATION
Fibrinolytic dysfunction mediates the increased risk of coronary artery disease in individuals with the metabolic syndrome (155). Adipose tissue induces thrombocyte activation by the production of adipokines, of which some such as leptin and adiponectin have been shown to directly interfere with platelet function. Increased adipose tissue mass induces insulin resistance and systemic low-grade inflammation, also affecting platelet function. It has been demonstrated that adipose tissue directly impairs fibrinolysis by the production of plasminogen activator inhibitor-1 and possibly thrombin-activatable fibrinolysis inhibitor (156). Adipose tissue may contribute to enhanced coagulation by direct tissue factor production, but hypercoagulability is likely to be primarily caused by altered hepatic synthesis of the coagulation factors fibrinogen, factor VII, factor VIII and tissue factor, by releasing free fatty acids and pro-inflammatory cytokines (TNF-α, IL-1 and IL-6) into the portal circulation and by inducing hepatic insulin resistance. Adipose tissue dysfunction could thus play a causal role in the prothrombotic state observed in obesity, by directly and indirectly affecting hemostasis, coagulation and fibrinolysis (157).
In type 2 diabetes, there are increased levels of fibrinogen and PAI-1, favoring both thrombosis and defective dissolution of clots once formed. Platelets in type 2 diabetic individuals adhere to vascular endothelium and aggregate more readily than those in healthy people. Loss of sensitivity to the normal homeostatic restraints exercised by prostacyclin (PGI2) and nitric oxide (NO) generated by the vascular endothelium presents as the major defect in platelet function. Insulin is a natural antagonist of platelet hyperactivity. It sensitizes the platelet to PGI2 and enhances endothelial generation of PGI2 and NO. Thus, the defects in insulin action in diabetes create a milieu of disordered platelet activity conducive to macrovascular and microvascular events (158). Patients with type 2 diabetes and abdominal fat patterning displayed higher plasma activities of clotting factors VII and VIII as well as increased plasma levels of fibrinogen and von Willebrand factor antigen, when compared with not only healthy normal weight controls, but also with diabetic patients at normal body weight (159, 160).
PAI-1 is elevated in subjects with the metabolic complications of obesity, and is expressed in the stromal fraction of adipose tissue, including endothelial cells (161–165). PAI-1 inhibits both tissue-type plasminogen activator and urokinase-type plasminogen activator through its serine protease inhibitor function, and thus contributes to a pro-thrombotic state (166). PAI-1 gene expression is controlled by TGF-β, which combines with phosphorylated SMAD and binds to the PAI-1 promoter (167). A second pathway is via TSP1. TSP1 is expressed in adipocytes (113) and inhibits angiogenesis, cell proliferation, and wound healing (112, 168). TSP1 is a major activator of TGF-β R1 (110), and PAI-1 activation by TSP1 has been described (169). A recent study demonstrated TSP1 expression largely by adipocytes compared with the stromal vascular fraction of adipose tissue, suggesting that TSP1 is a true adipokine(113). TSP1 expression was increased in obese, insulin-resistant subjects, was associated with plasma PAI-1 levels, and was positively associated with adipose tissue macrophage markers. In addition, TSP1 expression was decreased by treatment of subjects or adipocytes with the PPAR-γ agonist, pioglitazone.
Modeling Metabolic Syndrome
The ideal model for metabolic syndrome should be obese, hypertensive, insulin resistant and have the appropriate dyslipidemia. There is at present no perfect animal model of this human disease. The most common models to study metabolic syndrome are mice which can be based one of three strategies: obese mouse strains that mimic metabolic syndrome; mice fed high-fat diets to induce metabolic syndrome; or gene knockout mice that mimic metabolic syndrome. Insulin resistance in mice with differential susceptibility to diabetes and metabolic syndrome is preceded by differences in the inflammatory response of adipose tissue. This phenomenon may serve as an early indicator of disease and contribute to disease susceptibility and progression (170). The Lepob/ob, LepRdb/db and Ay/a mice are the three most commonly used spontaneously mutant obese mouse models. They display insulin resistance and may develop diabetes depending on the background strain. In addition, Ay/a mice have intact leptin signaling and display a delayed onset obesity that can be amplified by high-fat feeding, making them a good model for human obesity. All three models fall short of an ideal model for metabolic syndrome (171). Obese mouse models such as Ay/a, Lepob/ob and LepRdb/db have increased total plasma cholesterol levels; however, this is the result of increased HDL rather than increased VLDL and LDL levels. The increase in HDL makes these mice resistant to atherosclerotic lesion formation. These lipid changes do not mirror those seen in metabolic syndrome. Hypertensive mice do not make good models unless cross bred with obese mice (171). Care should thus be taken to choose the mouse model most appropriate for the scientific or mechanistic mechanism to be tested under metabolic syndrome conditions.
Conclusion
Metabolic syndrome represents a combination of synergistic vascular pathologies that lead to an accelerated atherogenic state that compromises the ability of the patient to satisfactorily respond to humoral, cellular and mechanical stresses. Delineating the contribution of insulin resistance and the role of the adipokines remains a focus of current research. Defining key signaling pathways may provide opportunities for new therapeutic targets and interventions. It represents a complex pathology that will require better definition prior to the development of satisfactory therapeutic interventions.
Acknowledgments
Supported by: U.S. Public Health Service HL086968
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daynene Vykoukal, Vascular Biology and Therapeutics Program, The Methodist Hospital Research Institute, Houston, Texas.
Mark G. Davies, Methodist DeBakey Heart and Vascular Center, Department of Cardiovascular Surgery, The Methodist Hospital.
References
- 1.Kahn R. Metabolic syndrome—what is the clinical usefulness. Lancet. 2008;371:1892–1893. doi: 10.1016/S0140-6736(08)60731-X. [DOI] [PubMed] [Google Scholar]
- 2.Belch J. Medical management of intermittent claudication. In: Greenhalgh RM, editor. Vascular and endovascular opportunities. London: WB saunders; 2000. pp. 361–388. [Google Scholar]
- 3.Fowkes FG, Housley E, Riemersama RA, et al. Smoking, lipids, glucose intolerance and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh arterial Study. Am J Epidemiol. 1992;135:331–340. doi: 10.1093/oxfordjournals.aje.a116294. [DOI] [PubMed] [Google Scholar]
- 4.Brandman O, Redisch W. Incidence of peripheral vascular changes in diabetes mellitus. Diabetes. 1953;2:194–198. doi: 10.2337/diab.2.3.194. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey L, Williams B, Johnston G, MacGregor G, Poston L, Porter J, et al. Guidelines for management of the British Hypertension Society: report of the workng party of the British Hypertension Society. J Hum Hypertens. 1999;13:569–592. doi: 10.1038/sj.jhh.1000917. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 7.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 8.Meigs JB, Wilson PWF, NAthan DM, D'Agostino RB, Sr, williams K, Haffner SM. Prevalence and characteristics of the metabolic syndrome in the San Antonio Heart and the Framingham offspring studies. Diabetes. 2003;52:2160–2167. doi: 10.2337/diabetes.52.8.2160. [DOI] [PubMed] [Google Scholar]
- 9.Park Y-W, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heyisfield SB. The metabolic syndome: prevalence and associated risk factor findings in the US popluation from the third national health and nutritional examination survey. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banne-Parikka P, Eriksson JG, Linstrom J, Hamalainen H, Keinanen-Kiukanniemi S, Laakso M, et al. Prevalence of he metabolic syndrome and its components: findings from a Finnish general population sample and the diabetes prevention study cohort. Diabetes Care. 2004;27:2135–2140. doi: 10.2337/diacare.27.9.2135. [DOI] [PubMed] [Google Scholar]
- 11.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 12.Gorter PM, Olijhoek JK, van der Graaf Y, Algra A, Rabelink TJ, Visseren FL, et al. Prevalence of the metabolic syndrome in patients with coronary heart disease, cerebrovascular disease, peripheral arterial disease or abdominal aortic aneurysm. Atherosclerosis. 2004;173:363–369. doi: 10.1016/j.atherosclerosis.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 13.Brevetti G, Schiano V, Sirico G, Giugliano G, Laurenzano E, Chiariello M. Metabolic syndrome in peripheral arterial disease: relationship with severity of peripheral circulatory insufficiency, inflammatory status, and cardiovascular comorbidity. J Vasc Surg. 2006;44(1):101–107. doi: 10.1016/j.jvs.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 14.Saely CH, Geiger K, Drexel H. Brown versus White Adipose Tissue: A Mini-Review. Gerontology. 2011 doi: 10.1159/000321319. (in press). [DOI] [PubMed] [Google Scholar]
- 15.Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37:753–768. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haffner SM. Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med. 2007;120:S10–S17. doi: 10.1016/j.amjmed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity - associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho CH, Koh YJ, Han J, Sung HK, Jong Lee H, Morisada T, et al. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ Res. 2007;100:e47–e57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 19.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, et al. HIF 1 alpha Induces Fibrosis and Insulin Resistance in White Adipose Tissue. Mol Cell Biol. 2000;117 doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landskroner-Eiger S, Qian B, Muise ES, Nawrocki AR, Berger J, Fine EJ, et al. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin Cancer Res. 2009;15:3265–3276. doi: 10.1158/1078-0432.CCR-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 24.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, DeFuria J, Jick Z, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 26.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005;29:1308–1314. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 27.Cho CH, Koh YJ, Han J, Sung HK, Jong Lee H, Morisada T, et al. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ Res. 2007;100:e47–e57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 28.Nils Halberg N, Wernstedt I, Scherer PE. The Adipocyte as an Endocrine Cell. Endocrinol Metab Clin North Am. 2008;37 doi: 10.1016/j.ecl.2008.07.002. 753–xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 30.Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr. 2008;100:227–235. doi: 10.1017/S0007114508971282. [DOI] [PubMed] [Google Scholar]
- 31.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 32.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007;455:479–492. doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;180(3):338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42(13–14):1331–1346. doi: 10.1016/j.clinbiochem.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Palomo I, Alarcón M, Moore-Carrasco R, Argilés JM. Hemostasis alterations in metabolic syndrome. Int J Mol Med. 2006;18(5):969–974. [PubMed] [Google Scholar]
- 36.Prieur X, Roszer T, Ricote M. Lipotoxicity in macrophages: evidence from diseases associated with the metabolic syndrome. Biochim Biophys Acta. 2010;180(3):327–337. doi: 10.1016/j.bbalip.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Assimacopoulos-Jeannet F. Fat storage in pancreas and in insulin-sensitive tissues in pathogenesis of type 2 diabetes. Int J Obes Relat Metab Disord. 2004;28 Supple 4:S53–S57. doi: 10.1038/sj.ijo.0802857. [DOI] [PubMed] [Google Scholar]
- 38.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32 Supple 3:14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 39.Belfort R, et al. Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes. 2005;54:1640–1648. doi: 10.2337/diabetes.54.6.1640. [DOI] [PubMed] [Google Scholar]
- 40.Gao Zea. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18:2024–2034. doi: 10.1210/me.2003-0383. [DOI] [PubMed] [Google Scholar]
- 41.Hajri T, et al. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest. 2002;109:1381–1389. doi: 10.1172/JCI14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye JM, et al. Peroxisome proliferator-activated receptor (PPAR)-a activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-g activation. Diabetes. 2001;50:411–417. doi: 10.2337/diabetes.50.2.411. [DOI] [PubMed] [Google Scholar]
- 43.Karastergioua K, Mohamed-Alia V. The autocrine and paracrine roles of adipokines. Mol Cell Endocrin. 2010;318:69–78. doi: 10.1016/j.mce.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 44.Matsuda M, Shimomura I. Adipocytokines and metabolic syndrome--molecular mechanism and clinical implication. Nippon Rinsho. 2004;62(6):1085–1090. [PubMed] [Google Scholar]
- 45.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 46.Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356:237–247. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 48.Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Jr, Rossetti L. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab. 2006;4:49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 51.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 52.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 53.Banga A, Bodles AM, Rasouli N, Ranganathan G, Kern PA, Owens RJ. Calcium is involved in formation of high molecular weight adiponectin. Metab Syndr Relat Disord. 2008;6:103–111. doi: 10.1089/met.2007.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Lam KS, Chan L, Chan KW, Lam JB, Lam MC, et al. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem. 2006;281:16391–16400. doi: 10.1074/jbc.M513907200. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008;409:623–633. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- 57.Kusminski CM, McTernan PG, Schraw T, Kos K, O'hare JP, Ahima R, et al. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 2007;50:634–642. doi: 10.1007/s00125-006-0577-9. [DOI] [PubMed] [Google Scholar]
- 58.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-α expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 60.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 61.Heidemann C, Sun Q, van Dam RM, Meigs JB, Zhang C, Tworoger SS, et al. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med. 2008;149:307–316. doi: 10.7326/0003-4819-149-5-200809020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes Metab Res Rev. 2006;55:249–259. [PubMed] [Google Scholar]
- 63.Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, et al. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007;27:3716–3731. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JY, van de WE, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rasouli N, Yao-Borengasser A, Miles LM, Elbein SC, Kern PA. Increased plasma adiponectin in response to pioglitazone does not result from increased gene expression. Am J Physiol Endocrinol Metab. 2006;290:E42–E46. doi: 10.1152/ajpendo.00240.2005. [DOI] [PubMed] [Google Scholar]
- 67.Bodles AM, Banga A, Rasouli N, Ono F, Kern PA, Owens RJ. Pioglitazone increases secretion of high-molecular-weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab. 2006;291:E1100–E1105. doi: 10.1152/ajpendo.00187.2006. [DOI] [PubMed] [Google Scholar]
- 68.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;274:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 69.Rasouli N, Raue U, Miles LM, Lu T, Di Gregorio GB, Elbein SC, et al. Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:E930–E934. doi: 10.1152/ajpendo.00522.2004. [DOI] [PubMed] [Google Scholar]
- 70.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to PPARγ-agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 71.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 72.Zhu W, Cheng KKY, Vanhoutte PM, Lam KSL, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci (Lond) 2008;114:361–374. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- 73.Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA. 2001;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 75.Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L. Disulfidedependent multimeric assembly of resistin family hormones. Science. 2004;304:1154–1158. doi: 10.1126/science.1093466. [DOI] [PubMed] [Google Scholar]
- 76.Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, et al. Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab. 2003;88:5452–5455. doi: 10.1210/jc.2002-021808. [DOI] [PubMed] [Google Scholar]
- 77.Heilbronn LK, Rood J, Janderova L, Albu JB, Kelley DE, Ravussin E, et al. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J Clin Endocrinol Metab. 2004;89:1844–1848. doi: 10.1210/jc.2003-031410. [DOI] [PubMed] [Google Scholar]
- 78.Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003;88:4848–4856. doi: 10.1210/jc.2003-030519. [DOI] [PubMed] [Google Scholar]
- 79.Vozarova deCourten B, Degawa-Yamauchi M, Considine RV, Tataranni PA. High serum resistin is associated with an increase in adiposity but not a worsening of insulin resistance in Pima Indians. Diabetes. 2004;53:1279–1284. doi: 10.2337/diabetes.53.5.1279. [DOI] [PubMed] [Google Scholar]
- 80.Hivert MF, Sullivan LM, Fox CS, Nathan DM, D'Agostino RB, Sr, Wilson PW, et al. Associations of adiponectin, resistin, and tumor necrosis factor-α with insulin resistance. J Clin Endocrinol Metab. 2008;93:3165–3172. doi: 10.1210/jc.2008-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kamin D, Hadigan C, Lehrke M, Mazza S, Lazar MA, Grinspoon S. Resistin levels in human immunodeficiency virus-infected patients with lipoatrophy decrease in response to rosiglitazone. J Clin Endocrinol Metab. 2005;90:3423–3426. doi: 10.1210/jc.2005-0287. [DOI] [PubMed] [Google Scholar]
- 82.Gavi S, Qurashi S, Stuart LM, Lau R, Melendez MM, Mynarcik DC, et al. Influence of age on the association of retinol-binding protein 4 with metabolic syndrome. Obesity (Silver Spring) 2008;16:893–895. doi: 10.1038/oby.2007.138. [DOI] [PubMed] [Google Scholar]
- 83.Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee MJ, et al. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. 2007;92:2590–2597. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mody N, Graham TE, Tsuji Y, Yang Q, Kahn BB. Decreased clearance of serum retinol-binding protein and elevated levels of transthyretin in insulin-resistant ob/ob mice. Am J Physiol Endocrinol Metab. 2008;294:E785–E793. doi: 10.1152/ajpendo.00521.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kitani T, Okuno S, Fujisawa H. Growth phase-dependent changes in the subcellular localization of pre-B-cell colony-enhancing factor. FEBS Lett. 2003;544:74–78. doi: 10.1016/s0014-5793(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 86.Samal B, Sun Y, G S, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 88.Berndt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54:2911–2916. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 89.Varma V, Yao-Borengasser A, Rasouli N, Bodles AM, Phanavanh B, Lee MJ, et al. Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipid and inflammation. J Clin Endocrinol Metab. 2007;92:666–672. doi: 10.1210/jc.2006-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dahl TB, Yndestad A, Skjelland M, Oie E, Michelsen A, Damas JK, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115:972–980. doi: 10.1161/CIRCULATIONAHA.106.665893. [DOI] [PubMed] [Google Scholar]
- 91.Kralisch S, et al. Proinflammatory adipocytokines induce TIMP-1 expression in 3T3-L1 adipocytes. FEBS Lett. 2005;579(6417–6422) doi: 10.1016/j.febslet.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 92.Kralisch S, et al. Tissue inhibitor of metalloproteinase 1 expression and secretion are induced by b-adrenergic stimulation in 3T3-L1 adipocytes. J Endocrinol. 2006;189:665–670. doi: 10.1677/joe.1.06645. [DOI] [PubMed] [Google Scholar]
- 93.Dusserre E, Moulin P, Vidal H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim Biophys Acta. 2000;1500:88–96. doi: 10.1016/s0925-4439(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 94.Van Harmelen V, Ariapart P, Hoffstedt J, Lundkvist I, Bringman S, Arner P. Increased adipose angiotensinogen gene expression in human adiposity. 2000;8:337–341. doi: 10.1038/oby.2000.40. [DOI] [PubMed] [Google Scholar]
- 95.Massiera F, Bloch-Faure M, Cellier D. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;5:2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- 96.De Souza CM, Yang RZ, Lee MJ, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–1661. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 97.Schaffler A, Neumeier M, Herfarth H, Furst A, Scholmerich J, Buchler C. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim Biophys Acta. 2005;1732:96–102. doi: 10.1016/j.bbaexp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 98.Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–E1261. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 99.Bozaoglu K, Bolton K, McMillan J, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 100.Goralski KB, McCarthy TC, Hanniman EA, et al. Chemerin: a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 101.Lee DK, George SR, O'Dowd B. Unravelling the roles of the apelin system: prospective therapeutic applications in heart failure and obesity. Trends Pharmacol Sci. 2006;27:190–194. doi: 10.1016/j.tips.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 102.Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci USA. 2005;102:10610–10615. doi: 10.1073/pnas.0504703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kloting N, Berndt J, Kralisch S, Kovacs P, Fasshauer M, Schon MR, et al. Vaspin gene expression in human adipose tissue: association with obesity and type 2 diabetes. Biochem Biophys Res Commun. 2006;339:430–436. doi: 10.1016/j.bbrc.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 104.Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Russe R, et al. Macropages in human visceral adipose tissue: increased accumulation in obesity and source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 105.Leinonen E, Hurt-Camejo E, Wiklund O, Hulten LM, Hiukka A, Taskinen MR. Insulin resistance and adiposity correlate with acute-phase reaction and soluble cell adhesion molecules in type 2 diabetes. Atherosclerosis. 2003;166:387–394. doi: 10.1016/s0021-9150(02)00371-4. [DOI] [PubMed] [Google Scholar]
- 106.Lin Y, Rajala MW, Berger JP, Moller DE, Barzilai N, Scherer PE. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J Biol Chem. 2001;276:42077–42083. doi: 10.1074/jbc.M107101200. [DOI] [PubMed] [Google Scholar]
- 107.Poitou C, Viguerie A, Cancello R, De Matteis R, Cinti S, Stich V, et al. Serum amyloid A: production by human white adipocytes and regulation by obesity and nutrition. Diabetologia. 2005;48:519–528. doi: 10.1007/s00125-004-1654-6. [DOI] [PubMed] [Google Scholar]
- 108.Schwartz RS, Osborne-Lawrence S, Hahner L, Gibson LL, Gormley AK, Vongpatanasin W, et al. C-reactiveprotein downregulates endothelial no synthase and attenuates reendothelialization in vivo in mice. Circ Res. 2007;100(10):1452–1459. doi: 10.1161/01.RES.0000267745.03488.47. [DOI] [PubMed] [Google Scholar]
- 109.Xu JW, Morita I, Ikeda K, Miki T, Yamori Y. C-reactive protein suppresses insulin signaling in endothelial cells. ---role of syk tyrosine kinase. Mol Endocrinol. 2006 doi: 10.1210/me.2006-0354. [DOI] [PubMed] [Google Scholar]
- 110.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, et al. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell Metab. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 111.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest. 2001;107:929–934. doi: 10.1172/JCI12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Varma V, Yao-Borengasser A, Bodles AM, Rasouli N, Phanavanh B, Nolen GT, et al. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes. 2008;57:432–439. doi: 10.2337/db07-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lteif A, Vaishnava P, Baron AD, Mather KJ. Endothelin limits insulin action in obese/insulin-resistant humans. Diabetes. 2007;56(3):728–734. doi: 10.2337/db06-1406. [DOI] [PubMed] [Google Scholar]
- 115.Ahlborg G, Shemyakin A, Bohm F, Gonon A, Pernow J. Dual endothelin receptor blockade acutely improves insulin sensitivity in obese patients with insulin resistance and coronary artery disease. Diabetes Care. 2007;30(3):591–596. doi: 10.2337/dc06-1978. [DOI] [PubMed] [Google Scholar]
- 116.Brownlee M, Cerami A, Vlassara H. Advanced Products of Non-enzymatic Glycosylation and the pathogenesis of diabetic vascular disease. Diabetes/Metabolism Reviews. 1988;4:437–451. doi: 10.1002/dmr.5610040503. [DOI] [PubMed] [Google Scholar]
- 117.Getz GS. Report on the workshop on diabetes and mechanisms of atherogenesis. Arterioscler Thromb. 1993;13:459–464. doi: 10.1161/01.atv.13.3.459. [DOI] [PubMed] [Google Scholar]
- 118.Agrawal DK, Bhimji S, McNeill JH. Effect of chronic experimental diabetes on vascular smooth muscle function in rabbit carotid artery. J Cardiovasc Pharmacol. 1987;9:584–593. doi: 10.1097/00005344-198705000-00013. [DOI] [PubMed] [Google Scholar]
- 119.MacLeod KM, McNeill JH. The influence of chronic experimental diabetes on contractile responses of rat isolated blood vessels. Can J Physiol Pharmacol. 1985;63:52–57. doi: 10.1139/y85-009. [DOI] [PubMed] [Google Scholar]
- 120.Koschinsky T, Bunting CE, Rutter R, Gries FAS. Vascular growth factors and the development of macrovascular disease in diabetes mellitus. Diabetes et Metabol. 1987;13:318–325. [PubMed] [Google Scholar]
- 121.Fuster V. Progression-regression of atherosclerosis: molecular, cellular and clinical bases. Circulation. 1992;86 suppl III:1–123. [Google Scholar]
- 122.Potenza MA, Addabbo F, Montagnani M. Vascular actions of insulin with implications for endothelial dysfunction. Am J Physiol Endocrinol Metab. 2009;297:E568–E577. doi: 10.1152/ajpendo.00297.2009. [DOI] [PubMed] [Google Scholar]
- 123.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 124.Uysal KT, Wiesbrock SM, Hotamisligil GS. Functional analysis of tumor necrosis factor (TNF) receptors in TNF-α-mediated insulin resistance in genetic obesity. Endocrinology. 1998;139:4832–4838. doi: 10.1210/endo.139.12.6337. [DOI] [PubMed] [Google Scholar]
- 125.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 126.Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-α induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939–2945. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- 127.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 128.Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R. Effects of an engineered human anti-TNF-α antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes. 1996;45:881–885. doi: 10.2337/diab.45.7.881. [DOI] [PubMed] [Google Scholar]
- 129.Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, Garcia-Porrua C, Sanchez-Andrade A, Martin J, et al. Anti-tumor necrosis factor-α blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83–86. [PubMed] [Google Scholar]
- 130.Seriolo B, Ferrone C, Cutolo M. Longterm anti-tumor necrosis factor-α treatment in patients with refractory rheumatoid arthritis: relationship between insulin resistance and disease activity. J Rheumatol. 2008;35:355–357. [PubMed] [Google Scholar]
- 131.Tam LS, Tomlinson B, Chu TT, Li TK, Li EK. Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clin Rheumatol. 2007;26:1495–1498. doi: 10.1007/s10067-007-0539-8. [DOI] [PubMed] [Google Scholar]
- 132.Sury MD, Frese-Schaper M, Muhlemann MK, Schulthess FT, Blasig IE, Tauber MG, et al. Evidence that n-acetylcysteine inhibits tnf-alpha-induced cerebrovascular endothelin-1 upregulation via inhibition of mitogen- and stress-activated protein kinase. Free Radic Biol Med. 2006;41(9):1372–1383. doi: 10.1016/j.freeradbiomed.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 133.Rask-Madsen C, Dominguez H, Ihlemann N, Hermann T, Kober L, Torp-Pedersen C. Tumor necrosis factor-alpha inhibits insulin's stimulating effect on glucose uptake and endothelium-dependent vasodilation in humans. Circulation. 2003;108(15):1815–1821. doi: 10.1161/01.CIR.0000091406.72832.11. [DOI] [PubMed] [Google Scholar]
- 134.Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem. 2000;275:15985–15991. doi: 10.1074/jbc.275.21.15985. [DOI] [PubMed] [Google Scholar]
- 135.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54 Suppl 2:S114–S124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- 136.Ruderman NB, Keller C, Richard AM, Saha AK, Luo Z, Xiang X, et al. Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes. 2006;55 Suppl 2:S48–S54. doi: 10.2337/db06-s007. [DOI] [PubMed] [Google Scholar]
- 137.Di Gregorio GB, Hensley LTL, Ranganathan G, Kern PA. Lipid and carbohydrate metabolism in mice with a targeted mutation in the IL-6 gene: absence of development of age-related obesity. Am J Physiol Endocrinol Metab. 2004;287:E182–E187. doi: 10.1152/ajpendo.00189.2003. [DOI] [PubMed] [Google Scholar]
- 138.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 139.Andreozzi F, Laratta E, Procopio C, Hribal ML, Sciacqua A, Perticone M, et al. Interleukin-6 impairs the insulin signaling pathway, promoting production of nitric oxide in human umbilical vein endothelial cells. Mol Cell Biol. 2007;27(6):2372–2383. doi: 10.1128/MCB.01340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 141.van Exel E, Gussekloo J, Craen AJ, Frolich M, Bootsma-VanDer Wiel A, Westendorp RG. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes: the Leiden 85-plus study. Diabetes. 2002;51:1088–1092. doi: 10.2337/diabetes.51.4.1088. [DOI] [PubMed] [Google Scholar]
- 142.Straczkowski M, Kowalska I, Nikolajuk A, Krukowska A, Gorska M. Plasma interleukin-10 concentration is positively related to insulin sensitivity in young healthy individuals. Diabetes Care. 2005;28:2036–2037. doi: 10.2337/diacare.28.8.2036. [DOI] [PubMed] [Google Scholar]
- 143.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 144.Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, et al. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53:1060–1067. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- 145.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 147.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kiefer FW, Zeyda M, Todoric J, Huber J, Geyeregger R, Weichhart T, et al. Osteopontin expression in human and murine obesity: extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology. 2008;149:1350–1357. doi: 10.1210/en.2007-1312. [DOI] [PubMed] [Google Scholar]
- 152.Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007;117:2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Touyz RM. Molecular and cellular mechanisms regulating vascular function and structure-implications in the pathogenesis of hypertension. Can J Cardiol. 2000;16(9):1137–1146. [PubMed] [Google Scholar]
- 154.Rahmouni K, Correia MLG, Haynes WG, Mark AL. Obesity-Associated Hypertension: New Insights Into Mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 155.Anand SS, Yi Q, Gerstein HC, Lonn E, Jacobs R, Vuksan V, et al. Relationship of metabolic syndrome and fibrinolytic dysfunction to cardiovascular disease. Circulation. 2003;108(4):420–425. doi: 10.1161/01.CIR.0000080884.27358.49. [DOI] [PubMed] [Google Scholar]
- 156.Aso Y. Plasminogen activator inhibitor (PAI)-1 in vascular inflammation and thrombosis. Front Biosci. 2007;12:2957–2966. doi: 10.2741/2285. [DOI] [PubMed] [Google Scholar]
- 157.Faber DR, de Groot PG, Visseren FL. Role of adipose tissue in haemostasis, coagulation and fibrinolysis. Obes Rev. 2009;10(6):554–563. doi: 10.1111/j.1467-789X.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- 158.Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001;24(8):1476–1485. doi: 10.2337/diacare.24.8.1476. [DOI] [PubMed] [Google Scholar]
- 159.Mertens I, Van Gaal LF. Obesity, haemostasis and the fibrinolytic system. Obes Rev. 2002;3(2):85–101. doi: 10.1046/j.1467-789x.2002.00056.x. [DOI] [PubMed] [Google Scholar]
- 160.Coca M, Cucuianu M, Hâncu N. Effect of abdominal obesity on prothrombotic tendency in type 2 diabetes. Behavior of clotting factors VII and VIII, fibrinogen and von Willebrand Factor. Rom J Intern Med. 43(1–2):15–26. 205. [PubMed] [Google Scholar]
- 161.Alessi MC, Peiretti F, Morange P, Henry M, Nalbone G, Juhanvague I. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes. 1997;46:860–867. doi: 10.2337/diab.46.5.860. [DOI] [PubMed] [Google Scholar]
- 162.Cigolini M, Tonoli M, Borgato L, Frigotto L, Manzato F, Zeminian S, et al. Expression of plasminogen activator inhibitor-1 in human adipose tissue: a role for TNF-α? Atherosclerosis. 1999;143:81–90. doi: 10.1016/s0021-9150(98)00281-0. [DOI] [PubMed] [Google Scholar]
- 163.Alessi MC, Bastelica D, Morange P, Berthet BIL, Verdier M, et al. Plasminogen activator inhibitor 1, transforming growth factor-β1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes. 2000;49:1374–1380. doi: 10.2337/diabetes.49.8.1374. [DOI] [PubMed] [Google Scholar]
- 164.Mertens I, Verrijken A, Michiels JJ, Van der Planken M, Ruige JB, Van Gaal LF. Among inflammation and coagulation markers, PAI-1 is a true component of the metabolic syndrome. Int J Obes (Lond) 2006;30:1308–1314. doi: 10.1038/sj.ijo.0803189. [DOI] [PubMed] [Google Scholar]
- 165.Kanaya AM, Wassel FC, Vittinghoff E, Harris TB, Park SW, Goodpaster BH, et al. Adipocytokines and incident diabetes mellitus in older adults: the independent effect of plasminogen activator inhibitor 1. Arch Intern Med. 2006;166:350–356. doi: 10.1001/archinte.166.3.350. [DOI] [PubMed] [Google Scholar]
- 166.Sprengers ED, Kluft C. Plasminogen activator inhibitors. Blood. 1987;69:381–387. [PubMed] [Google Scholar]
- 167.Song CZ, Siok TE, Gelehrter TD. Smad4/DPC4 and Smad3 mediate transforming growth factor-β (TGF-β) signaling through direct binding to a novel TGF-β-responsive element in the human plasminogen activator inhibitor-1 promoter. J Biol Chem. 1998;273:29287–29290. doi: 10.1074/jbc.273.45.29287. [DOI] [PubMed] [Google Scholar]
- 168.Esemuede N, Lee T, Pierre-Paul D, Sumpio BE, Gahtan V. The role of thrombospondin-1 in human disease. J Surg Res. 2004;122:135–142. doi: 10.1016/j.jss.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 169.Lundgren CH, Brown SL, Nordt TK, Sobel BE, Fujii S. Elaboration of type-1 plasminogen activator inhibitor from adipocytes. A potential pathogenetic link between obesity and cardiovascular disease. Circulation. 1996;93:106–110. doi: 10.1161/01.cir.93.1.106. [DOI] [PubMed] [Google Scholar]
- 170.Mori MA, Liu M, Bezy O, Almind K, Shapiro H, Kasif S, et al. A systems biology approach identifies inflammatory abnormalities between mouse strains prior to development of metabolic disease. Diabetes. 2010;59(11):2960–2971. doi: 10.2337/db10-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kennedy AJ, Ellacott KLJ, King VL, Hasty AH. Mouse models of the metabolic syndrome. Dis Model Mechan. 2010;3:156–166. doi: 10.1242/dmm.003467. [DOI] [PMC free article] [PubMed] [Google Scholar]